Dental stem cell-conditioned medium for tissue regeneration:Optimization of production and storage
Batoul Chouaib,Frédéric Cuisinier,Pierre-Yves Collart-Dutilleul
Batoul Chouaib,Frédéric Cuisinier,Pierre-Yves Collart-Dutilleul,Laboratory Bioengineering and Nanosciences UR_UM104,University of Montpellier,Montpellier 34000,France
Abstract BACKGROUND Mesenchymal stem cells(MSC)effects on tissue regeneration are mainly mediated by their secreted substances(secretome),inducing their paracrine activity.This Conditioned medium(CM),including soluble factors(proteins,nucleic acids,lipids)and extracellular vesicles is emerging as a potential alternative to cell therapy.However,the manufacturing of CM suffers from variable procedures and protocols leading to varying results between studies.Besides,there is no welldefined optimized procedure targeting specific applications in regenerative medicine.AIM To focus on conditioned medium produced from dental MSC(DMSC-CM),we reviewed the current parameters and manufacturing protocols,in order to propose a standardization and optimization of these manufacturing procedures.METHODS We have selected all publications investigating the effects of dental MSC secretome in in vitro and in vivo models of tissue regeneration,in accordance with the PRISMA guidelines.RESULTS A total of 351 results were identified.And based on the inclusion criteria described above,118 unique articles were included in the systematic review.DMSC-CM production was considered at three stages:before CM recovery(cell sources for CM),during CM production(culture conditions)and after production(CM treatment).CONCLUSION No clear consensus could be recovered as evidence-based methods,but we were able to describe the most commonly used protocols:donors under 30 years of age,dental pulp stem cells and exfoliated deciduous tooth stem cells with cell passage between 1 and 5,at a confluence of 70% to 80%.CM were often collected during 48 h,and stored at -80 °C.It is important to point out that the preconditioning environment had a significant impact on DMSCCM content and efficiency.
Key Words: Tissue engineering;Mesenchymal stem cells;Dental;Conditioned medium;Secretome;Regeneration
INTRODUCTION
Although mesenchymal stem cells(MSC)were initially isolated from bone marrow,MSC from dental and periodontal tissue(DMSC)have attracted international attention for future therapies because of their practical and technical advantages[1].Since 2000,when Gronthoset al[2]described a population of pluripotent progenitors in adult dental pulp,studies have shown that dental tissues can be an important resource of MSCs:dental pulp stem cells(DPSC),exfoliated deciduous tooth stem cells(SHED),apical papilla stem cells(SCAP)which are situated at the ends of growing dental roots[3],periodontal ligament stem cells(PDLSC),dental follicle stem cells(DFPC)located around the tooth germ,and responsible for cementum,periodontal ligament and alveolar bone formation during tooth development[4],gingiva-derived mesenchymal stem cells(GMSC)(Figure 1).For teeth at early stage of development(bell stage),multipotent progenitors from dental mesenchyme have been described,named as tooth germ progenitor cells(TGPC)[5].
An important advantage of these sources of MSC is the absence of morbidity and the fact that no additional surgical procedures is required[6].DMSC are obtained from exfoliated teeth,teeth extracted for orthodontic or medical needs,and supernumerary teeth.While generally discarded as medical waste,teeth could be an abundant source of mesenchymal stem cells.
Numerous studies have indicated that MSC effects on tissue regeneration are mostly mediated by their secreted substances[7]defined as secretome,or MSC-conditioned medium(MSC-CM),which are endowed with paracrine activity.These MSC-CM include soluble factors(proteins,nucleic acids,lipids)and extracellular vesicles(EV)[8].MSC-CM appears as a potential substitute for cell therapy,with considerable potential to be developed into pharmaceutical products for use in regenerative medicine[9].
Compared to non-dental MSC-CM,dental mesenchymal stem cell-conditioned medium(DMSC-CM)have greater amounts of transcriptional,metabolic and proliferation-associated proteins,neurotrophins and chemokines.They also present reduced levels of proteins required for extracellular matrix production and adhesion,and superior effects on cell differentiation,maturation and tissue regeneration[10].
Many research works have described the application of DMSC secretome for various diseases treatment and for tissue regeneration[8,10-12].Dental MSC-CM have been investigated for the repair of neurological disorders[13-18],cardiac lesions[19],diabetic disorders[20],liver diseases[21,22],pulmonary lesions[23],immunity problems[24-26],dental and bone defects[27-30],and growth of hair[31].These potentials are assigned to positive effects on cell proliferation-migration-protection,to specific effects such as anti-apoptotic,pro-angiogenic,immunomodulation,or to cell differentiation and further tissue regeneration like osteodifferentiation,neuron-like regeneration,dentin-pulp complex formation,periodontal regeneration(Figure 2 and Table 1).
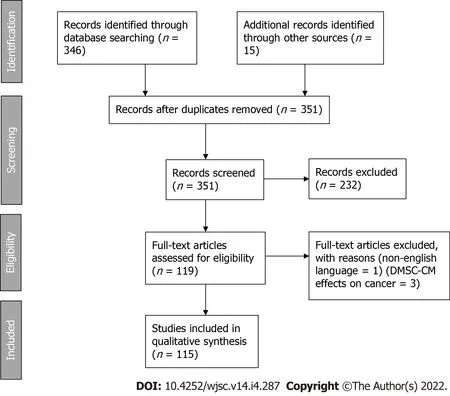
Figure 1 PRISMA 2009 flow diagram.DMSC-CM:Dental mesenchymal stem cell conditioned medium.
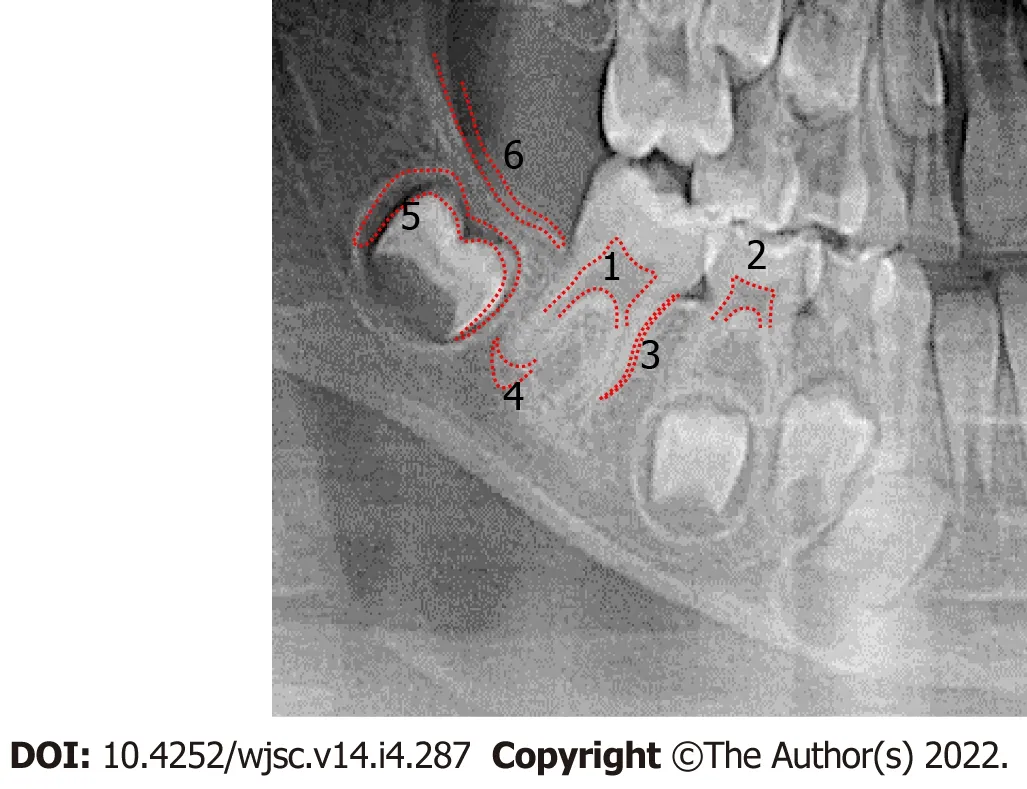
Figure 2 Orthopantomogram X-ray,with representative sources of various dental mesenchymal stem cells.(1)Dental pulp stem cells;(2)Stem cells from human exfoliated teeth;(3)Periodontal ligament stem cells;(4)Stem cells from apical papilla;(5)Dental follicle stem cells;and(6)Gingiva-derived mesenchymal stem cells.
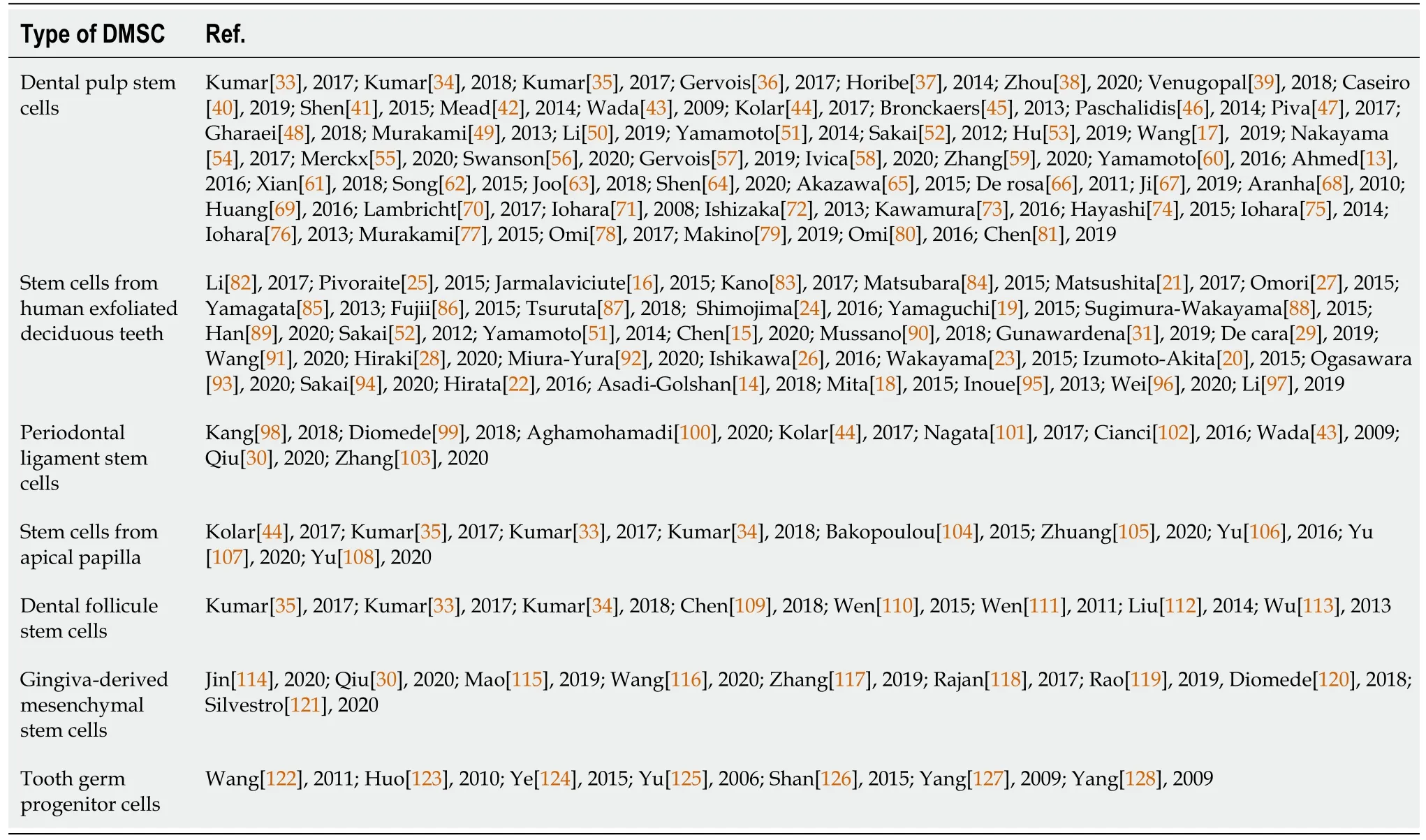
Table 1 Studies investigating the effects of secretomes from dental pulp stem cells,stem cells from human exfoliated deciduous teeth,periodontal ligament stem cells,stem cells from apical papilla,dental follicle stem cells,gingiva-derived mesenchymal stem cells and tooth germ progenitor cells for tissue regeneration
However,MSC-CM manufacturing suffers from variable procedures and protocols,leading to different results between studies.Besides,there is no well-defined optimized procedure targeting specific applications in regenerative medicine.
In the present article,we focus on conditioned medium produced from DMSC and their derivative products.We review the current parameters and DMSC culture conditions used in the manufacturing protocols.The ultimate objective is to facilitate the standardization and optimization of manufacturing procedures needed for the clinical translation of DMSC-CM and their derivative products.
MATERIALS AND METHODS
This systematic review was performed in accordance with the PRISMA guidelines[32].
Data sources and research strategy
We have selected all publications investigating the effects of dental MSC secretome(CM,EV,exosomes)inin vitroandin vivomodels of tissue regeneration by using the PubMed electronic database and the following search terms:((dental stem cells)AND((conditioned medium)OR(secretome)))/((dental stem cells)AND((extravesicles)OR(exosomes))).The bibliographic search considered articles meeting the inclusion criteria,and published between 2006 and July 2020.Articles taken into account had studied the DMSC secretome as a therapeutic agent,had analyzed the profiles of DMSC-CM,or included experiments based on DMSC-CM or its derivatives(EV,exosomes)to assess the paracrine activity of DMSC.Only articles in English were considered.All results were screened based on titles and abstracts.Full texts of the potentially selected records were obtained for definitive inclusion.
RESULTS
A total of 351 results were identified.And based on the inclusion criteria described above,118 unique articles were included in the systematic review.Flow diagram is available as Figure 1.
To organize the analysis and interpretation,DMSC-CM production was considered at three stages:Before CM recovery(cell sources for CM),during CM production(culture conditions)and after production(CM treatment).For these three steps,we identified key points that were often taken into account in the published experimental works.A schematic representation of these elements is shown in Figure 3.
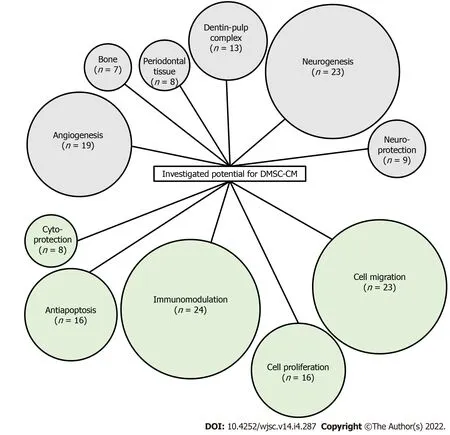
Figure 3 Schematic representation of the characteristics investigated as potential applications of dental mesenchymal stem cell conditioned medium.Number in brackets indicates the number of publications considering the activity.DMSC-CM:Dental mesenchymal stem cell conditioned medium.
Dental mesenchymal cells:Type of cells
Most of studies have investigated the secretome effects of DPSC and SHED.The other secretomes,from PDLSC,SCAP,DFSC,GMSC,or TGPC have been less investigated(Table 1).
Most of these studies analyzed DMSC to verify their stemness.Specific cell surface markers were verified before starting the experimentation:positive expression of CD90,CD73,CD29,CD44,and CD105(MSC markers),and absence of CD34,CD45,CD11b/c,CD31,CD144,and HLA-DR(endothelial/hematopoietic markers).Cell differentiation capacities were controlled for the adipogenic,chondrogenic,and osteogenic lineage.However,there were several studies in which stemness character was not controlled.And few studies did not considered stemness character for dental cells[58,65,111-113,129](Supplementary Table 1).
DPSC,SHED,PDLSC,SCAP,DFPC,GMSC and TGPC derive from oral and tooth related structures.However,the specific properties of these different dental stem cell populations are slightly different according to the location from which they are isolated,in term of marker expression and differentiation potencies[130].Thus,their secretome may also vary.A previously published study compared the neural potential of DMSC-CM obtained from three sources(DPSC,DFSC,and SCAP)with BMSC-CM:the results showed that neurite extension in neural cells were significantly lowered when cells were incubated in BMSC-CM,compared to DMSC-CM.However,among these three dental CM,a significant difference was observed,in term ofin vitroeffects and secretome profiles.Thus,neurite length was increased with neural cells incubated with DPSC-CM,compared to DFSC-CM and SCAP-CM.Besides,significantly different levels of neurotrophins and cytokines were observed in the three secretomes[35].Another study compared also the neural potential of DMSC-CM from three dental sources(DPSC,SCAP,and PDLSC)of the same donor;Although all CM significantly enhanced axon regeneration and showed neuroprotective effects on neurons,results were better with SCAP-CM.By protein quantification,it was revealed that the level of secreted proteins were very similar for DPSC-CM and SCAPCM,but different from PDLSC-CM[44].A third study,comparing SHED and DPSC,could not highlight any difference between SHED-CM and DPSC-CM,as both secretome promoted neurite extension[52].
DMSC-CM have been generally compared with ASC-CM and BMSC-CM.Small particles with molecular weights between 30 and 100 nm were more abundant in DPSC-CM,whereas the fraction of intermediate particles(100-300 nm)was larger in BM-MSC[55].DPSC-CM seemed to be more potent in neurogenesis[35,72],neuroprotection[13,39,42],angiogenesis[72,74],and migration[74]than ADCS-CM and BMSC-CM.Furthermore,the anti-apoptotic potential of DPSC-CM was found to be greater than that of BMSC-CM and ADSC-CM[39,74].However,endothelial cell chemotaxis andin ovoneovascularization were less observed with DPSC-CM than BM-MSC[55].Compared to umbilical cord mesenchymal stem cells(UMSC),some growth factors dominated in UMSC secretion,while others were more evident in DPSC,despite their overall similar profiles[40].
Similarly,SHED and other MSC differed in their basal expression of several biomolecules.Some relevant factors were abundant in SHED-CM but hardly detectable in ASC-CM[90].SHED-CM was more anti-inflammatory[19,21]and anti-apoptotic[19]compared to BMSC-CM and ASC-CM.It was more efficient than BMSC-CM for the treatment of arthritis[26]and diabetes[20],for neuroprotection[62]and repair[18].
Concerning SCAP-CM,studies have shown that compared to BMSC,SCAP secrete more proteins associated with metabolic processes and transcription,but less proteins involved in biological adhesion,immune function,and developmental processes.The amounts of chemokines and neurotrophins secreted by SCAP are significantly higher than those of BMSC,in contrast to extracellular matrix proteins and proangiogenic factors[106].
Cells origin:Donors
Aging has a clear influence on cell proliferation and capacity to differentiate.However,it is difficult to define a barrier discriminating optimal age for cell recovery.Decrease in dental MSC proliferation,migration,and differentiation has been correlated with age in the literature[131].But among the publications considered in this systematic review,only one investigated the impact of donor age and could not highlight any significant difference between DPSC-CM from aged(44-70 years old)and young donors(19-30 years old),in term of trophic effects[37].Taking the overall publications,donor age range in most of the studies is between 14 to 30 years old.Some studies considered donors over 30 years old[38,53,66,67,102],with good results overall.In general,the number of donors,their gender and medical status were not reported.Inter-donor variability has rarely been taken into account,although teeth have been extracted from several donors.In most cases,DPSC were isolated from erupted or impacted third molars,but they were also obtained from premolars,canines and incisors in some studies.SHED were always isolated from deciduous teeth,with donors under 12 years of age.CSDF were obtained mainly from molars and premolars.The developmental stages of the teeth were considered only in few studies,and their clinical status(decayed or not)were not systematically provided.
Some studies used dental cells from rats,dogs,and pigs,making comparison even more complicated,in term of donor.Thus,it is not possible to conclude about optimal donor age range,according to the available data.However,except for SHED,most of the investigations were conducted with donors under 30 years(Figure 4).Complete donors’ characteristics of studies investigating DMSC-CM are given in supplementary data(Supplementary Table 2).
Cell expansion
To our knowledge,the impact of cell passage number on their secretions has never been examined,while its effect on the concentration of secreted growth factors has only been noted once by Miura-Yuraet al[92].The passage number of cells ranged from 1 to 12 in studies investigating the potential of DMSC secretomes(Supplementary Table 3).Some studies did not even precise cell passage numbers[6,16,20,29,30,60,66,68,72,82,85,87,88,92,98].When considering cell expansion(through passage number),we can only observe that the majority of works were conducted with cells before passage 5,to prevent any risk of senescence during cell expansion(Figures 4 and 5).
Cell sorting
Significant variability in proliferative and differentiation capabilities have been observed for individual DPSC populations expanded from human teeth from donors within a similar age range.Inherent differences were even identified between DPSC populations derived from the same patient[132].In some studies,cell population was selected before investigating DMSC-CM.Side populations were used in some studies,targeting CD31 marker[72,74]or CD31/CD146 markers[71,133].These sorted populations from dental pulps are enriched in stem/progenitor cells and can significantly stimulate angiogenesis/vasculogenesis[133].Stem cell mobilization through G-CSF induction was also used in several studies to isolate stem cells[37,49,54,60,73,75-77].This technique generated a mobilized subpopulation of dental pulp stem cells that are stable and age-independent,with high proliferative,migratory and regenerative potentials.Single cell clones derived from human deciduous tooth pulp cells were used by Akazawaet al[65].Finally,De Rosaet al[66]selected DPSC based on their relative positivity profile for markers such as CD34,CD117,CD44,STRO-1,RUNX-2 and OC,before studying the secretome potential of osteodifferentiated DPSC.
No consensus could be described about cell sorting before CM recovery.This is not surprising as mesenchymal/stromal population are heterogeneous populations in which no specific/unique marker is described so far.
Conditioning period
In most studies investigating DMSC-CM effects from secretome,cells were harvested for 48 h,starting with DMSC at 70%-80% of confluency(Histograms in Figure 4 and Supplementary Table 4 in supplementary data).Paschalidiset al[46]followed DPSC-CM collections from 4 d to 24 d,and described most pronounced effects within the first collection.The conditioning period was always considered with cells incubated in serum-free media.No clear difference could be enlightened from the various studies about protein concentration or DMSC-CM effects,but 48 h was the most used duration.
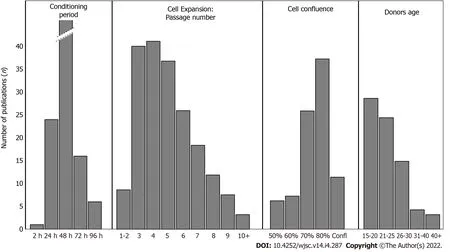
Figure 5 Histograms showing the number of publications for each parameter from the 4 specific key points:Conditioning period;Cell passage number;Cell confluence;Donor age.
Cell culture medium
The principal culture medium used for DMSC-CM processing was DMEM.This medium was used for processing indifferently of the targeted application,or clinical objective:cardiac injury[19],diabetes[20],lung[23]or liver[22]injuries,bone regeneration[27],nerve regeneration[60],dental regeneration[73],and cerebral ischemia[95].During the processing,in most of the studies,cells were kept in serum-free conditions,without Phosphate Buffer Saline(PBS)washing step before conditioning period(Supplementary Table 5).
Microenvironmental cues
It has been described that MSC could act as repair cells within the body when stimulated and recruited.This concept lead to MSC implication,not only by natural and constitutive secretion of regenerative factors,but also by producing specific factors in response to stimuli[134].Thus,the secretion of different potential therapeutic factors by MSC can be modulated according to various physical,chemical,and biological cues.
During DMSC-CM production,the effects of hypoxia,3D culture,LPS preconditioning,and osteodifferentiation were studied(Supplementary Table 6).Hypoxia is an environmental cue increasing VEGF levels[68,104]in the secretome.A study even described that hypoxia increased angiogenic potential of SCAP conditioned medium[104].It was shown that 3D culture increased anti-apoptotic effects of SHED conditioned medium,when used with dopaminergic neurons[16].LPS preconditioning increased DPSC conditioned medium potential for Schwann cells proliferation,migration,and odontogenic differentiation[50].Secretome with LPS preconditioning also increased PDLSC anti-inflammatory effects[98].The cytokine,chemokine and growth factor profiles of SHED-CM were significantly affected after osteoinduction of SHED cells[90],which also strengthened the effect of SHED-Exosomes on the osteogenic differentiation of PDLSC[91].Osteogenesis of amniotic fluid stem cells was also stimulated by the secretome of osteodifferentiated DPSCs[66].Kolaret al[44]stimulated DMSC using a previously published protocol[135],which increased significantly the secretion of VEGF-A and BDNF proteins and enhanced the effect of DMSC secretome on neurite outgrowth.
Each preconditioning regimen induced an individual expression profile with a wide variety of factors,including several growth factors and cytokines[136].
Secretome purification
DMSC-CM was concentrated before use in some studies and diluted to 50% in others[35,46,109,110].A 50% diluted DMSC-CM has sometimes been proposed as the most effective[46],probably because of an optimal balance achieved between paracrine stimulatory and metabolic inhibitory products[46,137].Only protein fractions with a molecular weight between 10 kDa and 3 kDa were able to protect neurons from induced neurodegeneration,indicating a possible responsibility of these microproteins for the neuroprotective character of DPSC-CM[39].Only the fraction smaller than 6 kDa of SHED-CM promoted neurite growth in dorsal root ganglion neurons[92].Finally,in most studies,filtration with 0.2μm pore filters was performed,in order to sterilize the CM and/or remove debris.Supplementary Table 7 summarizes the CM purification procedures in studies using DMSC-CM for tissue regeneration.
Numerous studies have been performed to assess if DMSC functions are associated with EV-enriched fractions or not.Merckxet al[55]demonstrated that the main angiogenic effect of growth factors secreted by DMSC was not associated with EVs.Nevertheless,Zhouet al[38]showed a decrease in the proangiogenic effects of DPSC-CM when EV secretion was blocked.DPSC exosomes in comparison with DPSC-CM showed similar neuroprotective efficacy,with superior anti-necrotic properties and implication in aging processes[39,138].SHED-exosomes,in contrast to SHED-derived microvesicles,have approved anti-apoptotic effects in dopaminergic neurons[16].However,they did not show the neuritogenic potential observed in SHED-CM[92].Supplementary Table 8 in supplementary data summarizes the methods used to purify the DMSC-EV in the literature.
Secretome storage
DMSC-CM was stored at -80 °C in most studies,at -20 °C in three studies[78-80]and at +4 in two studies[87,88].These different kind of storage did not affect the potentials of CM.Protease inhibitors were added to secretomes just in few studies[13,16,73,74].Supplementary Table 9 in supplementary data summarizes the storage conditions of DMSC-CM in literature.
Secretome characterization
Secretome characterization is necessary to identify DMSC-CM profiles and to confirm the reproducibility of manufacturing.Different technics were used in the literature(Supplementary Table 10).Protein concentration in the secretome was measured with the BCA and Bradford protein assays.Other techniques were used for qualitative and quantitative identification of CM.With the development of technology,proteomics has become a strong tool for identification,and mass spectrometry has emerged as the main technique applied for the detection of proteins in cell secretomes[139].A high protein coverage was obtained by mass spectrometry in a study conducted by Tachidaet al[140]to identify the secretion profile of DPSC-CM.In that study,CM was prepared with DPSC isolated from rat incisors,having a confluence of 80-90%.Three washes with PBS were done before starting the conditioning of serum-free alpha-MEM medium by DPSC for 72 h.A 0.2µm filtration and a 50-fold concentration with a 3 KDa MWCO centrifugal unit were then performed[140].The CM profile described in this study cannot represent DPSC-CM in general,as the CM preparation protocol is different from those used in other DMSC-CM studies.Comparing protein profiles and concentrations directly between studies is hampered by the variability of all the factors detailed above,and a production standardization may be the key to obtaining clear DMSC-CM profiles and ensure appropriate use of each CM based on its profile.
DISCUSSION
Among all the considered key points,at the 3 Levels of CM production,no consensus could be highlighted as evidence-based methods.We could only describe the most commonly used protocols.However,the various microenviroenmental cues were shown many times to have a significant impact on CM protein content,and improved effect on specifically targeted applications.Hypoxia enhanced the VEGF secretion and proangiogenic;3D culture increased anti-apoptotic effects on neurons;LPS preconditioning increased cell proliferation and migration,with enhanced anti-inflammatory activity;osteoinduction of DMSC enhanced the CM effect for further cell osteodifferentiation.Taken altogether,each preconditioning manipulation induced a specific protein expression profile.
More recently,we have demonstrated that specific preconditioning protocols could lead to DMSCCM which significantly increase neurite growth in sensory neurons[141].This neuroregenerative effect of CM due to preconditioning was linked to a change in secretome composition,with an increase of several factors involved in neurogenesis,neuroprotection,and angiogenesis.
CONCLUSION
We have reviewed here the different conditions and protocols used in the manufacturing of DMSC-CM and their derived products.This literature survey allowed us to describe that donors under 30 years of age are often used to produce CM.DPSC and SHED were the most commonly used cells,with cell passage between 1 and 5,and at a confluence of 70% to 80%.DMEM was the most commonly used culture medium for all applications.CM were often collected during the first 48 h,and frozen at -80 °C.The preconditioning environment(environmental cues)had a significant impact on DMSC-CM content and efficiency.Therefore,further studies should be conducted to confirm which environmental conditions specifically optimize the potentials of DMSC-CM for each application in tissue regeneration,and which parts of DMSC-CM are responsible for these potentials.
ARTICLE HIGHLIGHTS
Research background
Dental Mesenchymal stem cells are progenitor populations recovered from dental and periodontal tissues easily accessible when a tooth is extracted(and usually discarded as medical waste).One of the main effects of Mesenchymal stem cells on tissue regeneration is due to their paracrine activity,mediated by their secreted substances defined as secretome or conditioned medium.Conditioned medium represents a cell-free product with wide potential applications for various diseases treatment and tissue regeneration.
Research motivation
Conditioned medium manufacturing suffers from variable procedures and protocols.Results presented by various studies are difficult to compare,due to the different methods of production.Moreover,there is no well-defined optimized procedure to specifically target specialized tissue in regenerative medicine.
Research objectives
To describe potential consensus for the standardization and optimization of manufacturing procedures,mandatory for further clinical applications.In this systematic review,we focused on conditioned medium produced from Dental Mesenchymal stem cells.We explored the current parameters and culture conditions used in the manufacturing protocols.
Research methods
The bibliographic research was conducted in accordance with the PRISMA guidelines.All articles published between 2006 and 2020 investigating the effects of Dental Mesenchymal stem cells secretome on tissue regeneration were selected.We used the electronic PubMed database with these search terms:((dental stem cells)AND((conditioned medium)OR(secretome)))/((dental stem cells)AND((extravesicles)OR(exosomes))).Only publications in English were considered.
Research results
Based on the inclusion criteria,118 articles were included in the systematic review.Conditioned medium production was considered at three levels:before recovery(cell sources),during production(culture conditions)and after production(secretome treatment).We identified key points that were often taken into account in the published experimental works.
Research conclusions
Among all the considered key points,no consensus could be highlighted.However,some tendencies could be described:cells used were mainly from donors under 30 years of age,with cell passage between 1 and 5,at a confluence of 70% to 80%.Conditioned medium was usually collected during the first 48 h,and kept frozen at -80°C.The various microenviroenmental cues were shown many times to have a significant impact on protein content,and improved effects on specifically targeted applications:each preconditioning manipulation induced a specific protein expression profile.
Research perspectives
Standardization of procedures is of prior importance to develop clinical-grade products.However,the protein contents of secretome is more linked to preconditioning than to specific technical methods.The challenge to overcome in the near future is to define specific preconditioning protocols to produce tissue specific conditioned medium(i.e,osteogenic environement,neuronal environment,cell incubation with inflammatory proteins…).
FOOTNOTES
Author contributions:Chouaib B conducted the bibliographic research and selected the targetted articles;Chouaib B and Collart-Dutilleul PY analyzed the selected articles;Chouaib B and Collart-Dutilleul PY wrote the main draft;Cuisinier F corrected the manuscript and supervised the findings of this work;all authors discussed the results and contributed to the final manuscript.
Conflict-of-interest statement:All authors declare that they have no conflict of interest.
PRISMA 2009 Checklist statement:The authors have read the PRISMA 2009 Checklist,and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial(CC BYNC 4.0)license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See:http://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:France
ORCID number:Batoul Chouaib 0000-0002-5860-067X;Frédéric Cuisinier 0000-0001-6870-0490;Pierre-Yves Collart-Dutilleul 0000-0003-3017-6843.
S-Editor:Wang LL
L-Editor:A
P-Editor:Wang LL
 World Journal of Stem Cells2022年4期
World Journal of Stem Cells2022年4期
- World Journal of Stem Cells的其它文章
- Correlation between amino acid metabolism and self-renewal of cancer stem cells:Perspectives in cancer therapy
- Treatment of syringomyelia using uncultured umbilical cord mesenchymal stem cells:A case report and review of literature
- Skinny people serum factors promote the differentiation of multipotent stem cells into brown adipose tissue
