Desalted duck egg white nanogels as Pickering stabilizers for food-grade oil-in-water emulsion
Jingyun Zho, Xiohn Guo, Ze Chen, Ylei Di, Hongshn Ling,Qinhun Deng, Shugng Li, Bin Zhou,*
a Key Laboratory of Fermentation Engineering, Ministry of Education; National “111” Center for Cellular Regulation and Molecular Pharmaceutics;Hubei Key Laboratory of Industrial Microbiology; School of Biological Engineering and Food, Hubei University of Technology, Wuhan 430068, China
b College of Food Science and Technology, Huazhong Agricultural University, Wuhan 430070, China
c Key Laboratory of Oilseeds processing, Ministry of Agriculture and Rural Affairs, Wuhan 430062, China
Keywords:
Salted duck egg white
Desalination
Protein nanogels
Pickering emulsion
Stability
A B S T R A C T
Achieving the reuse of traditional egg by-products, salted duck egg whites (SEW), is an urgent problem to be solved. In this current work, we constructed a heat-induced gel-assisted desalination method for SEW.Subsequently, a top-down way was utilized to prepare desalted duck egg protein nanogels (DEPN) with uniformly distributed diameters and their application in the oil/water (O/W) interface system was explored.The results revealed that the increase of DEPN concentration could lower the droplet size, however, the size was negatively correlated with the oil phase fraction. Moreover, the effect of pH, ionic strength, and temperature on the emulsion stability demonstrated that the DEPN-stabilized emulsion displayed superior physical stability under different conditions. The addition of NaCl resulted in the significant decrease in droplet size of the emulsion, while further increasing the NaCl concentration, the droplet size did not decrease accordingly. Besides, heat-treatment and cold-treatment had little negative effect on the stability of the emulsion. Even if the droplet size of the emulsion increased at 80 °C for 3 h, the morphology of the emulsion remained unchanged. Our study demonstrated DEPN had great potential as a stabilizer for food-grade Pickering emulsions.
1. Introduction
Salted duck egg is one of the most popular preserved egg products in China, and egg yolks are widely used as food ingredient in moon cakes, pastries and some dishes owing to its unique flavor and desired texture [1-3]. However, the production of salted egg yolks results in the generation of huge amounts of SEW with high-salt content (7%-12%) [4].Plenty of researches have conducted from different perspective to reuse SEW. For instance, it could be used as an additive to partially substitute salt or to improve the gel-properties of foods [5,6].Besides, extraction of bioactive proteins, such as lysozyme, is also the main reuse method of SEW. However, the high-salt content limits its scope of application, resulting in colossal waste of highquality protein resources and serious environmental problems [7-9].Therefore, realizing the reuse and expanding the application scope of SEW is still an important issue in the poultry and egg industry.
Nowadays, the SEW after desalination are of widespread used in industry and other fields [10,11]. The typical desalination methods are electrodialysis, ion exchange [3], nanofiltration [12], reverse osmosis [13]. Although some of these methods have high desalination efficiency and can maintain the original functional characteristics of the materials, the SEW may adhere to the surface of the membrane due to its high viscosity, which makes the method unsustainable and costly. Therefore, looking for an efficient, convenient, and economical approach for desalting and making full use of SEW is of immense scientific interest. According to previous reports, there was a simple and low-cost approach that SEW was immersed to desalinate using the gel-forming property of egg whites after heating [14,15], while the reuse of the formed gel have thus far been limited.
In recent years, food-grade particles as the Pickering emulsion stabilizers have been brought into scholars’ focus, which avoided the use of some environmental-unfriendly surfactants [16]. It has reported that the sources of food-grade particles as stabilizers include some proteins and polysaccharides [17], among which egg white protein micro-/nano-particles with good emulsifying capacity are great material as a stabilizer for Pickering emulsion [11]. Herein, the objective of this study was to explore a simple and economical way to desalt SEW and reuse desalted duck egg protein nanogels (DEPN) via a physical “top-down” method as a food-grade material to stabilize the Pickering emulsion. In addition, the characterization of DEPN and the stability of the Pickering emulsion were investigated, as well as the effects of DEPN concentration, O/W fraction, storage, pH,ionic strength, and temperature stability on the Pickering structures.DEPN had extraordinary potential as a food-grade Pickering emulsion stabilizer, which broadened the scope of application of duck egg industry by reusing the by-products of SEW.
2. Materials and methods
2.1 Materials
Salted duck eggs were bought from a local market in Wuhan,China. Soybean oil was obtained from a local supermarket in Wuhan,China, without further purification before usage. Sodium azide and fluorescent dyes (Nile Blue A and Nile Red) were purchased from Invitrogen (USA). Water was purified by treatment with a Milli-Q apparatus, with a resistivity not less than 18.2 MΩ cm at 25 °C. All other chemical reagents used in this study were of analytical grade.
2.2 Desalination of SEW
2.2.1 Preparation of desalted duck egg white gel (DEWG)
The SEW were separated from the salted duck egg yolks, and the chalazae were removed by filtering with gauze to obtain the complete SEW. The prepared SEW were heated in a water bath at 90 °C for 30 min in order to form a SEW gel followed by breaking up into blocks of 1 cm × 1 cm × 1 cm. The blocks of SEW gel were directly immersed in water to remove salt for 6 h (water changed every 2 h),gently stirring during the process of desalination. Finally, the SEW gel after immersing and desalting was filtered using gauze to obtain the DEWG.
2.2.2 Determination of desalination rate
The content of sodium chloride (NaCl) in SEW gel and DEWG was referred to the method adopted by Zhou et al. [18]. The gel sample (1 g), 0.1 mol/L AgNO3(25 mL) and concentrated nitric acid(10 mL) were added to a 250 mL Erlenmeyer flask and gently boiled for 10 min. Water and 5 mL of ferric indicator (FeNH4(SO4)2·12 H2O)were added to the solution, which was then titrated by 0.1 mol/L KSCN standard solution, until the solution became permanent light brown. The NaCl content was calculated as follows:
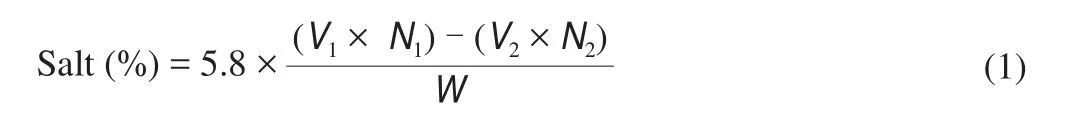
whereV1is the volume of AgNO3(mL);N1is the concentration of AgNO3(mol/L);V2is the volume of KSCN (mL);N2is the concentration of KSCN (N), andWis the weight of sample (g).
The desalination rate can be calculated as follows:
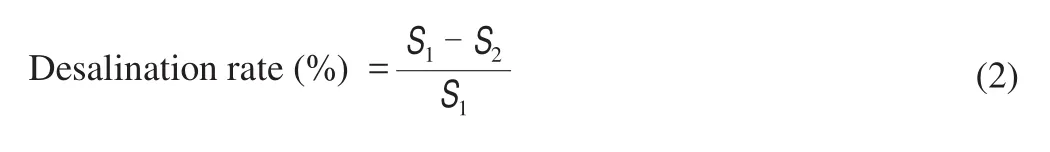
whereS1is the NaCl content in SEW gel before desalination andS2is the NaCl content in DEWG after desalination.
2.3 Preparation and characterization of DEPN
2.3.1 Preparation of DEPN
DEPN were prepared by a previous approach with some modifications [10]. The prepared DEWG (40 g in 100 mL water) was sheared by a homogenizer (T18 digital ULTRA-TURRAX®; IKA Works, Inc., Wilmington, NC, USA) at a speed of 15 000 r/min for 4 min followed by passing through the 160 mesh sieve (ca.97 μm) to obtain the initial broken DEWG particles. Next, the treated samples were homogenized by a microfluidizer (Microfluidics M-110L,Microfluidics Corp., Newton, MA, USA) operated at 9 000 psi to obtain DEPN.
2.3.2 Characterization of DEPN
Dynamic laser scattering (DLS) and zeta potential analysis:The particle size, zeta potential and polydispersity index (PDI) of the DEPN were measured by Zetasizer Nano-ZS90 (Malvern instruments,England) on the basis of DLS techniques at 25 °C [19].
The morphology of DEPN were observed by transmission electron microscopy (TEM) analyzer (JEM-2100F, Japan).
2.4 Preparation of Pickering emulsion
The Pickering emulsions were prepared by DEPN dispersions at varying concentration of 10%-40% (m/V) with relative variation of O/W fraction. As an example, the emulsions at O/W of 30% were prepared as follows: In brief, 6 mL of soybean oil was added to 14 mL of the DEPN suspensions with 0.02% (m/V) sodium azide in a glass vial, and the emulsions were achieved by shearing at 10 000 r/min for 3 min via a T18 homogenizer with the final pH value of 7.4 (T18 digital ULTRA-TURRAX®; IKA Works, Inc.,Wilmington, NC, USA). To study the storage stability, samples after preparation were stored for 30 days, which were characterized at different time intervals.
2.5 Characterization of Pickering emulsions
2.5.1 Droplet size of Pickering emulsions
The droplet size of Pickering emulsion was measured using a static light scattering instrument (Mastersizer 2000; Malvern Instruments Ltd. Worcestershire, UK) at 25 °C. The droplet size was showed as surface-weighted mean diameters (D3,2). All of the experiments were performed in three replicates.

wherenirepresented the number of droplets andDirepresented the particle diameter.
2.5.2 Zeta potential of Pickering emulsions
Zeta potential of Pickering emulsion was evaluated using Zetasizer Nano-ZS90 (Malvern instruments, England) at 25 °C. Prior to test, all the samples were diluted to the appropriate concentration.And all samples were balanced for 120 s and then tested over 20 cycles. The results were reported as averages of three readings.
The micromorphology of Pickering emulsion was observed using an optical microscope. Before observing the morphology, all samples were diluted with the continuous phase.
Besides, Leica TCS SP8 Confocal Laser Scanning Microscope(CLSM) (Leica Microsystems, Mannheim, Germany) was also used to observe the microstructure of Pickering emulsion at 25 °C.Protein phase and oil phase were stained with Nile Blue A and Nile Red respectively. The CLSM observation was conducted using dual-channel imaging with the laser excited at 633 and 488 nm,respectively.
2.5.4 Evaluation of emulsions stability
For the pH stability, all the DEPN (pH 7.4) was firstly adjusted to pH 3.0, 4.0, 5.0, 6.0, 7.0 and 9.0, and then prepared the emulsions according to the above mentioned method. Droplet size and size distributions as well as visual appearance of the emulsions were monitored periodically.
The prepared Pickering emulsions were mixed with different ratios of salt solution and water, reaching the final salt concentration ranging from 100 mmol/L to 500 mmol/L. Droplet size and size distributions as well as visual appearance of the emulsions were monitored, respectively.
The prepared Pickering emulsions were stored at 4, 40, 60 or 80 °C for 3 h, and then stored in room temperature prior to analysis.Droplet size and size distributions as well as visual appearance of the emulsions were monitored, respectively.
2.6 Statistical analysis
All experimental measurements were performed in three parallels.Statistical differences were assessed using one-way analysis of variance with Duncan’s test and significant differences were accepted withP< 0.05. All the values were shown as mean ± standard deviation (n= 3). And the entire statistical analysis was performed using SPSS statistical software (version 23; SPSS Inc., Chicago, IL).
式中:Pm表示汽车等速(60km/h)行驶时的功率;S为汽车续驶里程要求(km);W为电池可用能量(kW·h);ur为电动汽车行驶速度(km/h );Pm为汽车等速行驶时所需功率(kW);t为机械效率,本文取0.9;M为汽车质量(kg);f为滚动阻力系数;W为单个串联电池组可用电量;Q为电池总容量(Ah);Ubat为蓄电池单体电压(V);N1求得为30。
3. Results and discussion
3.1 Desalination rate
The desalination rate of SEW is an important indicator to illustrate the feasibility of the desalination method. In this study, SEW gels after heating were immersed in water for 2, 4, 6 h (water changed every 2 h) respectively to desalinate. According to the results, the desalination rates of salt DEWG significantly increased as the desalination time increased from 2 h to 4 h, reaching to(83.8 ± 3.8)% and (95.7 ± 2.2)% respectively. After 6 h desalination,the desalination rate reached to (98.6 ± 1.9)% (Data not shown),which was more efficient than that of (92.16 ± 0.21)% by ultrasound and microwave pretreatments [1], suggesting the immersion desalination method of the DEWG was more convenient and costeffective. Hence, it was determined that 6 h-desalination (water changed every 2 h) would be used in subsequent experiments.
3.2 Characterization of DEPN
The DEPN were prepared from DEWG via a physical topdown method. As can be seen in Fig. 1a, the particle size of DEPN were of narrow and unimodal distribution with the mean particle diameter of the DEPN at (387.9 ± 2.4) nm and the PDI at 0.21 ± 0.01,which suggested that the distribution of DEPN particle size was monodispersed. In addition, the zeta potential of DEPN was shown as (-19.2 ± 1.7) mV. As presented in TEM image in Fig. 1b, DEPN consisted of particles of size with hundreds of nanometers and all particles showed irregular spherical and irregular block-like structures, which were not uniform. This was probably because the treatment of high-speed shearing and high-pressure homogenization was random to the fragmentation of the gel, thus obtaining nanogels with non-uniform morphology.
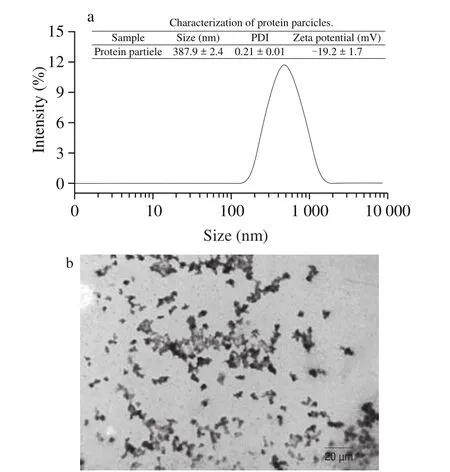
Fig. 1 The size distribution of DEPN (a). The TEM image of DEPN (b).
3.3 Characterization of Pickering emulsions
3.3.1 Droplet size of Pickering emulsions
The process of desalination of SEW and preparation of the Pickering emulsion stabilized by DEPN was presented in Fig. 2a. In previous studies, it was reported that the properties of the Pickering emulsion were affected by the properties of the stabilizers [20-22],while the emulsification efficiency or emulsification ability of the emulsions can generally be reflected by the droplet size of the emulsions. In general, the emulsification efficiency or ability increased as the droplet size decreased [23]. Figs. 2c-f showed the droplet size and size distribution of Pickering emulsions stabilized by different DEPN concentration ranging from 10% to 40%, with the fixed oil phase after different time storage. When the DEPN concentration increased from 10% to 40%, the droplet size of emulsion decreased gradually. This was probably because more particles were adsorbed on the interface of oil droplets as the protein concentration increased,in order to maintain the stability of the emulsion, which led to the decrease in the droplet size diameters of the emulsions. In addition,except for the emulsion stabilized by 10% DEPN, the droplet size and zeta potential of other emulsions changed slightly with the increase of storage days, which showed the great storage stability of emulsions.Especially, the phenomenon of the creaming behavior was noticeable at 10% DEPN, while this phenomenon did not occur when the DEPN concentration was 20%-40% (Fig. 2b). The optical microscope images of the emulsions in Fig. 3 agreed with this phenomenon.The connection between the droplets was closer and the droplet size was lager at lower DEPN concentration (10%), while the emulsions displayed good dispersibility and the droplet became relatively uniform with the increase of DEPN concentration (20%, 30%, 40%).Hence, we selected 20% DEPN to stabilize the emulsions in the subsequent experiment to examine the influence of different factors on the stability of the emulsion.
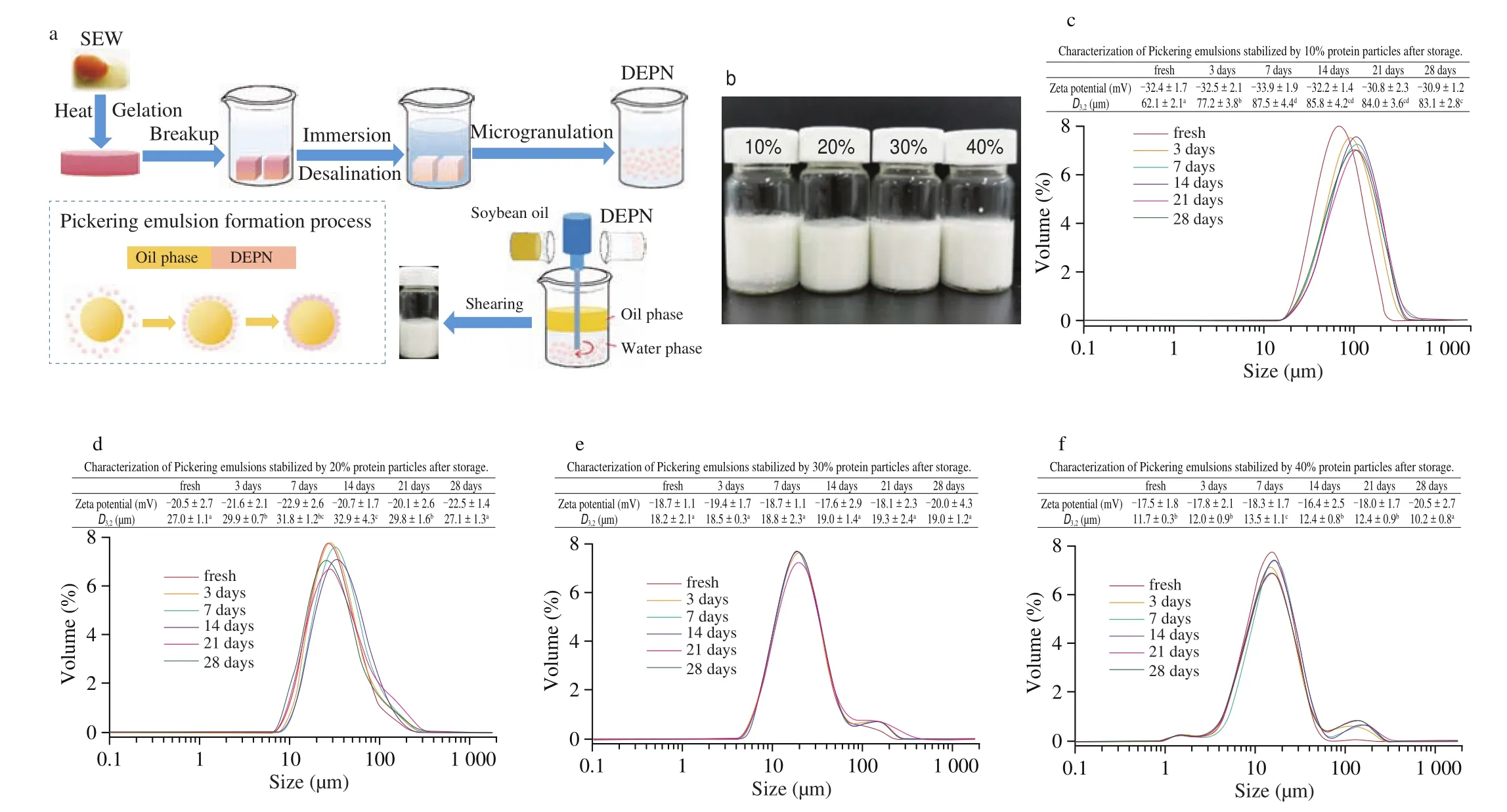
Fig. 2 Schematic diagram of Pickering emulsion formation (a). Photos of the emulsions with different concentration of DEPN (10%, 20%, 30%, 40%, m/m,without pH adjustment) with fixed oil fraction of 30% (V/V) (b). The size distribution of Pickering emulsions stabilized by 10% (c), 20% (d), 30% (e) and 40% (f)initial DEPN solution after different storage time with fixed oil fraction of 30% (V/V).
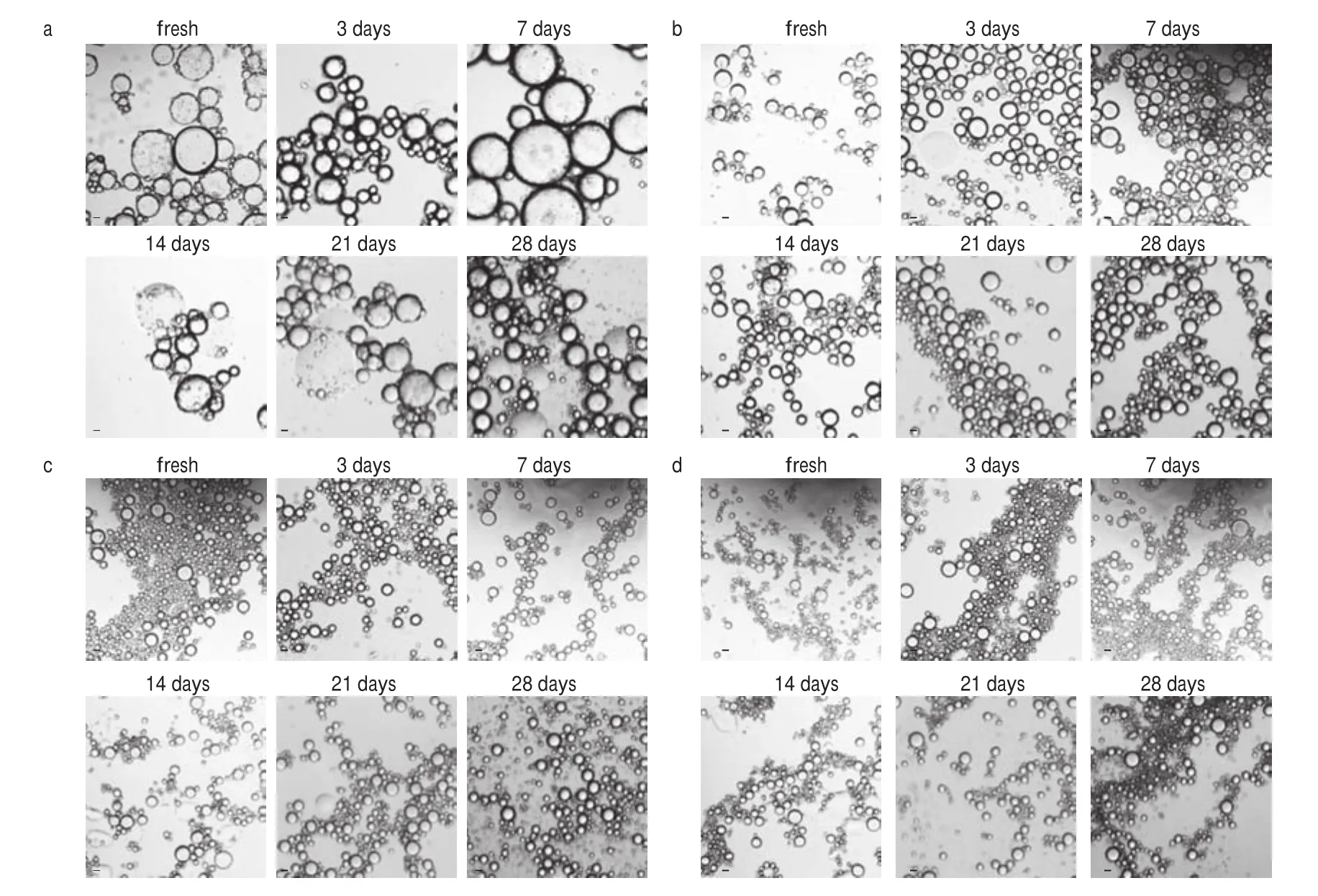
Fig. 3 Optical micrographs of the emulsion stabilized by 10% (a), 20% (b), 30% (c), 40% (d) initial DEPN solution with fixed oil fraction of 30% (V/V).
3.3.2 Microstructure
The microstructure of the emulsion was studied using CLSM.The oil and protein phases were stained green and red, respectively,using fluorescent dyes. The images in Fig. 4 showed the obvious contrast between bright green areas and red circles scattered around them, which indicated that the emulsions stabilized by DEPN were typical O/W emulsions [24], and the DEPN were adsorbed at the O/W interface to form a close-packed layer around the oil droplets,thus maintained the stability of the emulsions.
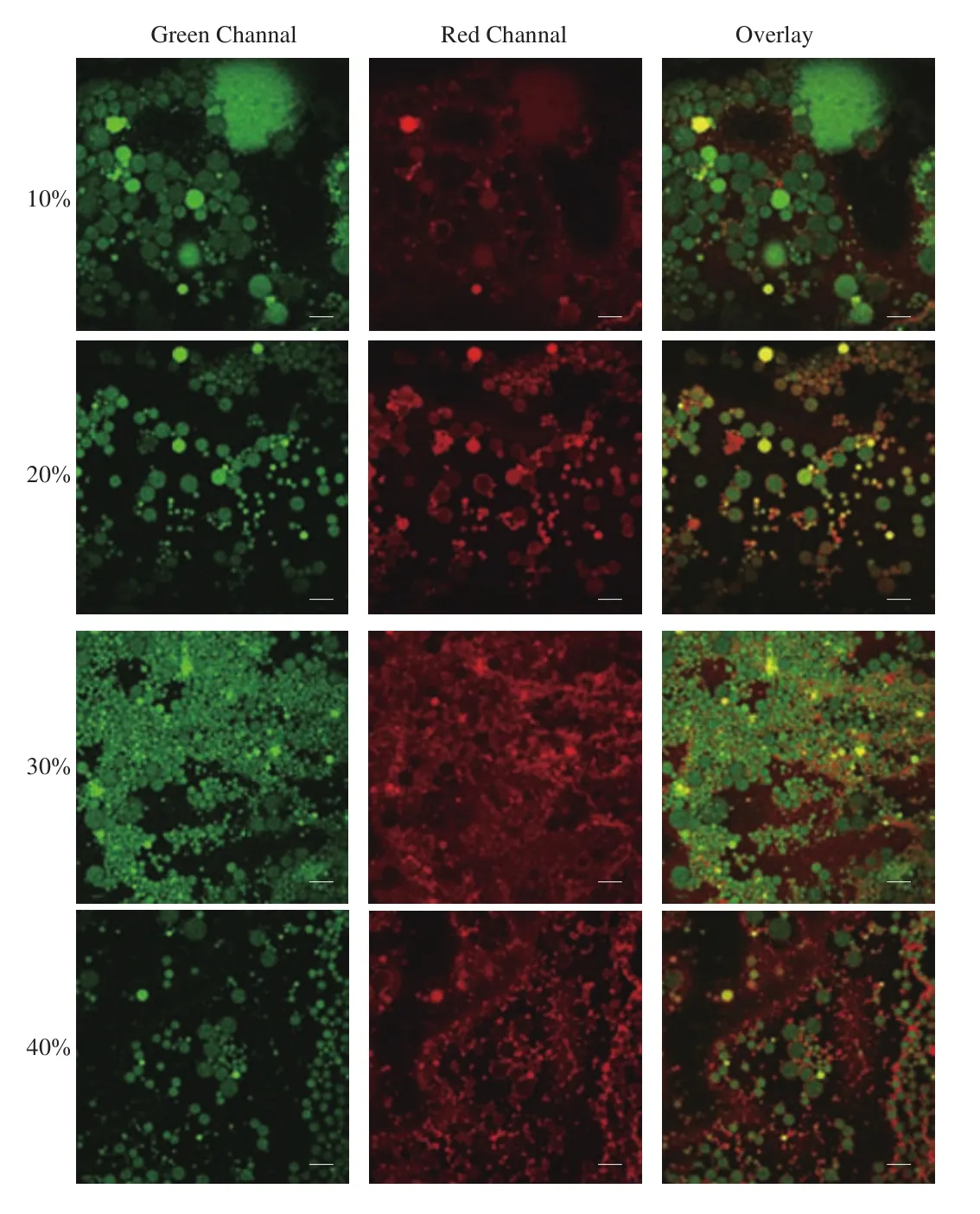
Fig. 4 CLSM images of emulsions stabilized by DEPN (10%, 20%, 30% and 40%, m/m, without pH adjustment) with fixed oil fraction of 30% (V/V).
3.3.3 Influence of oil phase
In Fig. 5a, as the oil fraction in the emulsion increased ranging from 40% to 60%, the droplet size (D3,2) also increased obviously from (65.0 ± 1.5) μm to (156.3 ± 8.2) μm which was consistent with the trend about emulsions stabilized by egg white protein [25]. With the volume of the oil phase increased, the droplet aggregation was promoted, resulting in the increase in droplet size, which could be interpreted by a phenomenon called limited coalescence [26,27].It could be inferred that the increase of oil fraction resulted in the increase specific surface area, so more DEPN were needed to adsorb on the interface against the instability of the emulsion,leading to insufficient DEPN to completely cover the surfaces of the oil droplets. In order to reduce the specific surface area of the droplets to maintain stability of the emulsion, the droplets aggregated due to strong interaction, which allowed more largesize particles to be detected. This could also be seen from the optical microscope image of the emulsion in Fig. 5c. Image of emulsions prepared with oil fraction of 40%, 50% and 60% were recorded in Fig. 5b. As the oil fraction increasing, the appearance of the emulsions did not change obviously, and the phenomenon of delamination did not happen.
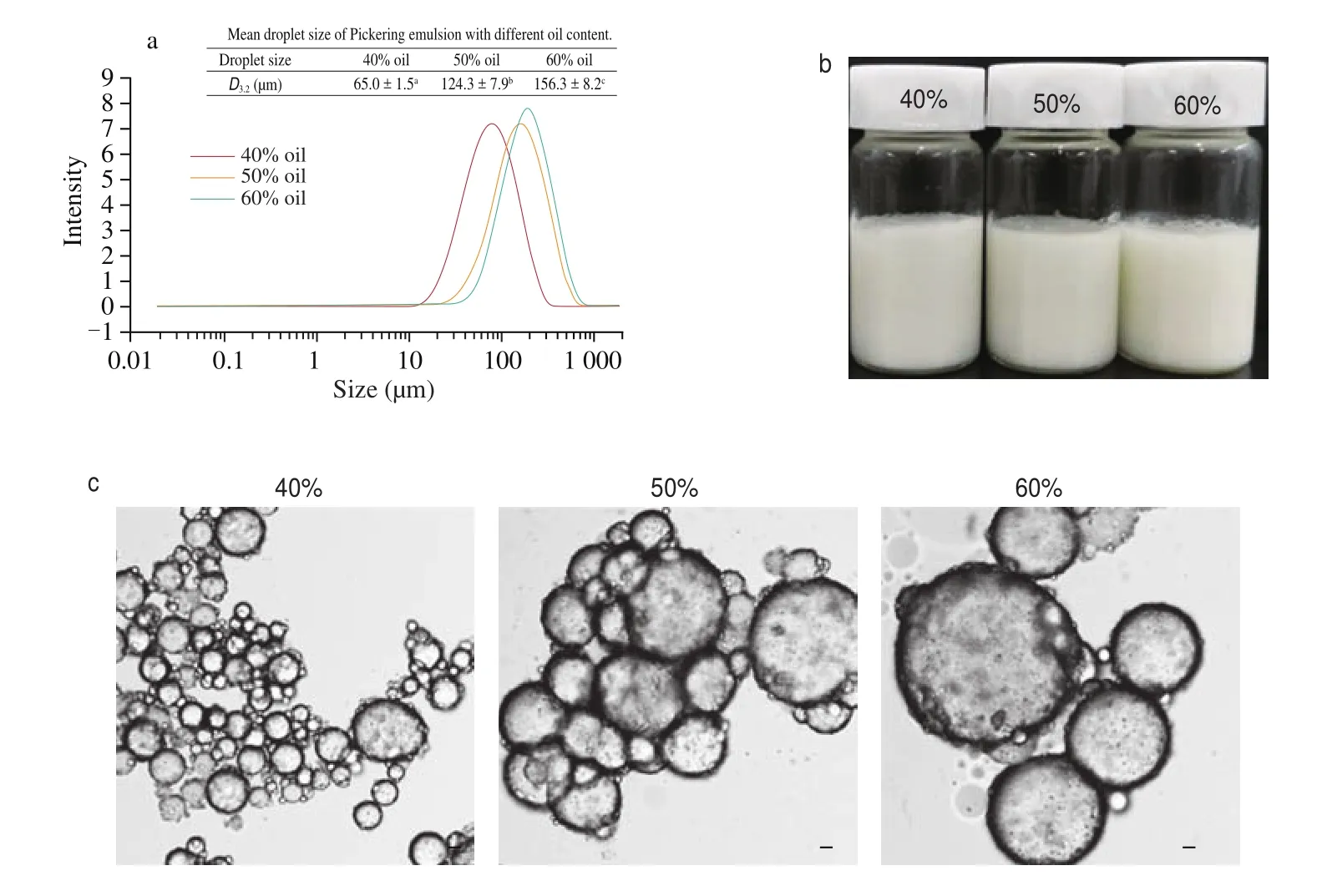
Fig. 5 The size distribution of Pickering emulsions with different oil fraction (40%, 50%, 60%, V/V) (a), Appearance of the emulsions with different oil phase(40%, 50%, 60%, V/V) (b), Optical micrographs of the emulsions with different oil phase (40%, 50%, 60%, V/V) (c). All the emulsions stabilized by 20% (m/m)DEPN without pH adjustment.
3.3.4 Effect of pH on emulsion stability
The pH value is one of the factors that affect the stability of the edible emulsion, because a food product usually undergoes a certain pH ranges during processing and digestion [19]. The droplet size and microstructure of the emulsions at various pH (3.0-9.0) were characterized. It was shown in Fig. 6 and Table 1 that the droplet size of the emulsions had no obvious change under different acidic environment (pH = 3, 4, 5, 6), while, it increased at pH 7 and pH 9,as well as the emulsion at pH 7.4 without adjustment. Besides, the optical microscope images of fresh emulsions and that of storage for 28 days at different pH values were displayed in Fig. 6. Apparently,DEPN-stabilized Pickering emulsions showed no noticeable differences in micromorphology at pH 3.0-7.4 and displayed more dispersed which may be due to DEPN exhibited a better stability against droplet aggregation at these pH values. It was noticeable that the droplet size of the emulsion increased at pH 9 and the aggregation happened between droplets, suggesting that pH may affect storage stability of the emulsion under alkaline conditions. In addition, as displayed in Table 2, with pH increasing from 4 to 5, Zeta potential of emulsions decreased from (8.4 ± 1.5) mV to (-15.1 ± 2.8) mV with the transformation from positive charge to negative change. It is known that the main protein in SEW is ovalbumin (OVA), and the isoelectric point (pI) of OVA is about 4.7 [28]. When dropping the pH down below pI, the surface of OVA mainly displays a positive charge, on the contrary, an increase in pH above pIcan changes to the electrostatic charge of protein surfaces, as a result of more negative charges of the protein, which is consistent with our results [29,30].Although the stability of the emulsions reduced under strong alkaline conditions, the pH of most food system is mainly in acidic, neutral and weakly alkaline conditions, which we can speculate that the emulsion prepared by DEPN it is very stable in wide pH 3.0-7.4 range and can be widely applied in some food.
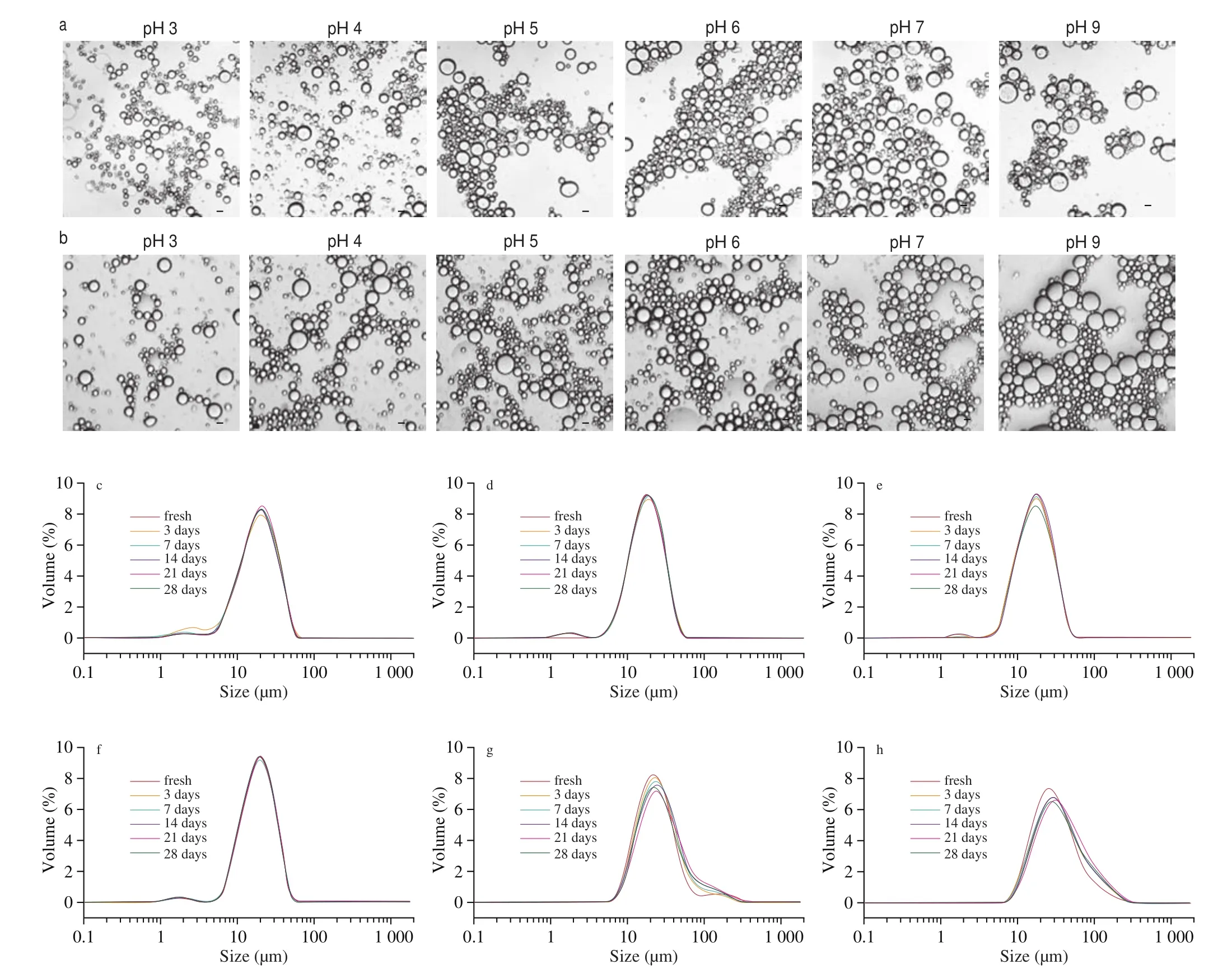
Fig. 6 Optical micrographs of the fresh emulsions (a) and stored emulsions after 28 days (b) in different pH value (3, 4, 5, 6, 7, 9), the size distribution of Pickering emulsions in different pH value (3, 4, 5, 6, 7, 9) (c-h). All the emulsions stabilized by 20% (m/m) DEPN with fixed oil fraction of 30% (V/V).
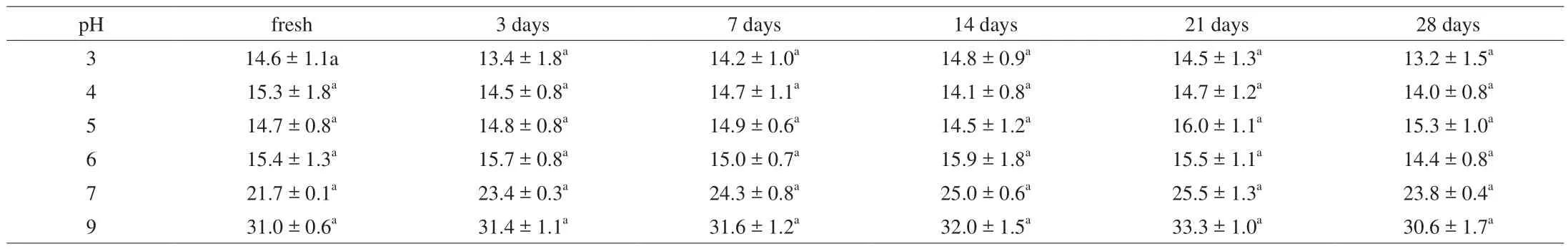
Table 1Mean droplet size (d3,2, μm) of Pickering emulsions with different pH value.

Table 2Zeta potential of Pickering emulsion with different pH value.
3.3.5 Ionic strength stability
Food systems often need to adapt to different ionic strength environments, so it is necessary to examine the performance of emulsions at different ionic strengths [31]. The ionic strength was adjusted by adding salt solution, and the influence of ionic strength was based on observation of the droplet size and the optical microscope images of the emulsions. Compared to the emulsion without adding NaCl, the droplet size of the emulsion decreased after adding NaCl, while futher increasing the NaCl concentration,the average droplet size of emulsions changed slightly as presented in Fig. 7g. This is probably because the electrostatic repulsion was sufficiently intense with the addition of NaCl to prevent the aggregation between droplets, and electrostatic repulsion significantly improved the emulsification efficiency of DDEP. Meanwhile, the increasing surface hydrophobicity of the DDPN may lead to the reduction of the droplet size as well [32]. Besides, Figs. 7b-f showed that the microstructure of DEPN-stabilized Pickering emulsions with different concentrations of NaCl displayed no obvious change, which corresponded to the results of the emulsion droplet size. In terms of ionic strength, it can be indicated that the DDPN-stabilized emulsion was not significantly affected by the concentration of NaCl, even the emulsion stability enhanced with the addition of NaCl, presenting the potential of application in food system.
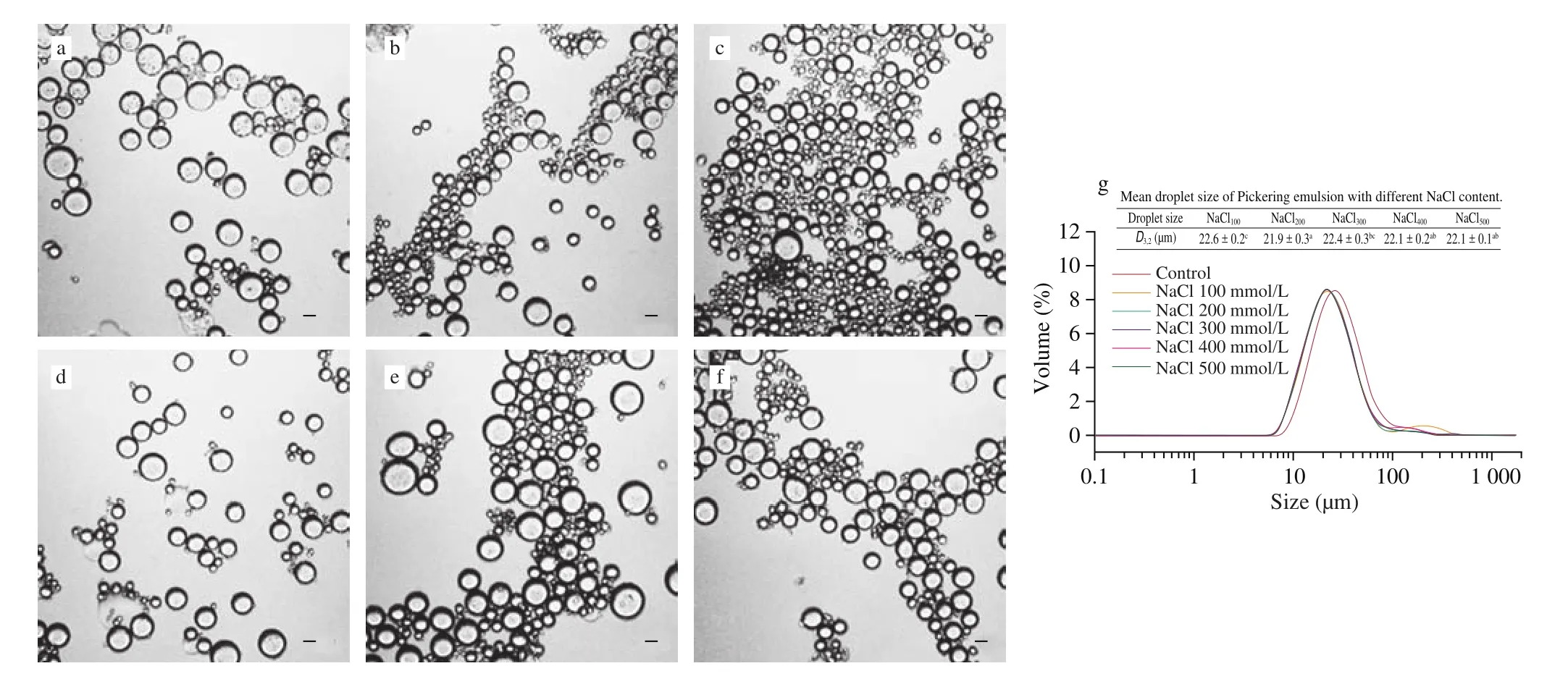
Fig. 7 Optical micrographs of the emulsions in different ionic strength: control (a), 100 (b), 200 (c), 300 (d), 400 (e), and 500 mmol/L (f). Size distribution of Pickering emulsions in different ionic strength (g). All the emulsions stabilized by 20% (m/m) initial DEPN solution with fixed oil fraction of 30% (V/V).
3.3.6 Temperature stability
In food processing, food productions usually undergo heat-treatment as an important step, therefore, the effect of temperature on the emulsion stability should also be investigated [33]. The Pickering emulsions were incubated at the temperature of 4, 40, 60, 80 °C, respectively, for 3 h followed by adapting to room temperature at 25 °C, compared with that of control at room temperature (25 °C) all the time. It can be seen in Fig. 8 that the droplet size and microstructure of the emulsion did not change obviously, whether it underwent thermal-treated (40, 60 °C)or cold-treatment (4 °C), except for the emulsion treated at 80 °C.However, the droplet size of the Pickering emulsion was enhanced when the treatment temperature was at 80 °C, which could be caused by the expanding of the partial protein and the alternation of surface hydrophobicity [14], but the visual appearance of the emulsion almost remained unchanged judging from the optical micrograph of the emulsion, which indicated that the emulsion can be considered as stable. In summary, the stability of the emulsion is insensitive to changes in various temperatures ranging from 4 °C to 80 °C.
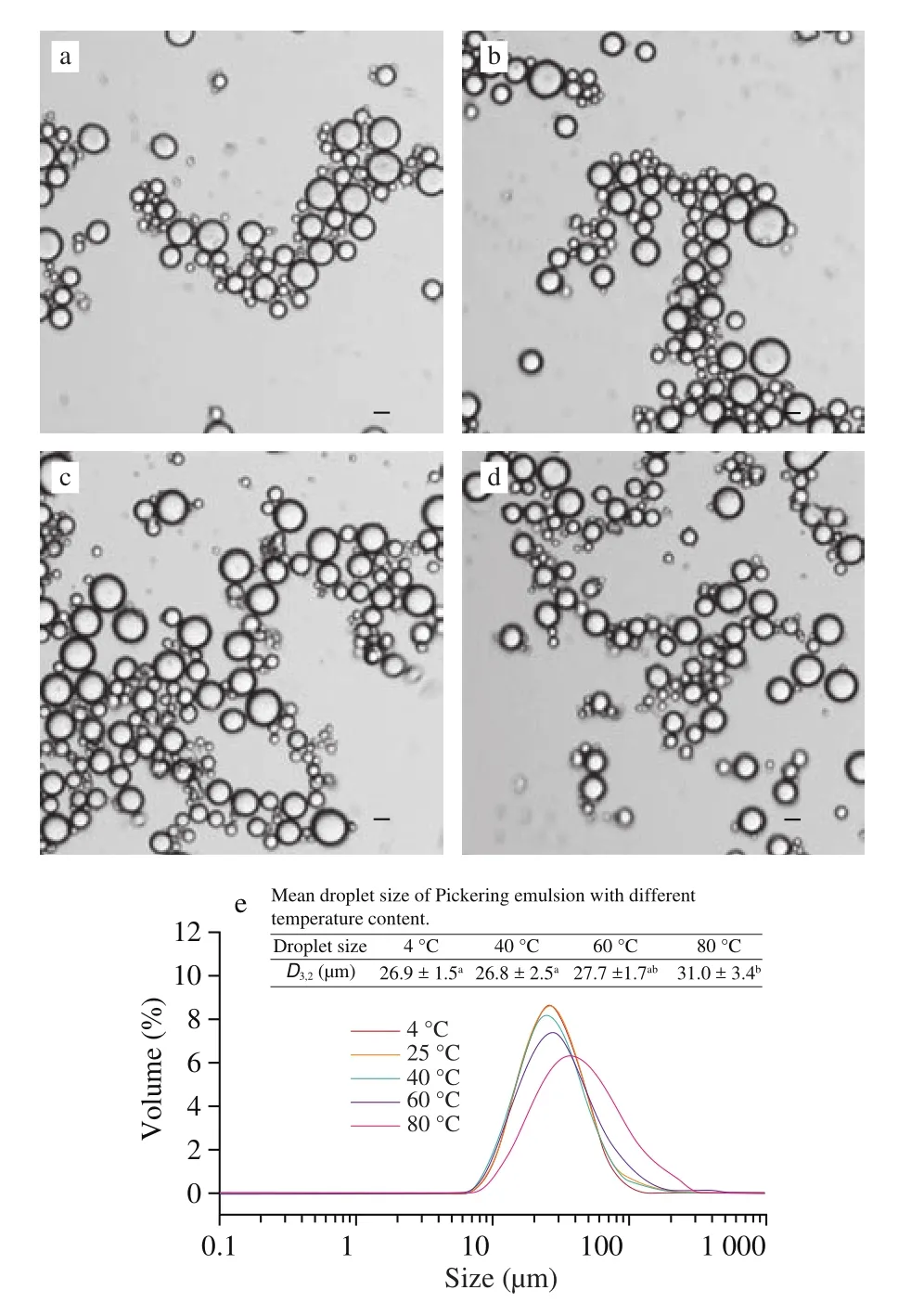
Fig. 8 Optical micrographs of the emulsions treated under 4 (a), 40 (b), 60 (c),and 80 °C (d) for 3 h. The size distribution of Pickering emulsions treated under different temperature (e). All the emulsions stabilized by 20% (m/m)initial DEPN solution with fixed oil fraction of 30% (V/V).
4. Conclusions
In this study, a simple method for desalting SEW was investigated,and DDPN-stabilized Pickering emulsions were characterized. The SEW gel was immersed for several hours to desalination, which method of desalination was efficient, convenient, and low-cost, with the desalination rate of (98.6 ± 1.9)% for 6 h (water changed every 2 h).The average particle diameter of the DEPN was (387.9 ± 2.4) nm with uniform distribution. The droplet size of the emulsion decreased as the increase of the DEPN concentration, and increased as the oil fraction increased. In general, the stability and storage stability of the emulsions performed superior stability, except for the emulsion stabilized by 10% DEPN. The microstructure of the emulsion indicated that the emulsion was an O/W Pickering emulsion, in which the DDPN were adsorbed to the surface of the oil droplets.This article also examined the effect of pH value, ionic strength, and temperature on the stability of the emulsion. The results demonstrated that the DEPN-stabilized emulsion displayed superior physical stability under acid condition (pH 3.0-7.4). Besides, heat-treatment and cold-treatment have little negative effect on the stability of the emulsion,even if the droplet size of the emulsion increased significantly at 80 °C for 3 h, the morphology of the emulsion remained unchanged. This discovery is of great significance for the development of a new food-grade biocompatible Pickering stabilizer.
conflict of interest
The authors declared there is no conflict of interest.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 32172354), the Opening Project of Key Laboratory of Oilseeds processing, Ministry of Agriculture and Rural Affairs (202003) and the Doctoral Research Startup Fund of Hubei University of Technology (BSQD2017027).
- 食品科学与人类健康(英文)的其它文章
- Yogurt-derived Lactobacillus plantarum Q16 alleviated high-fat diet-induced non-alcoholic fatty liver disease in mice
- The levels of osteopontin in human milk of Chinese mothers and its associations with maternal body composition
- Lactobacillus fermentum as a new inhibitor to control advanced glycation end-product formation during vinegar fermentation
- Screening and identification of purine degrading Lactobacillus fermentum 9-4 from Chinese fermented rice- flour noodles
- Characteristic and effect analysis of protein and peptide in Cantonese cured meat processing
- Formation of composite hydrogel of carboxymethyl konjac glucomannan/gelatin for sustained release of EGCG

