A review on current and future advancements for commercialized microalgae species
Jia Fei Wong, Hui Jing Hong, Su Chern Foo, Michelle Khai Khun Yap, Ji Wei Tan*
School of Science, Monash University Malaysia, Bandar Sunway 47500, Malaysia
Keywords:
Commercialized microalgae
Antioxidant
Anti-inflammation
Antimicrobial
Anticancer
A B S T R A C T
Microalgae are unicellular photosynthetic microorganisms that are commonly found in saline or freshwater environments. Over the years, microalgae represent promising sources of sustainable bioactivities with past literatures reflecting a growing interest in algae-based dietary supplements in the form of whole biomass.Notably, the bioactive molecules that can be identified and extracted in microalgae have scientifically proven to contain therapeutic properties which can be beneficial to human health. With the increasing occurrence of global health threats such as antimicrobial resistance and cancer, this has resulted in considerable attention for microalgae study especially in the medicinal field. Although studies have proved the therapeutic potentials of high-value bioproducts in microalgae, however, there is still room to understand their potential therapeutic properties on humans’ health, discovering novel microalgae-derived bioactive compounds, as well as translating the lab-based evidence to clinical trial studies. This review will focus on accessing the biochemical compositions of commercialised microalgae species from 2007 to 2020, and the activity of their biologically active molecules in eliciting selected therapeutic potentials which are anti-oxidative, anti-inflammatory,anti-microbial and anti-cancer properties. This review article will also be looking at the research gaps in addition to the above four major selected therapeutic potentials, and future prospective.
1. Introduction
Microalgae are photosynthetic and microscopic organisms commonly found in saline or freshwater environments, including prokaryotic and eukaryotic members. They are known as one of the earliest forms of living organisms that existed in the earth oceans over 3 billion years [1]. Microalgae are genetically diversified in a massive manner (> 200 000 species) with abundance in the divisions of Cyanophyta (blue-green algae), Chrysophyta (golden-brown algae)and Chlorophyta (green algae). Microalgae are known as versatile cell factories due to their ability to convert inorganic carbon dioxide,sunlight and water into organic algal. Microalgae biomass is rich in macronutrients (protein, lipid, carbohydrate), and micronutrients(carotenoids, phenolic acids, vitamins and minerals) [1,2]. Nowadays,microalgae species are receiving increasing attention in our daily life due to its beneficial applications. Many experiments have been conducted to explore the compositions of various microalgae in order to elucidate their biological activities in eliciting biotechnological(e.g. wastewater bioremediation, biodiesel production) and therapeutic properties [2]. They also provide a significant source of diverse numbers of essential nutrients. They have been used as food supplement and alternative medicine to prevent or treat some diseases [3]. For example, the most extensively studied microalgae,Arthrospira platensis(spirulina) is a “super food” that is full of nutritional wonders and is generally regarded as safe (GRAS) by the United States Food and Drug Administration (FDA). Other than being utilised as nutritional supplements, it was also scientifically proven that bioproducts fromA. platensiswere associated with certain therapeutic effects such as cholesterol reduction, anti-cancer properties, neuroprotective role, and immune system enhancement [4].Besides that, another microalgae species,Chlorella vulgaris, which is rich in various multifunctional micro- and macro-nutrients (e.g.omega-3 polyunsaturated fatty acids, vitamins, polysaccharides) also contributes significantly in ameliorating hyperlipidaemia as well as preventing oxidative stress, chronic obstructive pulmonary disease and carcinogenesis [5].
However, unlike macroalgae such as seaweeds which had a long historical use of food and medicine, there is limited information on the use of microalgae in human health [6]. Microalgae (~100 μm) is 10-4times smaller than macroalgae (~1 m) and remained unnoticed until recent decades. Besides their inconspicuous size, microalgae are associated with limitations that restrict their widespread usage such as time-consuming isolation process for microalgal bioactive compounds, harvesting costs, the availability of effective and low-cost mass production techniques [2]. Besides, previous studies involving microalgae were mainly focusing on the potential use of microalgae in environmentally friendly fuels production such as biodiesel, ethanol as well as molecular hydrogen [3]. Therefore, the industrial development of microalgae is still in its infancy; hence,more investigations are still needed to improve the current knowledge of microalgae extraction and processing techniques to establish the value chain in their therapeutic potential especially in medical and pharmaceutical fields.
Nevertheless, the current culturing and harvesting technologies still allow the development of microalgae therapeutic research in recent years and provides opportunities for health and nutritional studies. A growing number of microalgae species are acquiring biotechnological interest as they can produce several natural and novel bioactive substances such as polyunsaturated fatty acids,β-carotene and other pigments, sulphated polysaccharides,phycobiliproteins, and sterols which reported to have high nutritional value as well as its therapeutic effect towards human health [6].These microalgae products had been widely used in human nutrition as a high-protein supplement as well as nutraceutical purposes such as production of superfoods and medicinal drugs [7]. All these therapeutically useful bioactive metabolites from microalgae species gained great interest so that more promising remedial agents can be produced to resolve global health concerns such as infectious diseases and cancers. Thus, this literature review aims to assess various therapeutic effects of 8 commercialised microalgae species, which areC. vulgaris,A. platensis,Dunaliella salina,Phaeodactylum tricornutum,Haematococcus pluvialis,Isochrysis galbana,Scenedesmus obliquusandTetraselmis suecica. Firstly, the primary biochemical composition of these microalgae will be discussed.Secondly, the anti-oxidative, anti-inflammatory, anti-microbial and anti-cancer properties of the microalgae species will be explored with emphasis on identified knowledge gaps and future recommendations for continuous improvement.
2. Biochemical composition of microalgae
Microalgae have been proved to contain substantial quantities of carbohydrates, proteins, lipids and other biochemical compositions such as carotenoid pigments, dietary fibres [5]. As such, it had led to an enormous interest for their various applications in health-food,pharmaceutical and medicinal industries. This is because microalgae have been identified as a natural source of a healthy diet that is rich in carbohydrates, lipids, proteins, nucleic acids, dietary fibres and other valuable organic substances including essentials vitamins and minerals. Due to the specific strain of each microalga as well as the physiological response to various abiotic and biotic factors such as light intensity, temperature, growth phase, nutrients and photoperiod,the cellular content of each microalgae species were different [2].
Table 1 summarises the biochemical composition of macronutrients that present in the 8 microalgae that are being studied in this literature review. Based on Table 1,A. platensisrecorded the highest protein content (50%–70%) whereas redH. pluvialiswasassociated with the highest carbohydrates (36%–40%)and lipids (32%–37%) composition among the 8 species. It is noteworthy that most microalgae species listed have relatively high protein levels that are close to those present in traditional protein sources (eggs, soybeans, milk) except redH. pluvialisandI. galbana[5]. Thus, microalgae are regarded as an alternative viable protein source as they are having greater productivity (higher yield per unit area), better protective system against harsh environments and oxidative stresses, and comparable nutritional value when compared to traditional protein-rich crops such as soybeans and nuts [8]. Moreover, the high carbohydrates and lipids content in redH. pluvialisalso potentiated its capability as a safe colouring additive in feeding shrimps and salmonids as well as being utilised as active cosmetic compounds for skin softness [9]. Besides, Table 1 also illustrates the lipids compositions in each microalgae species. For example,I. galbanaandD. salinacontain 10.5% and 6.0% of lipids respectively. It further demonstrated the differences in the levels of protein, carbohydrates or lipids among various microalgae species.
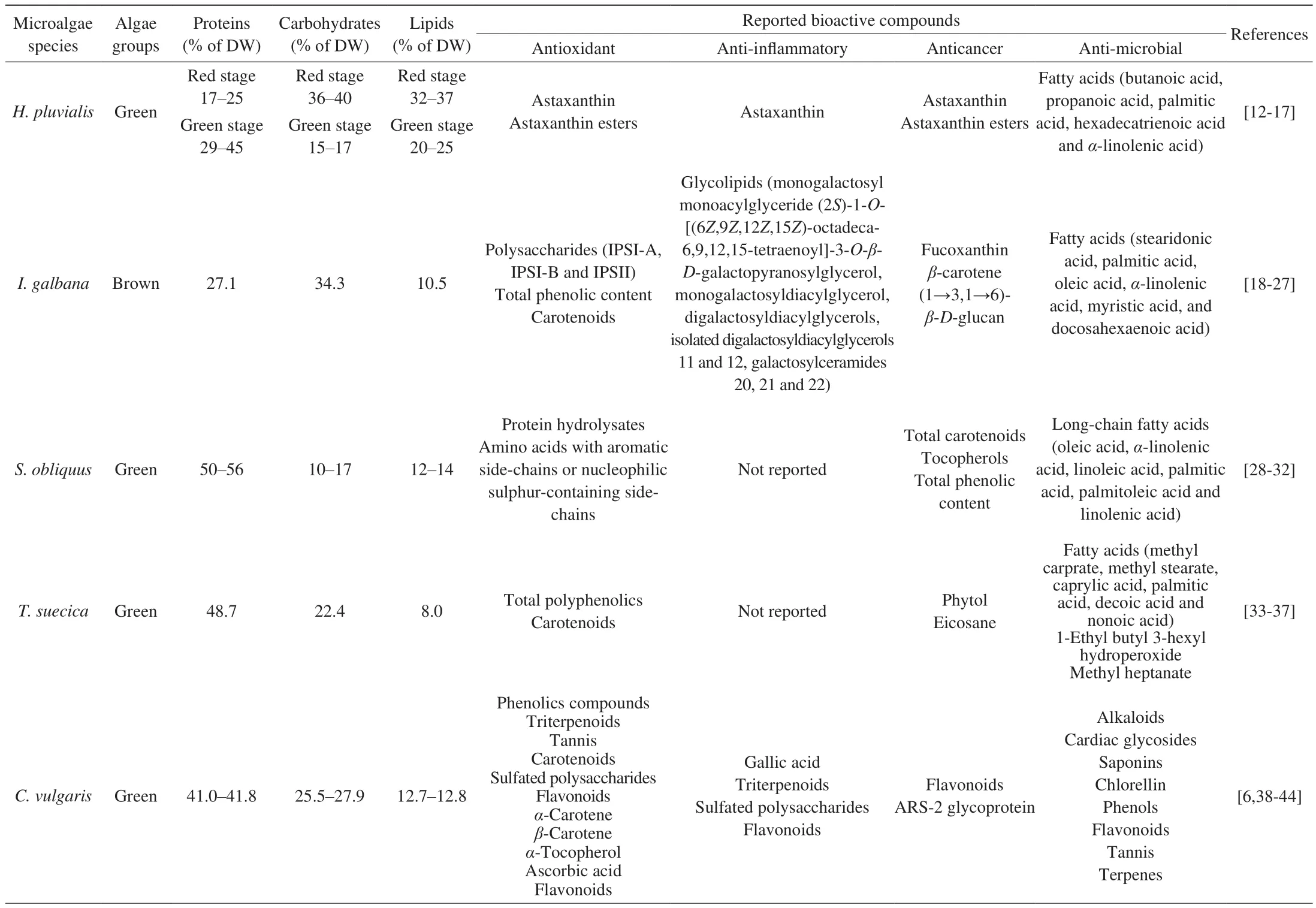
Table 1The primary biochemical composition of 8 commercialised microalgae species expressed on a dry weight basis.
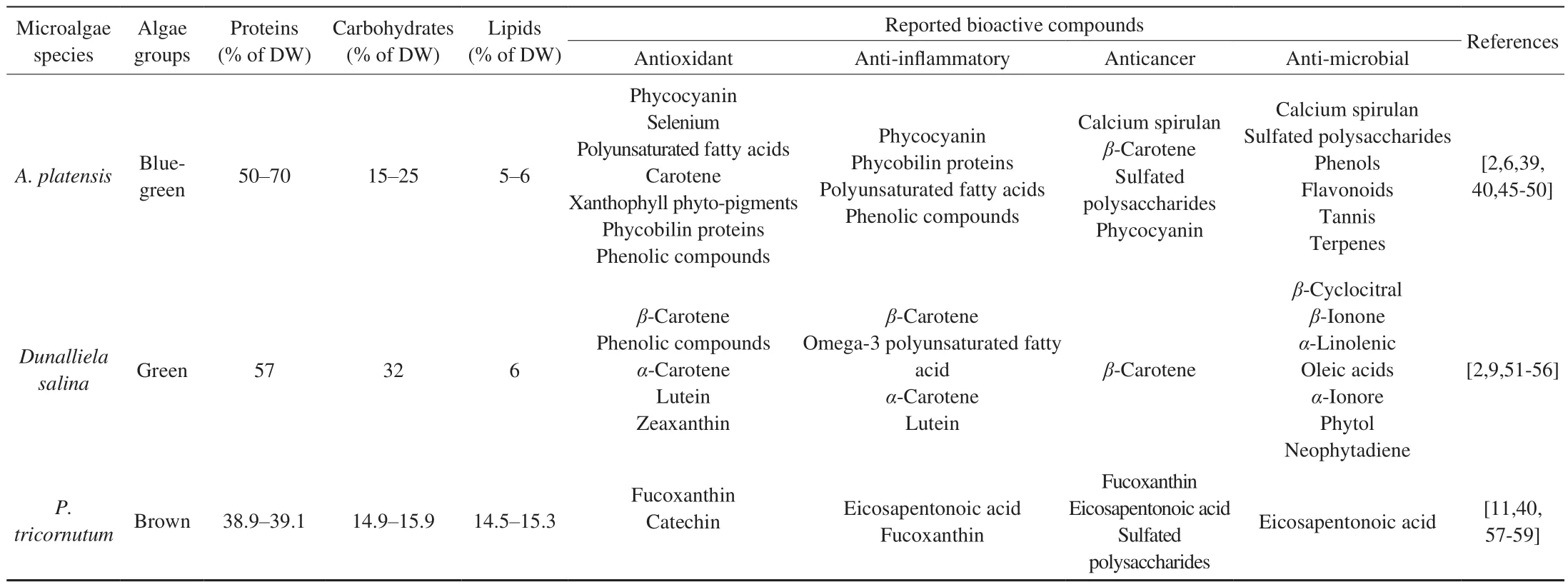
Table 1 (Continued)
Besides, microalgae also synthesize various bioactive metabolites (Fig. 1) which are attributed to various beneficial therapeutics properties such as antioxidant, anti-inflammatory,anticancer and antimicrobial properties. For example, phytochemicals such as chlorophyll, carotenoids extract and phenolic compounds played a crucial role in the anti-inflammatory and antioxidant activity of microalgae whereas eicosapentaenoic acid mainly attributed to the antimicrobial activity of microalgae [10,11]. Due to the limitation of assay available for detection of microalgae compounds,the information regarding each bioactive component present in microalgae is still limited. Therefore, more research is needed in this area to study the cellular content in microalgae which allowed various researchers to identify the peculiarities of some microalgae species to produce high number of desired compounds including carotenoids,protein and long-chain fatty acids, as well as to identify the possible application of these bioactive compounds in different sectors.
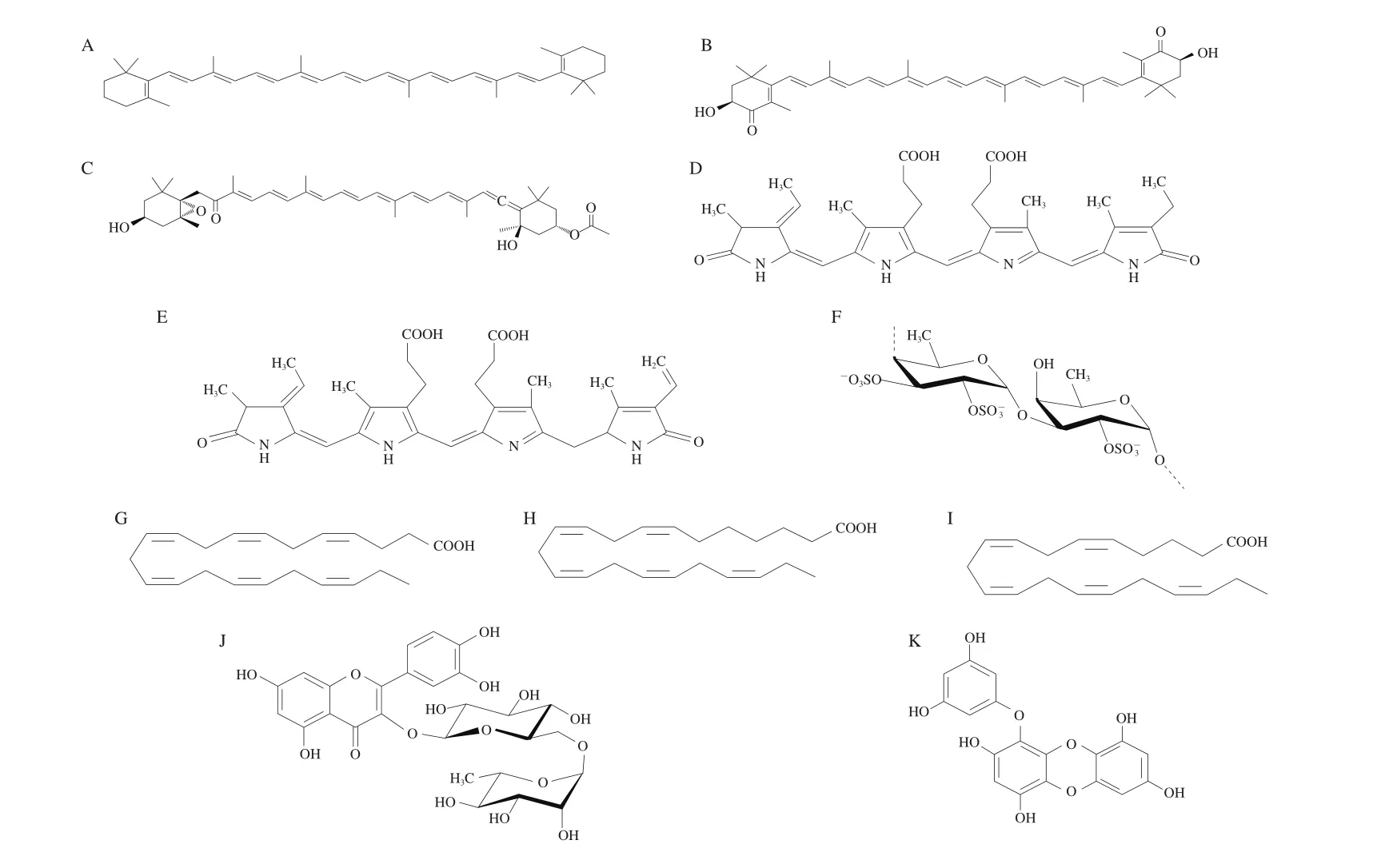
Fig. 1 Chemical structure of common bioactive compounds found in most microalgae species [2]. (A) Astaxanthin; (B) astaxanthin; (C) fucoxanthin; (D) phycocyanin; (E)phycoerythrin; (F) sulphated-polysaccharides; (G) docosahexaenoic acid; (H) docosapentaenoic acid; (I) eicosapentaenoic acid; (J) rutins; (K) eckol.
3. Therapeutics properties of microalgae
Traditionally, microalgae species includingC. vulgaris,H. pluvialis,A. platensisandD. salinahave always been consumed as functional foods in different forms (e.g. powder,solutions, capsules and tablets) in Asian countries such as China and Japan [2,60]. Moreover,P. tricornutum,T. suecica,S. obliquusandI. galbanaare used as feeds for aquatic and terrestrial animals [3], where the richness of unsaturated fatty acids inP. tricornutumandI. galbanais utilised as food additives or act as an alternative source of polyunsaturated fatty acids in nutraceutical food products [61]. Besides that, the presence of numerous bioactive components in aforementioned microalgae species is being actively researched and the recent results indicated various therapeutic potentialities of these species [60,61]. As shown in Fig. 2, the chosen 4 therapeutic benefits of the commercialised microalgae are in great interest and were well-explored over the past decade. In addition,these 8 important commercialised microalgae were reported to elicit these four therapeutic properties in common where their respective bioactive properties will be further discussed below.
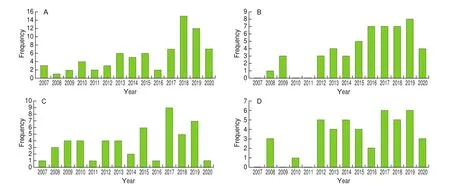
Fig. 2 Timeline regarding published studies on therapeutics properties of 8 different commercial microalgae species from 2007 to 2020. (A) Antioxidant,(B) anti-inflammatory, (C) anticancer, (D) antimicrobial, properties.
3.1 Antioxidant activity
Oxidative stress is defined as an imbalance between antioxidants and oxidants that result in reactive oxidative species (ROS) from the electron transport chain activity in mitochondria [62]. This was due to the leakage of electrons from mitochondria during the stimulation of NADPH via oxidative phosphorylation [63]. These ROS can disrupt and destroy molecules in biological systems resulting in various disorders and diseases including diabetes mellitus, ischemic disease,hypertension, atherosclerosis and malignancy.
Today, the study on the antioxidant activity of microalgae has attracted much attention from various researchers. Various studies had been proved in bothin vitroandin vivothat microalgae possessed antioxidant activity due to the presence of effective antioxidant phytochemicals such as carotene, phycocyanin and phenolics [2].The antioxidants properties can keep the body free from ROS and free radicals due to its radical’s scavenger activity scavenge and thus suppressing the development of several chronic diseases.For example, the antioxidant activity of the polysaccharides fromC. vulgarishad been reported by Yu et al.[62].C. vulgariswasa type of green microalga which is well-documented as medicine,food additive and fodder [62]. This was becauseC. vulgariswhich is rich in various phytochemicals such as chlorophyll, tocopherols,phenolic compounds and carotenoids attributed to its antioxidant properties and found to be beneficial to humans [10]. Thein vivostudy conducted by Sikiru et al. [63]revealed thatC. vulgarisable to stimulate the expression of immune modulation and transcripts genes in rabbits particularly enhancing the activity of antioxidant enzyme such as superoxide dismutase, glutathione peroxidase, catalase enzyme (CAT) and increase the concentration of non-enzymatic antioxidant such as glutathione. These antioxidant enzymes are considered as the primary defence that prevented the oxidative damage of biological macromolecules [63]. Besides,C. vulgarisextract also proved to exert chemoprotective properties by modulating the lipid peroxidation and antioxidant status in serum, kidney and liver of the mice [43]. Thus, dietary supplementation ofC. vulgariscan act as a complementary medicine for prevention of oxidative stress.
Raoet al. [13,14]indicated that astaxanthin and astaxanthin esters inH. pluvialisare potent antioxidants which offered protection against ultraviolet light-induced skin pigmentation in the former study and showed hepatoprotective role in the latter study. Antioxidant properties ofH. pluvialisin both studies were illustrated by normalising up the level of reduced antioxidant enzymes such as CAT, glutathione peroxidase (GPx) and superoxide dismutase (SOD), restoring the increased glutamate pyruvate transaminase, glutamate oxaloacetate transaminase and alkaline phosphatase in serum to normal level, as well as enhancing the inhibition on lipid peroxidation [13,14]. It is noteworthy that astaxanthin and astaxanthin esters show similar anti-oxidative and hepatoprotective potency [14]; but astaxanthin esters with higher bioavailability had a greater potential than astaxanthin in preventing skin cancer [13]. Another study by Katagiri et al. [64]also proved that anti-oxidative potential from astaxanthin-richH. pluvialiswas capable in improving cognitive function of human subjects. This study is significant in providing future insights for the neuroprotection role ofH. pluvialisas it proved that this microalga not only illustrated potent anti-oxidative effects onin vitrorat models, but also showing great anti-oxidative potential in clinical trials on humans.
A lot ofin vitrostudies had been identified whereA. platensisand its extracts possess potential antioxidant activity. For example,after the exposure of SH-SY5Y neuroblastoma cells to iron-induced oxidative stress, Bermejo-Bescos et al. [47]expressed thatA. platensisable to suppress lipid peroxidation, maintenance of antioxidant enzyme cellular activities such as CAT, GPx, SOD as well as increased the concentration of non-enzymatic antioxidant such as glutathione reductase (GR). This revealed that the protein extract fromA. platensiscan act as an effective antioxidant agent through a complex mechanism such as interfering with the radical-mediated cell death. Hence, the antioxidant activities ofA. platensisand the anti-oxidative enzymes were interrelated. The antioxidant activity ofA. platensiscan be further con firmed in anin vivostudy that involved the use of Wistar rat model [45]. The authors found thatA. platensiswas able to reduce oxidative stress and had effective protection against cyclophosphamide-induced reproductive damages in rat rather than vitamin E [45]. Therefore,A. platensiscan also act as a potential medicine to treat diseases that were provoked by ROS, reproductive disorder as well as treatment for neurodegenerative disorder.
A study conducted by Saranya et al. [25]proved that the elevation in both carotenoid and total phenolic content ofI. galbanawill cause a direct increase in its total anti-oxidative activity. Particularly, this study suggested that methanol extraction fromI. galbanacontains a significant higher total phenolic content and carotenoid content than that from hexane or acetone extraction; hence resulting in the highest antioxidant capacity [25]. However, this study only illustrated the significant contribution of both carotenoid and total phenolic content onto the antioxidant activity ofI. galbana. Therefore, more future research is required in elucidating the anti-oxidative effect among these 3 extractions from this microalga. Besides that, Sun et al. [26]indicated that 3 polysaccharides (IPSI-A, IPSI-B and IPSII) inI. galbanawere potent antioxidant compounds with strong reducing capacity which can scavenge hydrogen peroxide (H2O2), superoxide radicals and hydroxyl radicals in a concentration-dependent manner.This study also highlighted the novelty of IPSII to be recognised as a more potent anti-oxidative compound than IPSI-A and IPSI-B due to its distinctive molecular weight (15.93 kDa) and chemical characterisation (β-type heteropolysaccharide with a pyran group and predominantly consisting mannose with varying quantity of galactose,glucose and rhamnose) [26]. Insufficient data from carbon-13 nuclear magnetic resonance (13C NMR) caused a limitation in this study, where the relationship between the structure and antioxidation mechanisms of IPSII cannot be interpreted completely. As such, more attention should be given towards solving this issue in any future studies.
A strong correlation between the antioxidant activity of microalgae and total carotenoid extracts such as zeaxanthin,α-carotene,β-carotene and lutein has also been detected. In Hu et al. [56],the antioxidant capacities ofD. salinacarotenoid extract were evaluated and they found that the algal carotenoid extracts played an important role in its antioxidant capacity. Additionally, animal-based study as well as clinical studies had examined the antioxidant activity ofD. salinacarotenoid extracts [58,65]. These reported studies proved thatβ-carotene contains notable protective roles against oxidation as well as oxidation mediated disease by restoring the hepatic enzyme activity including SOD, CAT and peroxidase [65]. The microalgae antioxidant activities can also be attributed to fucoxanthin, which is a type of carotenoid that can be extracted from various microalgae includingP. tricornutum. A previous reportedin vitrostudy showed that fucoxanthin isolated fromP. tricornutumprovides beneficial effect in inhibiting oxidative burst in human polymorphonuclear leukocytes and scavenge radicals [58]. However, the reported antioxidant capability of fucoxanthin must be considered as the result of the combined effect of several other compounds present in the microalgae including phenolic acids and chlorophylls.
ForT. suecica, Lee et al. [35]utilised various carbohydrases and proteases in extracting antioxidants from this microalga and their antioxidant potential were evaluated. This study indicated the importance of using enzymatic digestion in effectively extracting natural water-soluble antioxidants from microalgae. Overall,Kojizyme and Neutrase digests fromT. suecicawere identified as the 2 most potent water-soluble antioxidants to be utilised in food-related industries as they showed highest anti-oxidative activity in scavenging alkyl radicals, 2,2-diphenyl-1-picrylhydrazyl (DPPH)and H2O2. Particularly, Neutrase digests with high polyphenols content significantly increase the scavenging rate of intracellular hydrogen peroxide; thus, alleviating H2O2-induced cell damage and resulted in remarkable cell survival rate of 69.55% at 200 μg/mL in Vero cells [35].Additionally, Sansone et al. [37]determined thatT. suecicawith high carotenoids level effectively reduced DPPH radicals up to 98% at highest concentration (200 μg/mL). At cellular level, the reduction in cell viability of A549 lung cells induced by H2O2damage can also be efficiently recovered by the strong anti-oxidative effect ofT. suecica.Furthermore, this study is also the first study that reported this marine green microalga’s extract could significantly inhibit prostaglandin E2release in H2O2-induced A549 cells at the same time increasing the gene expression of dehydrocholesterol reductase-24 and prostaglandin reductase 1 in the cells [37]. These findings are crucial as they firstly correlated the beneficial effects of antioxidants in marine microalgae with their ability in relieving inflammatory responses via the inhibition of prostaglandin release.
Another study by Guedes et al. [28]determined that protein hydrolysates ofScenedesmus obliquus(150 mg/mL) produced by trypsin (Sd2Try) and papain (Sd1pa) exhibited great anti-oxidative capacity in scavenging DPPH and 2,2-azinobis-3-ethylbenzothiazoline-6-sulfonate (ABTS) radicals. These hydrolysates were identified to be abundant in lysine, arginine, histidine, aspartic acid and alanine. This article showed that hydrolysis of protein is mandatory to free the antioxidative peptides fromS. obliquusso that they can exhibit respective antioxidant activities to scavenge free radicals [28]; however, the precise mechanism is yet to be investigated. As such, Norzagaray-Valenzuela et al. [31]predicted the mechanism of action might be that the anti-oxidative peptides or protein hydrolysates utilise their hydrophobicity in interacting hydrophobically with membrane bilayers in order to facilitate their entry into target organs; hence eliciting powerful antioxidant capacity for radicals scavenging. Specifically, amino acids with aromatic side-chains (e.g. tyrosine, phenylalanine) or nucleophilic sulphur-containing side-chains (e.g. cysteine) were known to elicit most effective radicals-scavenging activity; but in general, every amino acid is potential antioxidant which can interact readily with free radicals and disarm them [66].
The scientific evidence described above demonstrate that, the selected commercialized microalgae species show anti-oxidative potency as these microalgae can significantly enhance or maintain the antioxidant enzymes activity, increase the concentration of non-enzymatic antioxidant, suppress lipid peroxidation, and scavenge H2O2as well as superoxide radicals and hydroxyl radicals [13,14,26,63]. These selected microalgae species also proved to exert chemoprotective properties, hepatoprotective effect,protection against radicals induced by reproductive damages, as well as a notable protective role against oxidation and oxidative mediated disease including ageing, cancer and skin inflammatory disease [14,43,45,63,65]. Therefore, these commercialized microalgae can be further extended and exploited in the development of new alternative cosmeceutical products and nutritional supplements,application in food additives, or a potential complementary medicine to treat various diseases including skin cancer, reproductive disorder,neurodegenerative disorder, and other diseases that are triggered by ROS. However, the insufficient study in the effect of microalgae towards immunomodulatory action which includes the expression of transcripts and immune modulation gene, investigation on the biological activity of the bioactive compounds (e.g. xanthophylls)present in microalgae extract as well as the structural and mechanism of action study related to antioxidant behaviour of various microalgae extract limits the understanding of the anti-oxidative effects of microalgae. It is recommended that these suggestions are carried out in future experiments to correlate and further warrant the beneficial effects of antioxidants in microalgae. For example, a study reported by Carballo et al. [67]exhibited not only the antioxidant capacity and cytotoxicity, but also the immunomodulatory activity of chrysolaminarin, which is a type ofβ-glucan extracted fromP. tricornutum. The results showed that injection of chrysolaminarin-enrichedP. tricornutumin flatfish (Senegalase sole) rapidly activated the expression of pro-inflammatory cytokine (il1b), chaperone(hsp90aa) and the antimicrobial peptide (hamp1) in the three organs analysed (intestine, kidney and spleen). This further stimulated a series of immune reactions including the activation of generalized downstream responses through NF-κB and MAPK signalling pathway followed by the secretion of cytokines as well as the activation of migration and phagocytic activities of macrophage [67].Therefore, such reported study will be able to provide a better understanding on antioxidative efficacy and the immunomodulatory effects of microalgae which will be relevant in the future to establish the potential use of microalgae as a beneficial nutraceutical (e.g.,supplements) for animals and humans. Apart from that, clinical trials can be considered to provide a better explanation for the application of microalgae in food, feed, nutraceutical and pharmaceutical formulation as the above conclusion were mostly based onin vitroexperiments and animal studies.
3.2 Anti-inflammatory activity
The antioxidant effect and anti-inflammatory activity of microalgae are closely interrelated in the pathogenesis of several human diseases as both share a common trigger i.e., oxidative stress.The abnormal production of proinflammatory mediators and free radicals caused by oxidative stress may stimulate inflammation and further lead to extreme cellular damage [38]. According to Abu-Serie et al. [38], ROS and reactive nitrogen species (RONS)can stimulate the expression of proinflammatory genes by induced intracellular signalling cascade and lead to the release of various inflammatory mediators. Prolonged activation of inflammatory mediators is harmful as they play a major role in the pathogenesis of various diseases including inflammatory bowel disease, rheumatoid arthritis, chronic asthma, multiple sclerosis and even cancer [68].
In general, inflammation is a natural physiological response from the immune system towards injuries or infections [38].Consequently, inflammation can be treated by suppressing the activity of inflammatory cells or inhibiting the inflammatory mediator’s production. In this case, medicinal plants or anti-inflammatory diets have been extensively exploited to prepare new drugs with potent anti-inflammatory effects [69]. Similarly, studies also suggested that various microalgal compounds (e.g. carotenoids) can be utilised as alternative sources in eliciting anti-inflammatory properties [2].Guidetti et al. [12]showed that astaxanthin inH. pluvialiscould strongly reduce the generation of interferon-γ (INF-γ) (a key pro-inflammatory cytokine) in T lymphocytes of human and canine after the T cells were treated with oxytetracycline drug to exhibitin vitroinflammatory responses. This article sheds new light for future researchers in developing innovative functional foods and planning nutraceutical diets as it proved that astaxanthin is useful in alleviating the toxicity and inflammatory responses induced by oxytetracycline,a hypothetical toxic substance in food [12]. Thus, anti-inflammatory properties of this microalgae can be further explored to improve the standard pharmacological treatments in treating and preventing inflammatory episodes. Similarly, other microalgae species, such asC. calcitransrich in fucoxanthin could follow the same exploratory route to reveal potency as anti-inflammatory agents. Next, Yeh et al. [17]reported that diabetes-induced oxidative stress could stimulate the activity of transcription factor nuclear factor-kappaB (NF-κB).Activated NF-κB binds to the promoters to elevate the transcription of downstream inflammatory mediators (fractalkine, monocyte chemoattractant protein-1 and intercellular adhesion molecule-1) in ocular tissues; causing retinal inflammation in diabetic rats [17]. It was found treatment with astaxanthin significantly lessened the level of inflammatory mediators transcribed thereby inhibiting NF-κB activity. Correspondingly, Li et al. [70]also showed similar results as the studies above, where they verified that astaxanthin prevented the increase in pro-inflammatory cytokines (e.g. tumour necrosis factor-α(TNF-α) and interleukin-6 (IL-6)) as well as suppressing the NF-κB and mitogen-activated protein kinases (MAPK) signalling pathways which modulate anti-inflammatory responses. All these articles show that astaxanthin is useful against inflammatory responses as it selectively targets the regulatory pathways for inflammation(NF-κB and MAPK) and lowers the level of various pro-inflammatory cytokines [12,17,70]. Hence, they are remarkable for future references in evidencing the powerful anti-inflammatory properties of microalgae.
Abu-Serie et al. [38]conducted anin vitrostudy using lipopolysaccharides (LPS)-stimulated white blood cells and found that the extract of another chlorophyte,C. vulgarisexerted antiinflammatory activities. Their study showed significant effects in the suppression of various inflammatory mediators including nuclear factor-kappa (NF-κ), inducible nitric oxide synthase (iNOS),cyclooxygenase (COX)-2 and TNF-α. This mainly attributes to the presence of natural antioxidant and anti-inflammatory phytochemical compounds such as phenolics, carotenoids, tannins, carotenoids and terpenoids which are produced byC. vulgaristhat can inhibit the production of inflammatory mediators [38].
The aqueous Cyanophyta extract (ACE) from microalgae species in the class cyanophyceae,A. platensisis also well known for its’anti-inflammatory COX-2 inhibitor phycocyanin (PC) and some non-PC bioactive compounds, proven to possess anti-inflammatory effect [49]. PC inhibits COX-2 production whereas non-PC fractions inhibit the enzymatic activity of inflammatory mediator lipoxygenase. It is beneficial to consume the rich extract fromA. platensiswhich help in the reduction of inflammatory status,without further purification steps. Moreover,A. platensiswhich is also rich in high contents of γ-linolenic acid, proteins, vitamins and minerals, was proved as an effective treatment for diabetes associated with its anti-inflammatory activities [2,6]. For example, anin vivostudy accomplished by Joventino et al. [71]using diabetic rats’ model proved thatA. platensiswas able to decrease carcinogenic-induced paw oedema in mice. Besides, the results obtained showed a vital decrease in glycemia, triglycerides, total cholesterol level, TNF-α level in inflamed paw and the myeloperoxidase released from neutrophils. Due to the interrelation of anti-inflammatory action ofA. platensisalong with its anti-diabetic effect, the authors suggested thatA. platensiscan act as a potential medicine to treat diabetes due to the presence of various bioactive components in the microalga that exhibited many biological activities [71].
In vitrostudy on the anti-inflammatory effect of carotenoid extract fromD. salinarevealed the carotenoids extracts which composed ofα-carotene,β-carotene, lutein and zeaxanthin showed significant suppressive effect towards LPS-induced pro-inflammatory mediators in murine macrophage cells [72].This is because the carotenoid extracts fromD. salinais able to inhibit the production of IL-1β, IL-6, TNF-α, iNOs, COX-2, and secretion of nitric oxide (NO) and prostaglandin [72].In vitrostudy onthe omega-3 fatty acid concentrate isolated fromD. salinaable to reduce the LPS-induced IL-6 and TNF-α production in peripheral blood mononuclear cells (PBMCs). Besides, the studied omega-3 fatty acid concentrate was also proven as a potential COX-2 blocker and prevented expression of matrix metalloproteinase(MMP-2 and MMP-9) which were the principal isoform involved in inflammation [51].
Recently, a research group investigated the anti-inflammatory properties of microalgaeP. tricornutumwhich is rich in omega-3 fatty acids and other beneficial nutrients including carbohydrates,vitamins, proteins and carotenoids [73]. The research group conducted anin vitrostudy usingP. tricornutumto treat LPS-stimulated human PBMCs and murine macrophage and found that the aqueousP. tricornutumextract significantly suppressed the pro-inflammatory cytokines, IL-6, TNF-α, IL-1β, and COX-2 production in a dose-dependent manner [73]. Moreover, a study performed by Samarakoon et al. [74]further reinforced their claim that the methanol and aqueous extracts as well as fractionsn-hexane, chloroform and ethyl-acetate fractions from various microalgae species includingP. tricornutumpossessed strong anti-inflammatory activity as it was able to inhibit the production of NO which acts as an important signalling molecule that was induced in macrophage during inflammation.
Meanwhile, Rodríguez-Luna et al. [23]demonstrated that the topical administration of acetone-dissolved glycolipids monogalactosyl monoacylglycerol (2S)-1-O-[(6Z,9Z,12Z,15Z)-octadeca-6,9,12,15-tetraenoic]-3-O-β-D-galactopyranosyl glycerol (MGMG-A) and monogalactosyldiacylglycerol fraction(MGDG) fromI. galbanacould alleviate skin inflammation symptoms(oedema and thickened epidermis) by reducing pro-inflammatory cytokine generation such as TNF-α, IL-6 and IL-8 in epidermis layer of skin. This study is valuable as it firstly reported the preventative anti-inflammatory effect of topical application of glycolipids fromI. galbana. Besides, to overcome the limitations of acetone application onto the skin (e.g. difficulty in sample application, spreading of formulations, heterogeneity of dose in contact with skin) [75],this study suggested that cream-formulation of MGDG would have better transdermal drug delivery than ointment and hydrogel; as well as having better permeation than its acetonedissolved version and MGMG-A cream [23]. The scientists also elucidated the molecular mechanism of anti-inflammatory effect of MGDG-cream, proposing that this glycolipid fraction markedly suppressed the expression of cyclooxygenase-2 (responsible for overproduction of pro-inflammatory prostaglandins) in epidermal layer of inflamed skin [23]. The results are valuable for forthcoming experiments as they successfully proved the effectiveness ofI. galbana-glycolipids(MGDG-cream) and it could emerge as an important therapeutic in treating chronic inflammatory disorders such as psoriasis.Another study by de Los Reyes et al. [22]also investigatedI. galbanafor the anti-inflammatory activity of its glycolipids content, specifically glycosylceramides and glycosylglycerides. It is reported that monogalactosyl diacylglycerols (fraction I-MGDG),digalactosyldiacylglycerol (fraction I-DGDG), isolated DGDGs 11 and 12 as well as galactosyl ceramides 20, 21, and 22 illustrated significant inhibition on the generation of pro-inflammatory cytokine (TNF-α) in macrophages that differentiated from THP-1 cells;while galactosyl ceramides 20, 21, and 22 only acted as moderate inhibitors. Therefore, as suggested by the positive results above, one can concludes thatI. galbanais a marine microalga which is rich in polyunsaturated fatty acids where the anti-inflammatory properties of its monogalactosyl diacylglycerols and digalactosyldiacylglycerol fractions deserve further exploration [22].
Other than pro-inflammatory cytokines, iNOS in macrophages are also important mediators that are involved in acute and chronic inflammation. iNOS will synthesise nitric oxide (NO) that plays a vital role in the pathogenesis of inflammatory responses [76]. For this,Jo et al. [77]showed that 80% methanolic extraction fromT. suecicaelicited the most powerful anti-inflammatory effect on macrophages whereas water-extraction ofT. suecicashowed no inhibition on NO production. The aforementioned anti-inflammatory effects were illustrated by a significant inhibition on the production of TNF-α, IL-6 and NO in lipopolysaccharide-stimulated RAW264.7 macrophages.Furthermore, 80% methanolicT. suecicaextract illustrated a clear concentration-dependent pattern for the strongest anti-inflammatory activity, suggesting that the major portion of anti-inflammatory bioproducts were extracted in less-polar solvent than water [77].This important result proposed that some useful anti-inflammatory bioproducts can be further determined and purified fromT. suecicaby utilising organic solvents with lower polarity index in forthcoming studies. Although the efficacious use of anti-inflammatory properties in microalgae has been substantially investigated, insufficient interest has been given to microalgae as they are difficult to be cultivated and isolated, and are expensive in production [78]. Therefore, the study by Jo et al. [77]paved new path for the development of a novel anti-inflammatory agent as they successfully discovered the functional anti-inflammatory activities ofT. suecica; yet at the same time, this species can be cultivated at vast scale with low production cost [79].
Generally, microalgae displayed anti-inflammatory activity according to various studies reviewed in this section. All of them are proven to inhibit the release of prominent pro-inflammatory cytokines and inflammatory mediators such as iNOS, TNF-α, IL-6,INF-γ and others [12,23,73,79]. However, the differences in the extract concentrations, cell lines, inducer used in initiating inflammation, as well as the route of administration in anin vivomodel provide difficulties when it comes to comparing the anti-inflammatory effectiveness across studies using the same species of microalgae. Nonetheless, the anti-inflammatory activities of various commercial microalgae species as well as their extracts are important antecedents and successful milestones in warranting extract health benefits and its’ safety when consuming as supplements, as most of the aforementioned studies have not revealed any significant toxic effects or abnormalities on tested human cell lines and animals [38,72,73].Furthermore, additional research involvedin vivo,ex vivoand well-structured pre-clinical investigations are highly required to further examine the anti-inflammatory efficacies. For example, Heard [80]suggested thatex vivoskin model could offer comparable predictability toin vivostudies in determining the inflammation modulation; yet,ex vivoapproach is a less expensive option and could provide direct information relating to pro- or anti-inflammatory activity with minimal ethical restrictions. This presents a novel research direction for ongoing study in tackling inflammatory responses. Besides that, the molecular mechanism of the microalgal bioactive compounds in eliciting the anti-inflammatory activity are not well-defined in most studies above, whilst several studies suggested that the pivotal anti-inflammatory effects from microalgal bioactive compounds were closely related to the depression of NF-κB signalling pathway and the inactivation of c-Jun NH2-terminal kinase (JNK) signalling pathway [17,70,72]. With these identified evidence gaps, further investigations are needed to clearly elucidate the anti-inflammatory pathway exerted by microalgae extracts.
3.3 Anticancer activity
The important biological molecules including lipid, protein and DNA synthesis may be affected by the free radicals and ROS generated by microsomal metabolism and further lead to various degenerative disease conditions for example cancer [81]. Cancer is the most common type of disease worldwide with high mortality and morbidity rate leading to uncontrolled cell proliferation while inducing a large group of pathologies in the body [82]. Therefore,bioprospection of potent anti-cancer compounds from natural sources such as microalgae is extremely vital in providing novel remedial preparations. Firstly, it was reported that astaxanthin and astaxanthin esters fromH. pluvialiscan notably suppress the proliferation of skin cancer cells and its anti-cancer properties was well correlated to the powerful anti-oxidative capacity of astaxanthin and astaxanthin esters [13,83]. The skin cancer cell lines which were treated with astaxanthin showed an increased expression on tumour suppressor gene (p53) whereas the activity of proto-oncogeneBcl-2(an anti-death gene which inhibits apoptosis) was hindered [83].Furthermore, Rao et al. [13]suggested than astaxanthin esters may provide an enhanced anti-skin cancer potency than astaxanthin as astaxanthin esters were having higher bioavailability thus yielded more retinol, an active compound that is converted from astaxanthin and astaxanthin esters responsible for the anti-cancer performance.Additionally, astaxanthin-richH. pluvialisalso imposed strong anticancer effects on human colon cancer cells (HCT-116) by halting cell cycle progression and up-regulating apoptotic processes. The arrested cell cycle progression was illustrated by an increase in the activity of tumour suppressor gene p53 and cyclin kinase inhibitors p21, p27 (prevent over-proliferation of cancerous cells); while the enhanced apoptosis was illustrated by a down-regulation in the expression of Bcl-XL and Bcl-2 (inhibitors of programmed cell death)as well as phosphorylated Akt (cell survival-related proteins) [13,84].Interestingly, the more pronounced anti-tumoral effect fromH. pluvialisextracts than purified astaxanthin strongly suggested thatH. pluvialisextracts could be utilised for human supplementations since high purification cost could be precluded. In parallel, our previous work on anti-cancer activity exerted by the xanthophyll,fucoxanthin fromChaetoceros calcitransevidenced by the downregulation ofBclandBaxgenes [107].
The anticancer effect ofC. vulgariswas reported by Wang et al. [81]in anin vitrostudy using human lung cancer cell line. In this study,the effective production of microalgal biomass from newly isolated indigenousC. vulgarisstrain (namelyC. vulgarisC-C) which extracts using supercritical carbon dioxide (SC-CO2) extraction method showed that theC. vulgarisC-C significantly inhibits human lung cancer cell lines in a dose-dependent manner [81]. In ordinary mammalian physiological conditions, the cell migration played a crucial role in the development and maintenance of physiological homeostasis. Dysregulation of cell migration will result in metastasis and invasion of malignant tumour cells. In this case, theC. vulgarisC-C extracts may effectively reduce the migration of tumour cells,thus suggesting thatC. vulgarisC-C extract can be used as an easily available resource for the development of anticancer drugs to inhibit metastasis [81]. Moreover, anotherin vitrostudy conducted by Yusof et al. [44], successfully determined the apoptotic and antiproliferative effects of hot water extracts ofC. vulgarison the hepatoma cell line. They found thatC. vulgarisis able to induce apoptosis signalling cascades of tumour suppressor protein P53 and a host of pro- and antiapoptotic protein such as Bax, caspases-3,caspases-8 and Bcl-2 protein [44]. This will subsequently lead to an increase in DNA damage and apoptosis which in turn inhibit or delay cancer cell growth. All the above studies showed that the C-C and hot water extracts ofC. vulgarisare cytotoxicity against human lung carcinoma and hepatoma cells, suggesting thatC. vulgarisdo exhibit potential anticancer activity.
Next, Matos et al. [85]showed that the biomass (IC50= 0.32 mg/mL)and ethanolic extracts (IC50= 0.28 mg/mL) fromI. galbanaexhibited appreciable cytotoxicity on HeLa human cervical cancer cells by reducing the cell viability by 50%, but its aqueous extraction only associated with low cytotoxicity. This indicated that the anti-cancer bioactive compounds are presumably to be constituents in membrane,but not polyphenols with high hydrophilicity or polysaccharides with high molecular weight. Moreover, instead of focusing on the anti-cancer potential of isolated microalgal compounds, this study successfully proved that theI. galbanabiomass itself also elicited substantial cytotoxicity on HeLa cancerous cells [85]. This valuable finding can be utilised to enhance human health since microalgal biomass is consumed as nutraceutical supplements. In agreement with the results reported by other authors, the anti-carcinogenesis effect ofI. galbanawas attributed by the significant quantity of fucoxanthin andβ-carotene [19,21]. Additionally, an interesting highly branched chemical compound, (1→3,1→6)-β-D-glucan was identified inI. galbanaby Sadovskaya et al. [24]. As the biological activities ofβ-glucans (e.g. anti-cancer properties, immunomodulatory properties)rely on their chain length and the degree of branching (1→3 and 1→6 linkages ratio), the study claimed that the high proportion of 1→6 linkages in this glucan madeI. galbanaa potent anti-tumour microalga that exhibited direct inhibition on the cell proliferation of human leukemic monocyte lymphoma cells (U937) [24].Since the anti-cancer activity ofI. galbanawas in resemblance with the that fromE. bicycliswhich also enriched with 1→6 linkages and elicited powerful inhibition against human melanoma cells (SK-MEL-28) [86], thus, it was proposed that (1→3,1→6)-β-D-glucan fromI. galbanamay possess anti-cancer potential in combating myeloid cells-derived cancers.
Anticancer agents can either inhibit the growth of cancer cells or stimulate apoptosis of cancer cells. Numerousin vitrostudies had been conducted to evaluate the anticancer activity ofA. platensisand its extracts. For example,A. platensiswater has possess an excellent antiproliferative activity in hepatocellular carcinoma cells and human colon carcinoma cells as it was able to suppress the growth of these cancer cells [87]. In addition,A. platensisalso inhibits the growth of human non-small-cell lung carcinoma A549 [88]. The anticancer properties of microalgae are correlated with the antioxidant effects. Microalgae which contained various phytochemicals such as carotenoids, chlorophyll and phycocyanin evaluated all antioxidant related enzymes activities [88]. Apart from that, phycocyanin that is isolated from microalgae is also reported as an important pigment in treating cancer in animals. Recently, Hernandez et al. [89]evaluated the phycocyanin and polysaccharide present in the water extract ofA. platensispossessed anticancer activity towards acute leukaemia Kasumi-1 and chronic leukaemia K-562 cancer cell lines in a dose-dependent manner. In this study, phycocyanin is able to interfere with the tumour cells DNA synthesis and also bind to the mitogen receptor on the tumour cell membrane to stimulate the cellular apoptotic signal transduction. Besides, water-soluble polysaccharide fromA. platensisis able to increase the repair activity of unscheduled DNA synthesis as well as radiation DNS excision [89].
A recent study by Marrez et al. [30]suggested thatS. obliquusfrom diethyl ether extraction demonstrated the highest anti-cancer activity against HepG2 human liver cancer cell lines (IC50=42.8 mg/mL), HCT116 colon cancer cell lines (IC50= 24.6 mg/mL)and MCF7 breast cancer cell lines (IC50= 93.8 mg/mL). Similarly,Abd El Baky et al. [90]also highlighted the anti-cancer significance ofS. obliquuswhere it stated that the lipids extract ofS. obliquusfrom chloroform:methanolic (2:1) extraction also contains significant proliferation inhibition against HepG2 liver cancer (IC50=14.5 mg/mL), HCT116 colon cancer (IC50= 15.22 mg/mL) and MCF7 breast cancer cell lines (IC50= 11.62 mg/mL). Comparing these two studies, it is apparent thatS. obliquusobtained with chloroform: methanol extraction is having a generally lower IC50concentrations to elicit anti-cancer effect against all the three cancer cell lines than its diethyl ether extract [30,90]. This comparison revealed that chloroform:methanol (2:1) would be a more promising organic solvent option in extracting anti-cancer compounds fromS. obliquus. Lastly, Hussein et al. [34]indicated thatT. suecicafrom chloroform extraction exhibited noticeable cytotoxicity (growth arrestment and increased apoptosis) against 4T1 and MCF-7 breast cancer cell lines with IC50of 83.17 and 46.77 μg/mL, respectively.Intriguingly, whenT. suecicachloroform extract was co-applied with silver nanoparticles in 2:1 ratio, a significant lower IC50concentration was achieved against 4T1 (53.7 μg/mL) and MCF-7 (6.60 μg/mL)breast cancer cell lines [34]. Most importantly, both applications demonstrated that there is no cytotoxicity towards the normal Vero cell lines. This article has opened a new window on the formulations of anti-cancerT. suecica, suggesting that the co-application ofT. suecicachloroform extract with silver nanoparticles in appropriate ratio and concentrations would be an efficacious low cost theragnostic agent in breast cancer therapeutic that associated with minimal or no side effects. There are also other reported studies that supported the findings from Hussein et al. [91,92]where the anti-cancer effect ofT. suecicais attributed to its organic constituents such as phytol and eicosane.
The mechanism of anticancer action includes oxidative stress,cell cycle arrest, autophagy and apoptosis [52]. Jayappriyan et al. [93]evaluated theβ-carotene isolated fromD. salinashowed an effective apoptosis effect on PC-3 human prostate cancer cell line. Moreover,the antiproliferative and proapoptotic effect ofD. salinaalso proved by Chiu et al. [52]and showed that phytochemical presence inD. salinaable to suppress the proliferation of KB human oral squamous carcinoma cells. Anotherin vitrostudy on the ethanol extract ofD. salinainvestigated the proliferation and apoptosis in the A549 human lung cancer line. Results demonstrated thatD. salinaextracts which were a well-knownβ-carotene source that had anticancer potential against the particular cancer cell line as it can significantly increase the cyclin-dependent kinase (CDK) inhibitors expression on p21 and p53 along with death-receptor proteins Fas and FasL [52]. This will then induce the cell cycle arrest, cell apoptosis and inhibit the cell proliferation. Apart fromin vitrostudies,severalin vivostudies had also explored the anticancer properties ofD. salina. Chuang et al. [94]used syngeneic leukaemia-implanted mice (BALB/c and WEHI-3) to evaluate the antileukemia activity ofD. salinaby feeding them withD. salinawhich is suspended by distilled water. The authors found thatD. salinacan inhibit spleen metastasis and lengthen the survival in BALB/c mice that had accepted an intravenous injection of WEHI-3 cells via modulation of the immune response. This is because oral administration ofD. salinawasable toreduce spleen enlargement, enhance the cytotoxicity of natural killers as well as increase the phagocytic activity of macrophages [94]. Therefore, the above studies suggested thatD. salinacan be used not only as a dietary supplementation, but also a potential anticancer treatment.
Numerous studies had targeted the beneficial effects of fucoxanthin, which is a type of carotenoid fromP. tricornutumfor its anticancer effect. A recent reported study by Neumann et al. [58]evaluated thein vitroantiproliferative effect of fucoxanthin isolated fromP. tricornutum. Their studies showed that cancer cells (Caco-2,HeLa and HepG2) that were incubated with different concentrations of fucoxanthin will have their metabolic activity to be remarkably reduced in a dose-dependent manner. The authors also determined the caspase 3 and 7 activity which is an essential event during apoptosis and further con firmed that a reduced in cell metabolic activity may lead to an increase in apoptosis through both caspases’ activities [58].Anotherin vitrostudy also investigated the anticancer effect of sulfated polysaccharides extracted fromP. tricornutum[59]. The results obtained suggested that the polysaccharides extract fromP. tricornutumhad notable anticancer activity in a dose-dependent manner as it can inhibit the proliferation of HepG2 cells by initiate apoptosis without affecting the mitosis and normal cycle of HepG2 cells [59]. This further suggested thatP. tricornutummight serve as a possible drug for cancer treatment in the future.
In summary, from the various reported studies, microalgae species selected above proved to elicit the anticancer potency as these microalgae can significantly increase the expression on tumour suppression gene (p53), cyclin kinase inhibitors (p21), Bax and caspase-3 protein [13,44,52,58]. Reported studies also proved the anticancer potency of marine microalgae is related to its ability to inhibits cancer cells metastasis, cancer cells invasion and also proliferation, interfered the activity of proto-oncogene (Bcl-2) as well as induce cellular apoptosis effect [13,44,52,83,84]. Hence,these microalgae species can be further explored for their potential application in pharmaceutical (e.g. chemopreventive drug discovery),food, nutraceutical and health supplement [81,83,86,88]. Nonetheless,the underlying mechanism and signalling pathway regarding the anti-proliferative, anti-metastasis and anti-apoptotic effects of some bioactive compounds extracted from microalgae towards cancer cell lines are currently not studied in great detail and thus, narrow the understanding of the microalgae anticancer research. This additional information is required as it may provide a better explanation for the potential use of microalgae on cancer therapy research. Besides,the comparison of the anticancer effect of microalgae from different extraction method can be done in the future to provide a new window of discovery on the best solvent option (e.g. water, organic or inorganic) in extracting the anticancer compounds from microalgae.For example, a recent study reported by Marrez et al. [30]evaluated the anti-cancer activity ofS. obliquususing 8 different extraction methods which are water, methanol, ethanol, chloroform, acetone,ethyl acetate, hexane, and ethyl ester. The author indicated thatS. obliquusfrom diethyl ether extract exhibits the highest anti-cancer activity towards 3 different cancer cell lines (HepG2, HCT116,MCF7) compared to other extraction methods. Apart from the different extraction methods, morein vivoexperiments and clinical trials should be done in any future studies to further elucidate the anti-cancer effects of these microalgae towards humans and animals.
3.4 Antimicrobial activity
Microalgae are ultimate sources of structurally importance and biologically active metabolites. The primary and secondary metabolites that formed from these microalgae species may be a possible biologically active constituents of interests in the pharmaceutical industry [95]. Nowadays, the dramatic increase in antibiotic resistance bacteria, the episodes of viral infections and infectious disorders indicate the necessity in developing alternative therapeutics in combating these global health challenges. Microalgae produce several secondary metabolites associated with antibacterial and hormonal effects that can influence other organisms in the vicinity and are thought to be phylogenetic. Since microalgae had been used as traditional medicine over the years, there were numerous reports on compounds or extracts derived from microalgae that had been found to have antifungal, antibacterial, antiviral and antiprotozoal activity [96].
In this respect, Santoyo et al. [15,97]indicated an environmentally friendly technique, pressurized liquid extraction (PLE) that could effectively extract antiviral and antimicrobial compounds from natural microalgal source,H. pluvialis. Following this extraction method,Santoyo et al. [15]further determined that the ethanol extract fromH. pluvialisin its red hematocysts form (without flagella) was showing a remarkably higher anti-microbial activity on various clinically important microorganisms (Staphylococcus aureus,Escherichia coli,Candida albicansandAspergillus niger) when compared to the extracts from green motile form ( flagellated) and those from hexane extraction. Additionally, the stronger anti-microbial activity in redH. pluvialiswas attributed to their higher composition (> 56%) of more complex short-chain fatty acids such as butanoic and propanoic/lactic acids [15]. Meanwhile, Santoyo et al. [97]displayed similar results whereH. pluvialisethanol extracts also exerted stronger inhibition on herpes simplex virus type 1 (HSV-1) infection than extracts obtained with water and hexane. Both studies indicated that high-polarity ethanol is a better solvent choice in extracting antimicrobial or antiviral compounds from microalgae than low-polarity hexane [15,97]. Intriguingly, other than disrupting the attachment step of virus, Santoyo et al. [97]even proved thatH. pluvialisethanol extracts elicited powerful antiviral activity by posing magnificent inhibition on intracellular replication of HSV-1. This study also reported that the anti-viral properties ofH. pluvialisis contributed by the abundant quantity of short-chain fatty acids (propanoic/lactic and butanoic acids). Additionally, they also proposed that a significant quantity of palmitic acid, hexadecatrienoic acid andα-linolenic acid inH. pluvialisethanol extract may give rise to its substantial anti-viral activities [97].
Das et al. [48]investigated the antibacterial properties of aqueous, ethanolic and methanolic extracts from different microalgae species includingC. vulgarisandA. platensis. The result obtained demonstrated both ethanolic and aqueous extracts ofC. vulgariswere active against the pathogenic gram-negative strains ofVibriosp.,Pseudomonassp.,E. coli,andAeromonas hydrophilawhereas only ethanolic extracts ofA. platensiswere active against the pathogenic microorganism selected [48]. This concluded thatC. vulgarispossessed a stronger antibacterial activity when compared toA. platensis. Another research conducted by Uma et al. [95]further proved that methanolic, acetone, DMSO and ethanolic extract of green microalgae (C. vulgaris) possessed antimicrobial activity.This was because the microalgae extracts were active against various gram-positive and gram-negative strains bacteria as a zone of inhibition can be observed using the disc diffusion method. This further con firmedC. vulgarispossessed antimicrobial activity against various pathogenic bacteria. Moreover, Anchang et al. [98]had planned a research to investigate the antibacterial activity of bothC. vulgarisandA. platensispowder. The authors found that the ethanolic extract of bothC. vulgarisandA. platensiswas able to suppress the growth ofE. coliwhen conducting the antibacterial screening of both microalgae extracts. However, theC. vulgarisextracts have higher antibacterial activity compared withA. platensisas the radius of the zone of inhibition was much larger inC. vulgariscompared withA. platensis[98]. This further supported by study which revealed thatC. vulgarishave higher antibacterial activity thanA. platensismay be due to the quantity and quality of phytochemical composition such as phenolic compounds, flavonoids, tannins and terpenes [39].
Another study by Molina-Cárdenas et al. [20]discovered thatI. galbanawas capable in synthesising anti-bacterial fatty acids which could notably limit the growth of pathogenicVibriospecies such asV. alginolyticus,V. campbellii, andV. harveyi, exceptV. parahaemolyticus. However, the mechanism of these anti-bacterial fatty acids is scarcely investigated [99]. AsIsochrysisspecies were reported to be rich in omega-3 fatty acids and polyunsaturated fatty acids [100], the anti-bacterial fatty acids inI. galbanawere identified to be stearidonic, palmitic, oleic,α-linolenic, myristic, and docosahexaenoic acids [20]. Importantly, these fatty acids exhibited bactericidal properties which can be fully utilised in controllingVibrio-strained pathogens in the aquaculture system. Apart from that,anti-algal effect is another antimicrobial property fromI. galbana.Sun et al. [27]showed that 1-[hydroxyl-diethyl malonate]-isopropyl dodecenoic acid is a strong growth inhibitor that could be isolated fromI. galbanaand prominently suppressed the growth of co-existing microalgae species (e.g.C. vulgaris,D. salina,P. elliptica) as well as limiting their production of chlorophyll and proteins. This bioactive compound also acted as an auto inhibitor which would limit the own growth ofI. galbanaitself. Thus, the significant findings from this study not only introduced a natural algaecide which can be used to control noxious algal blooms, but also established the chemical structure of growth-inhibitor inI. galbana[27].The latter information is highly useful for future scientists in investigating an effective method to remove this growth-inhibitor from culture media ofI. galbanaso that its mass production in high or ultra-high-density cultures can be established.
In other previous studies, Herrero et al. [55]has determined the antimicrobial effects of different pressurized extracts as well as solvent extracts fromD. salinaagainst several gram-positive and gram-negative bacteria. The authors proposed that the antibacterial property ofD. salinamainly attributed to various fatty acids includingα-linolenic, palmitic and oleic acid. Besides, the carbon dioxide extracts ofD. salinaare also active against various bacteria includingE. coli,C. albicans,A. nigerandS. aureus[55]. Besides that, this study also indicated thatD. salinaextracts that obtained from ethanol, petroleum ether and hexane generally exhibited a better anti-microbial activity than its aqueous extract with the lowest extraction yield. The antimicrobial property ofD. salinawas mainly attributed to the existence of indolic derivative, polyunsaturated fatty acids and compounds associated with carotene metabolism such as neophytadiene andβ-ionone [101]. As the indolic derivative which isolated from the carbon dioxide extract ofD. salinahad never been reported, further study can be done to understand the exact antimicrobial mechanism of the particular microalgae species.
Apart from short-chain fatty acids, Catarina Guedes et al. [29]demonstrated that among various microalgae and cyanobacteria, the high level of long-chain fatty acids (e.g. oleic,α-linolenic, linoleic,palmitic, palmitoleic and linolenic acids) inboth extracellular and intracellular extracts ofS. obliquusillustrated the best anti-bacterial performance against most food-borne pathogens e.g.P. aeruginosa,E. coliandS. aureus, but notSalmonellaspecies. The result showed thatS. obliquusextracts can be used as a more promising anti-bacterial agent in preserving foods as some cyanobacteria or microalgae species would exhibit potential toxicity [29]. Thus, further investigations could be focused on evaluating the possible deleterious effects that may appear on the bioavailability of the aforementioned anti-bacterial long-chain fatty acids along with the procedures of food processing or storage in order to ensure its safety as a consumable food preservative.
Another study by Bai et al. [33]also displayed the anti-bacterial significance of various fatty acids such as methyl caprate, methyl stearate, caprylic acid, palmitic acid, decanoic acid and nonanoic acid fromT. suecicaextract. This study has identified two interesting chemical constituents (1-ethyl butyl 3-hexyl hydroperoxide and methyl heptanoate) inT. suecicawhich were known to exhibit anti-bacterial effects and these bioactive compounds deserve further investigations. Overall,T. suecicathat extracted with methanol and chloroform in a 1:1 ratio elicited appreciable growth inhibition on gram-negativeProteusspecies and gram-positiveS. pyogenes[33].According to the studies above, it is noteworthy that various fatty acids compounds from organic extracts of microalgae are directly accountable for different antimicrobial potentials. Although efforts in identifying these bioactive compounds have been in progress, it is still relatively incipient owing some new compounds classes in order to further elaborate on the prominent applications of microalgal fatty acids in combating infectious disorders or contributing to antimicrobial therapy.
The cell lysates activity of marine diatoms,P. tricornutumagainst gram-positive and gram-negative bacteria was attributed to eicosapentaenoic acid (EPA) which is a type of polyunsaturated fatty acids that isde novosynthesizedbyP. tricornutum[11]. This EPA is a type of polar lipid that can be found in the cell membrane which played a major role in microalgae defence. The study showed that the EPA was able to inhibit both gram-positive and gram-negative species and was active againstS. aureus[11]. This further raised the attention of various researchers on the use of eukaryotic microalgae as an exploitable source of antimicrobial compounds against diverse bacteria including the inhibition of multidrug-resistantS. aureus(MRSA) strains [11]. Similarly, hexadecatrienoic acid (HTA) isolated fromP. tricornutumwas also proven to be active against gram-positive pathogens especiallyS. aureus[102]. Another study conducted by Desbois et al. [103]successfully identified high levels of palmitoleic acid and other bioactive fatty acids in fusiform morphotype ofP. tricornutumcompared with oval morphotypes ofP. tricornutum. The authors proved that fatty acid isolated from fusiform morphotype ofP. tricornutumexpressed high antimicrobial activity and was able to inhibit numerous non-marines, gram-positive human pathogens in comparison with oval morphotype [103].However, the exact mechanism of action of these various bioactive fatty acids found inP. tricornutumhas yet to be explored. Therefore,future studies are needed to investigate the in-depth mechanism behind its antimicrobial properties.
Taken together, the above findings enable the discovery of new derivatives as well as provide new insights for finding new antimicrobial drugs through utilization of different methods used in drug discovery. Nowadays, the emergence of multiple-drugs resistant pathogenic bacteria is a global issue causing the gaining of research interest by the healthcare institute. In this case, the discovery and development of new drugs from these microalgae species will provide an alternative bioactive reservoir with target functions such as controlling vector infections by inhibiting bacteria growth beyond any adverse effects and in a more specific manner.Therefore, these microalgae may act as potential antibiotic agents with various biodegradable compounds to antimicrobial resistance.According to the studies above, the solvent used in the extraction step significantly affects the extraction yield and the presence of anti-microbial activity in microalgae. The studies above indicated that better anti-microbial activity was generally present in ethanolic extract of most microalgae species [15,48,97], while some studies showed a better extraction using chloroform: methanol in appropriate proportion of 1:1 [33]or 1:2 [100]. It is suggested that the microalgal compounds with an anti-microbial activity against pathogenic bacteria or viruses are mostly hydrophobic and can be more readily extracted with organic solvents. This suggestion is likely to be the possible cause as the molecular identification in most studies above showed that the antimicrobial agents in microalgae are mainly hydrophobic long-chain/short-chain fatty acids or phytochemicals with hydrophobic structures [11,15,20,33,39,97,101]. Particularly, SC-CO2pressurized liquid extraction method which is environmentally clean, allows quicker extraction and contains higher selectivity towards compounds of interest has gained increasing attention [55]. In addition, this greener technology utilises non-toxic and readily available CO2would be an excellent option for the extraction of antimicrobial compounds or other natural constituents from microalgae to inhibit the growth of foodborne pathogens in the food industry. Apart from this,literature search showed that only limited studies have been carried out to examine the antifungal activity of microalgae although over 400 fungi species have been incriminated as opportunistic human pathogens [104]. The detrimental effects caused by fungi (e.g. black gill infection [105], allergic and asthmatic diseases [106]) undoubtedly encourage a more comprehensive screening of microalgal species for the discovery of potential antifungal compounds.
4. Conclusion
From this review, it is evidenced that various bioactive molecules fromC. vulgaris,A. platensis,D. salina,P. tricornutum,H. pluvialis,I. galbana,S. obliquusandT. suecicacan be developed into promising and effective therapeutic agents with bright future in eliciting anti-oxidative, anti-inflammatory, anti-microbial or/and anti-cancer effects. However, most of the present studies were mainly conducted usingin vitrocell lines and animal models. Hence, more pre-clinical studies should be established in the future to understand the actual mechanisms of microalgal-derived bioactive molecules and their potential harm to guarantee the safety of microalgal products in medicinal industries. Besides that, other therapeutic effects of microalgae such as anti-diabetic, anti-aging and neuroprotective potential also deserve more attention and substantial investigation.It is noteworthy that the solvent being used in the extraction step can significantly influence the therapeutic capability of the extracted microalgal bioactive compounds. Finally, this review will act as an antecedent for under-discovered microalgae strains with potential to supersede the current strains in the future. No doubt there will be challenges such as time-consuming and high cost in isolation, process optimization in production, harvesting and biorefinery to close the loop for a circular economy for microalgae. However, with closer cooperation and determination from stakeholders, these challenges can be overcome for a better and stronger value chain. It is envisioned that this value chain will propel us all towards better health and well-being (United Nations sustainable development goal No. 3) by responsible consumption and production (United Nations sustainable development goal No. 12).
conflicts of interest
The authors declare that there is no conflict of interest.
Acknowledgement
This study was supported by Fundamental Research Grant Scheme (FRGS) 2019 from Ministry of Higher Education of Malaysia(FRGS/1/2019/STG05/MUSM/03/2) and Honours Study Consumable Fund 2020 from School of Science, Monash University Malaysia.
- 食品科学与人类健康(英文)的其它文章
- Hypoglycemic natural products with in vivo activities and their mechanisms: a review
- Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health
- Capsular polysaccarides of probiotics and their immunomodulatory roles
- Natural compounds may contribute in preventing SARS-CoV-2 infection: a narrative review
- A comprehensive review on the effects of green tea and its components on the immune function
- Effects of silkworm pupa protein on apoptosis and energy metabolism in human colon cancer DLD-1 cells

