固相萃取净化-气相色谱-负化学源质谱法测定近岸海洋环境中的甲氧基多溴联苯醚
郜文,孙秀梅,金衍健,郝青,傅宇,叶茂盛,杨承虎,郭远明
(1.浙江海洋大学 海洋与渔业研究所,浙江 舟山 316021; 2.浙江省海洋水产研究所浙江省海洋渔业资源可持续利用技术研究重点实验室,浙江 舟山 316021)
固相萃取净化-气相色谱-负化学源质谱法测定近岸海洋环境中的甲氧基多溴联苯醚
郜文1,2,孙秀梅2*,金衍健2,郝青2,傅宇1,2,叶茂盛1,2,杨承虎2,郭远明2
(1.浙江海洋大学 海洋与渔业研究所,浙江 舟山 316021; 2.浙江省海洋水产研究所浙江省海洋渔业资源可持续利用技术研究重点实验室,浙江 舟山 316021)
甲氧基多溴联苯醚(methoxypolybrominated diphenyl ethers, MeO-PBDEs)广泛存在于生物体和海洋环境。以象山海域的生物体和沉积物为样本,采用固相萃取净化-气相色谱-负化学源质谱法,检测了6种MeO-PBDEs,结果显示,当目标分析物浓度为0.1~20.0 μg∙L-1时,线性关系良好(R2gt;0.999),检出限为0.13~0.22 μg∙kg-1,定量限为0.42~0.72 μg∙kg-1,实际样品的平均回收率为71.2%~116.2%。MeO-PBDEs的分布状况调查结果显示,藻类样品中仅检出6-MeO-BDE-47,且浓度较低,其他生物体中检出3种MeO-PBDEs,检出率为31.3%,浓度为0.21~2.72 μg∙kg-1。沉积物中无MeO-PBDEs被检出。
甲氧基多溴联苯醚;气相色谱-负化学源质谱;固相萃取净化;海洋环境
甲氧基多溴联苯醚(methoxypolybrominated diphenyl ethers,MeO-PBDEs)是持久性有机污染物多溴联苯醚(PBDEs)的甲氧基结构衍生物。多溴联苯醚是一种广泛用于纺织、塑料和电子产品等的添加型溴系阻燃剂[1],由于其在生产、使用过程中不易挥发且难降解,对环境及生物造成持久影响,被《斯德哥尔摩公约》列为具有持久性、迁移性和生物累积性的持久性有机污染物。已有研究显示,MeO-PBDEs是天然有机物,主要源自海绵、藻类等生物,也可由PBDEs、OH-PBDEs通过生物转化、光转化等生成[2-5]。由于PBDEs、OH-PBDEs和MeO-PBDEs具有相似的结构和性质[6-7],并可相互转化,因此普遍认为MeO-PBDEs可能具有PBDEs和OH-PBDEs的毒性,影响甲状腺效应、免疫毒性和神经行为[8],并改变生殖行为,导致胚胎出现致命性或非致命性畸形,降低繁殖成功率[9],其对雌性激素的抑制作用已得到证实[10]。
在世界各地环境中均已检出MeO-PBDEs。在亚洲地区,如我国渤海、辽东湾和长江流域的各个营养层级生物体及我国香港和日本人体的乳汁、血清和肝脏中,均曾检出MeO-PBDEs[5],浓度为2~420 μg∙kg-1。在欧洲地区,如瑞典的鱼卵中,英国北海海域的海豹、海豚体内,均检出了6-MeOBDE-47和2apos;-MeO-BDE-68,浓度最高达483 μg∙kg-1[15-16]。在非洲突尼斯地区,人体的乳汁、血清中,检出了6种MeO-PBDEs,海洋生物体内检出了8种MeO-PBDEs,浓度为0.39~286 μg∙kg-1[17-18]。在澳洲南部海岸,发现了89种天然生成的MeO-PBDEs[17]。SUN等[18]在极地地区海洋食物网中发现了MeO-PBDEs的生物富集和营养转移。在美国、加拿大等地的环境中也存在MeO-PBDEs[19-21]。
常见的MeO-PBDEs检测方法有,气相色谱-负化学源质谱(GC-NCI/MS)[22]、气相色谱-电子捕获检测器(GC-ECD)[23]、气相色谱-高分辨质谱[24]、气相色谱-负化学电离源-质谱(GC-ECNI-MS)等仪器分析方法。ZHANG等[3]采用GC-NCI/MS方法结合液液萃取技术检测到BeWo细胞内含6-MeO-BDE-47、2apos;-MeO-BDE-68等MeO-PBDEs,回收率为88.9%±18.5%。LIU等[12]采用固相萃取、酸性硅胶柱、无水硫酸钠净化等方法,对海洋生物样品和沉积物进行液相色谱-电喷雾电离-三重四级杆质谱分析,回收率为71%~113%,方法检测限为2 μg∙kg-1(干重)。基于此,本实验选择固相萃取,辅以EMR-Lipid柱以及浓硫酸净化的方法,通过相应洗脱剂对样品进行选择性洗脱,达到分离净化的目的。依据MeO-PBDEs溴代数和结构性质特征,选择气相色谱-负化学源质谱进行检测,分析象山海域生物体和沉积物中MeO-PBDEs的累积特征。
1 实验
1.1 主要仪器与试剂
7890B-7000C型气相色谱-质谱联用仪(Agilent);R210旋转蒸发仪(瑞士Buchi公司);Centrifuge5810高速离心机(德国Eppendorf公司);N-EVAP-112氮吹仪(美国Organomation公司);超声波清洗器(上海科导超声仪器有限公司);MS3涡混合器(德国IKA公司);12通道固相萃取装置(美国Suplco公司);恒温振荡器。
正己烷、二氯甲烷和丙酮(农残级);浓硫酸;有机微孔滤膜(0.22 µm,上海安谱科学仪器有限公司);弗罗里硅土柱(500 mg,3 mL)、中性氧化铝柱(500 mg,3 mL)、EMR-Lipid固相萃取柱(500 mg,3 mL)、硅胶柱(500 mg,3 mL)均购自上海安谱科学仪器有限公司。
目标化合物包括6种MeO-PBDEs标准品:3apos;-MeO-BDE-28、3-MeO-BDE-47、5-MeO-BDE-47,浓度均为10 μg∙mL-¹;2apos;-MeO-BDE-68、6-MeO-BDE-47、4-MeO-BDE-42,浓度均为50 µg∙mL-¹。所有标准品均购自美国AccuStandard公司。
1.2 标准溶液的配制
准确移取6种MeO-PBDEs混合标准品(1.0 μg∙mL-1)0.1 mL,分别溶于5 mL正己烷溶液,配制成浓度为20 μg∙L-1的混合标准储备溶液,-20 ℃贮存,备用。用正己烷溶液配制质量浓度分别为0.1,0.2,0.5,1.0,2.0,10.0,20.0 μg∙L-1的MeO-PBDEs混合标准工作溶液。
1.3 样品收集
在象山海域采集20份沉积物样品和102份海洋生物样品,涉及20种鱼类、12种甲壳类(9种虾、2种蟹和1种龟足)、4种头足类、1种腹足类和3种藻类,如表1所示。现场采集足够数量的完好生物样品,冷冻后运回实验室,取鱼类的肌肉,甲壳类、腹足类和头足类的软组织以及藻类制样,真空冷冻干燥。采集沉积物样品后,滤去水分,剔除砾石、木屑、杂草及贝壳等动植物残体,搅拌均匀后装入棕色玻璃瓶中,冷藏运回实验室,经实验室真空冷冻干燥后,避光保存。所有样品密封后,于-20 ℃贮存。
表1 象山海域生物样品信息Table 1 Sampling information of organisms in Xiangshan sea area
1.4 样品前处理
1.4.1 样品的提取
准确称取0.5 g海洋生物样品(干样)于50 mL具塞离心管中,加入10 mL二氯甲烷/正己烷(体积比为1∶1),涡旋2 min,超声提取5 min,以6 000 r∙min-1离心,取上清液转移至离心管。重复提取一次,合并提取液于同一离心管中,加入2 mL水,再缓慢加入5 mL浓硫酸,封口,于恒温(20 ℃)振荡箱中振荡2 h。将酸化样品离心,取上清液转移至旋蒸瓶,加入10 mL二氯甲烷/正己烷(体积比为1∶1),重复提取一次,合并上清液,并旋蒸至近干,用1 mL正己烷复溶,待净化。
准确称取0.5 g沉积物样品(干样)于50 mL具塞离心管中,提取酸化过程同海洋生物样品,在提取液中加入1 g活化后的铜粉,超声15 min、静置2 h后转移至旋蒸瓶,旋蒸至近干,用1 mL正己烷复溶,待净化。
1.4.2 样品的净化
用EMR-Lipid固相萃取柱对提取样品进行净化。小柱用5 mL丙酮活化,提取液上样,用5 mL二氯甲烷洗脱,收集洗脱液氮吹浓缩至近干,加入1 mL正己烷定容。定容后的溶液过微孔滤膜,收集于气相小瓶,待进样分析。
1.5 仪器条件
1.5.1 气相色谱条件
色谱柱为DB-5MS毛细管柱(30 m×0.25 mm×0.25 μm);色谱柱升温程序:初始温度为70 ℃,以30 ℃∙min-1升至210 ℃,再以5 ℃∙min-1升至300 ℃,保持10 min;进样口温度为280 ℃;进样量为1 μL;进样方式为不分流进样。
1.5.2 质谱条件
电离方式为电子轰击电离(EI)源、负化学电离(NCI)源;离子源温度为150 ℃;四极杆温度为150 ℃;转移管温度为280 ℃;溶剂延迟时间为5 min;碰撞气流速为1.5 mL∙min-1,淬灭气流速为2.25 mL∙min-1;扫描模式为选择离子扫描(SIM)。
2 结果与讨论
2.1 检测条件的优化
为确定最佳检测条件,对气相色谱-串联质谱的进样口温度、检测器温度、升温程序和离子源等因素进行实验。比较EI源和NCI源对目标化合物的分析结果。先对6种MeO-PBDEs标准溶液进行EI源分析,再用SIM模式建立定量方法,其中6-MeO-BDE-47、2apos;-MeO-BDE-68、3-MeO-BDE-47、5-MeO-BDE-47、4-MeO-BDE-42、3apos;-MeO-BDE-28的扫描离子质荷比(m/z)分别为340.7,355.8,515.7,262.9,277.9,435.7,其他色谱条件与NCI源模式相同。各目标组分在EI源模式下基线不稳定,灵敏度较差,部分目标峰在监测总离子流时色谱图响应较低。NCI源的电离方式是电离反应气,使其与样品发生反应,使样品分子电离,对于含有较强吸电子基团的化合物,负离子的检测灵敏度较高。各目标组分在NCI源模式下峰形好,基线平滑,检测灵敏度高,且分离效果较好。因此,选择NCI源模式。6种MeO-PBDEs标准品在NCI源模式下的总离子流色谱如图1所示。
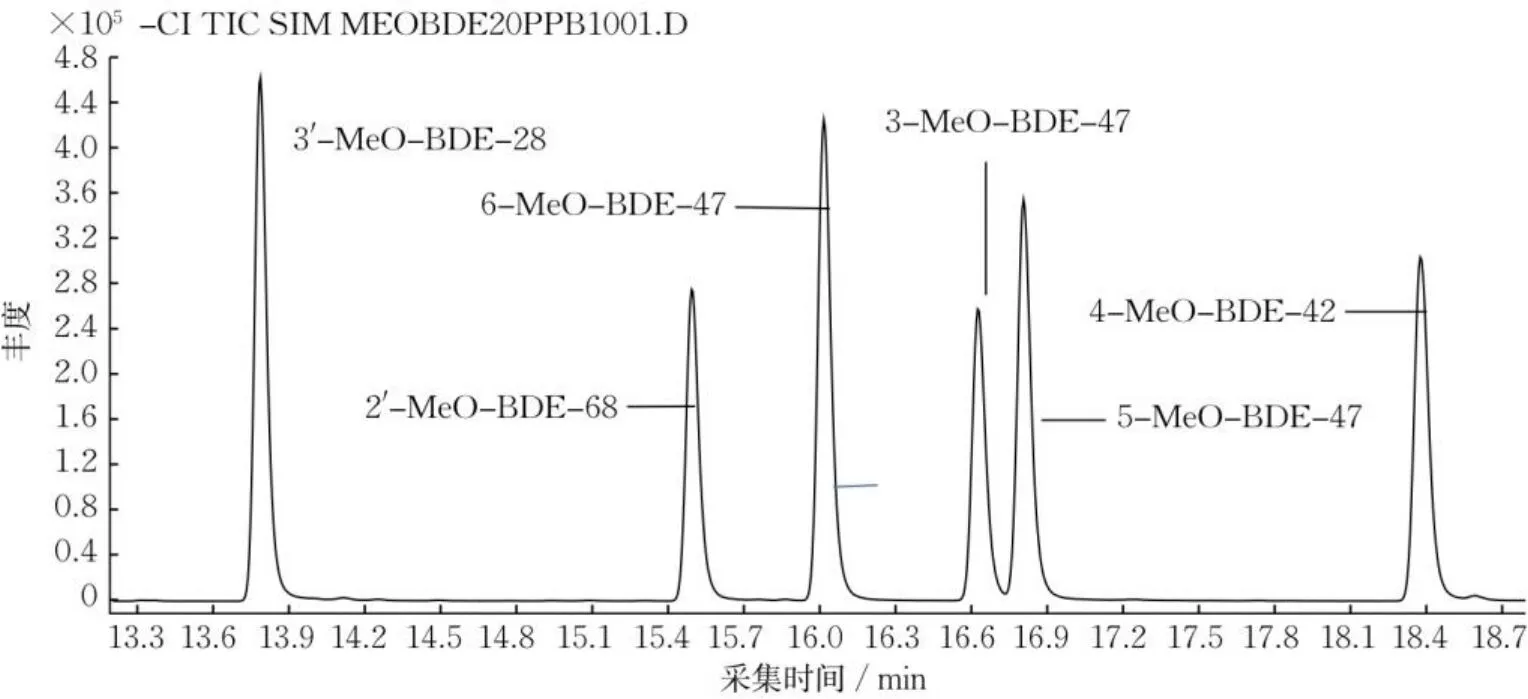
图1 6种MeO-PBDEs标准品的总离子流色谱(20 μg∙L-1)Fig.1 Total ion chromatogram of six MeO-PBDEs diphenyl ethers (20 μg∙L-1)
2.2 样品提取方式的选择
通过对比加速溶剂萃取[25]、索氏提取[26]和超声提取3种提取方式的加标回收率,确定沉积物样品中MeO-PBDEs的最佳提取方式。图2(a)(b)(c)分别为不同提取方式下低、中、高浓度水平目标化合物的加标回收率,可知,加速溶剂萃取、索氏提取、超声提取的平均加标回收率分别为77.5%~109.8%,83.9%~111.2%,76.3%~101.9%,均能较好地提取目标化合物。其中,超声提取耗时短,溶剂使用量小,且能满足提取效率需求,符合绿色原则。因此,选用超声提取法。
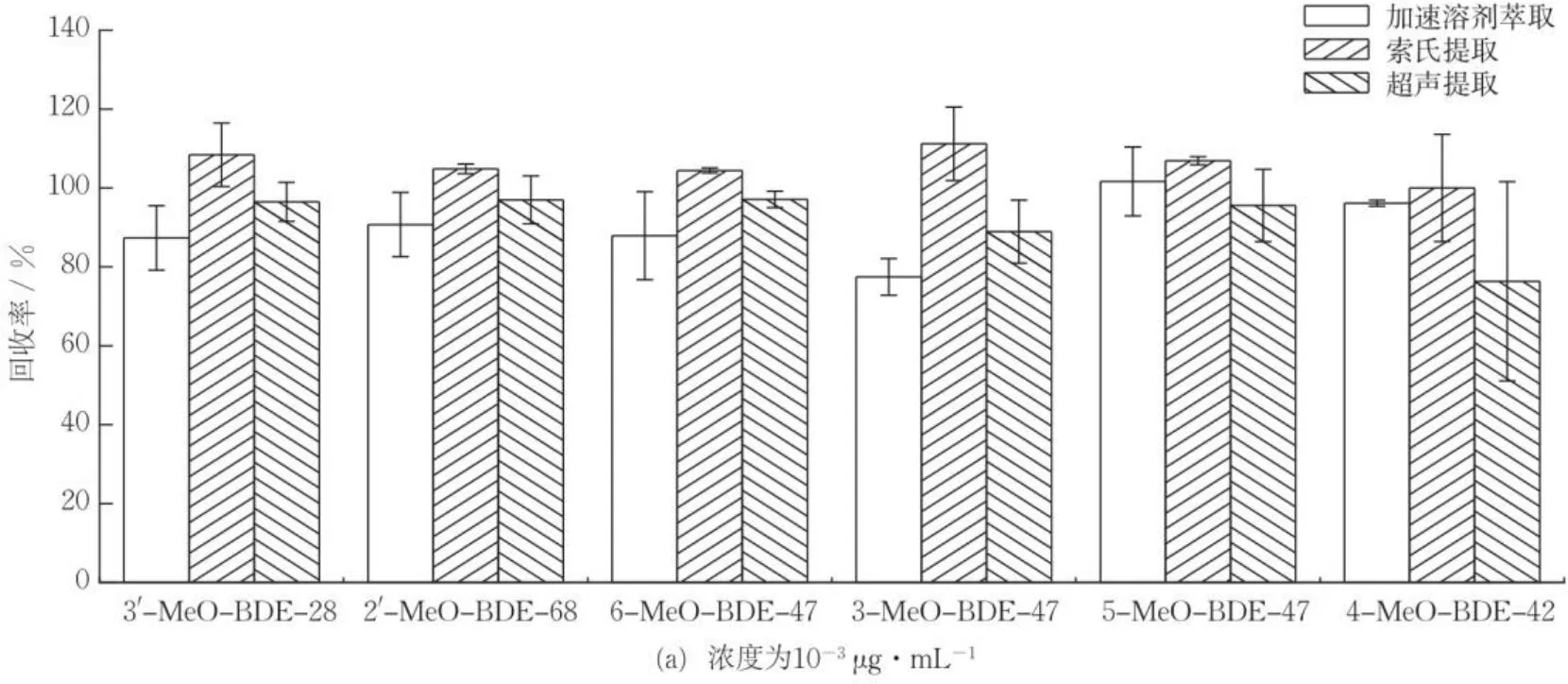
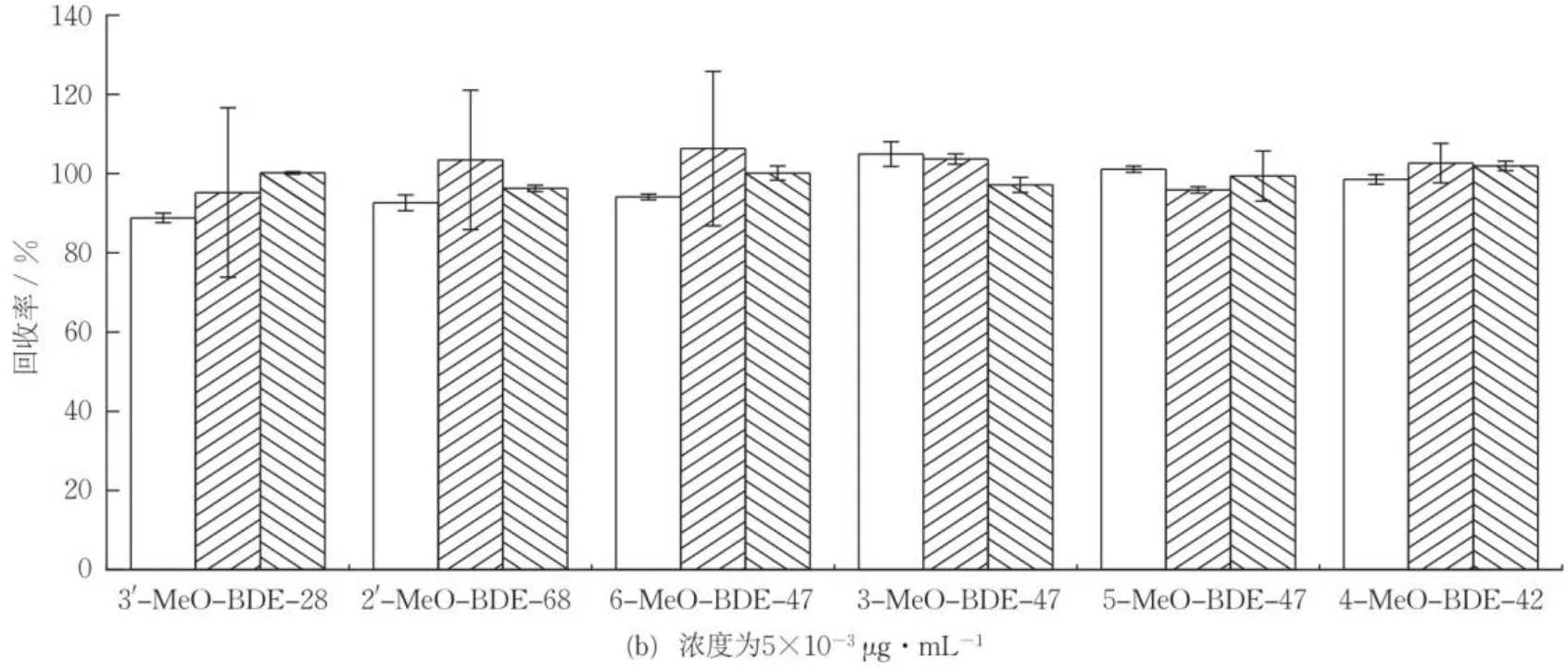
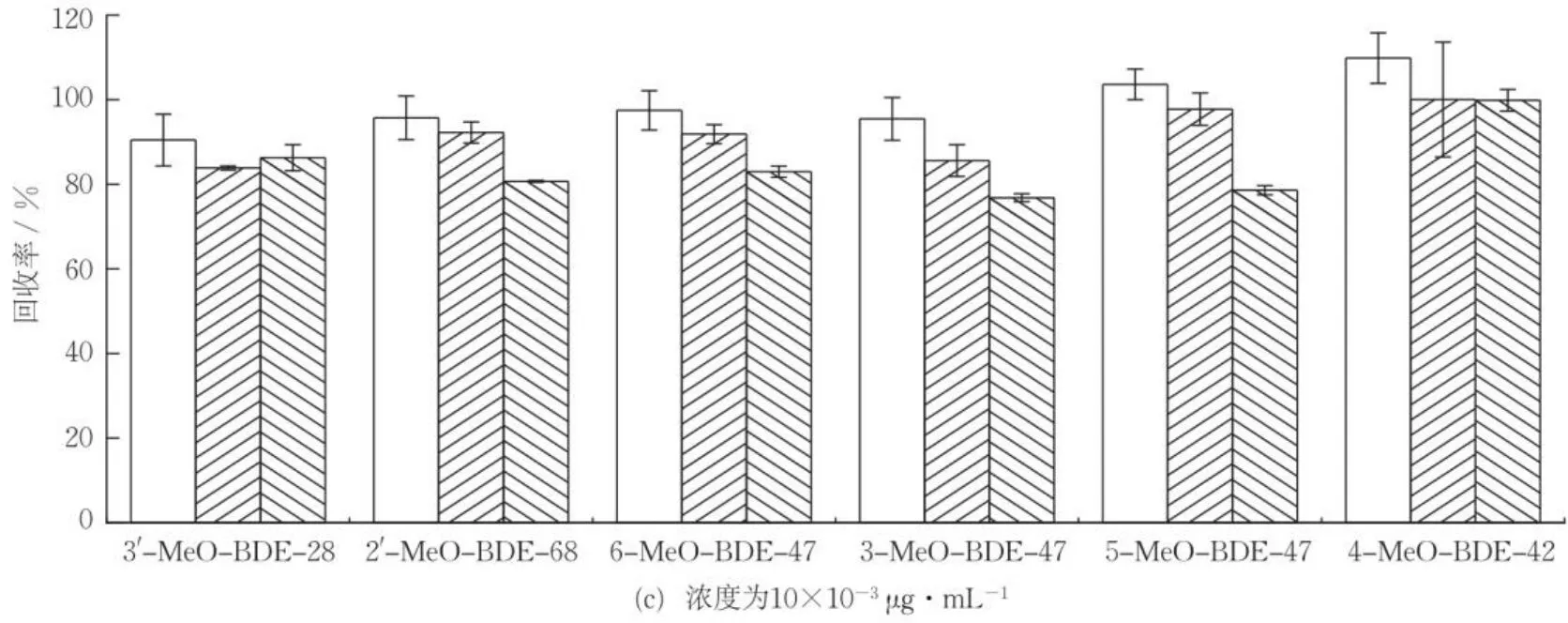
图2 不同提取方式对沉积物样品中MeO-PBDEs回收率的影响(n=3)Fig.2 Recoveries of MeO-PBDEs in sediments by different extraction methods (n=3)
2.3 净化条件的优化
2.3.1 固相萃取柱的选择
考查中性氧化铝柱、硅胶柱、弗罗里硅土柱、EMR-Lipid固相萃取柱等对样品回收率的影响。称取12份干燥后的样品各0.5 g,分为4组,每组3个平行样,每组样品加入1 mL 浓度为10 μg∙L-1的6种MeO-PBDEs混合标准溶液,采用超声提取法提取MeO-PBDEs。用5 mL丙酮活化、保持柱体湿润,上样后收集流出液,用5 mL二氯甲烷洗脱。
不同固相萃取柱对生物体样品中MeO-PBDEs的平均回收率的影响如图3所示。可知,中性氧化铝柱难以去除脂肪,样品难以通过萃取柱导致萃取柱堵塞,净化过程耗时过长,样品平均回收率为28.1%~55.2%,相对标准偏差(relative standard deviation,RSD)为10.3%~71.9%。硅胶柱的样品平均回收率也较低,为39.1%~71.0%,RSD为3.6%~46.6%。弗罗里硅土柱净化过程相对较短,样品平均回收率为37.2%~78.9%,但受基质影响较大,且对脂肪和藻类中色素的净化能力较弱。EMR-Lipid固相萃取柱的样品平均回收率为78.3%~99.7%,RSD为4.8%~6.5%,由于EMR-Lipid固相萃取柱是一种新型油脂吸附剂,结合前期酸化处理,能更好地降低基质干扰,减少实验时间,提高实验效率和平均回收率。
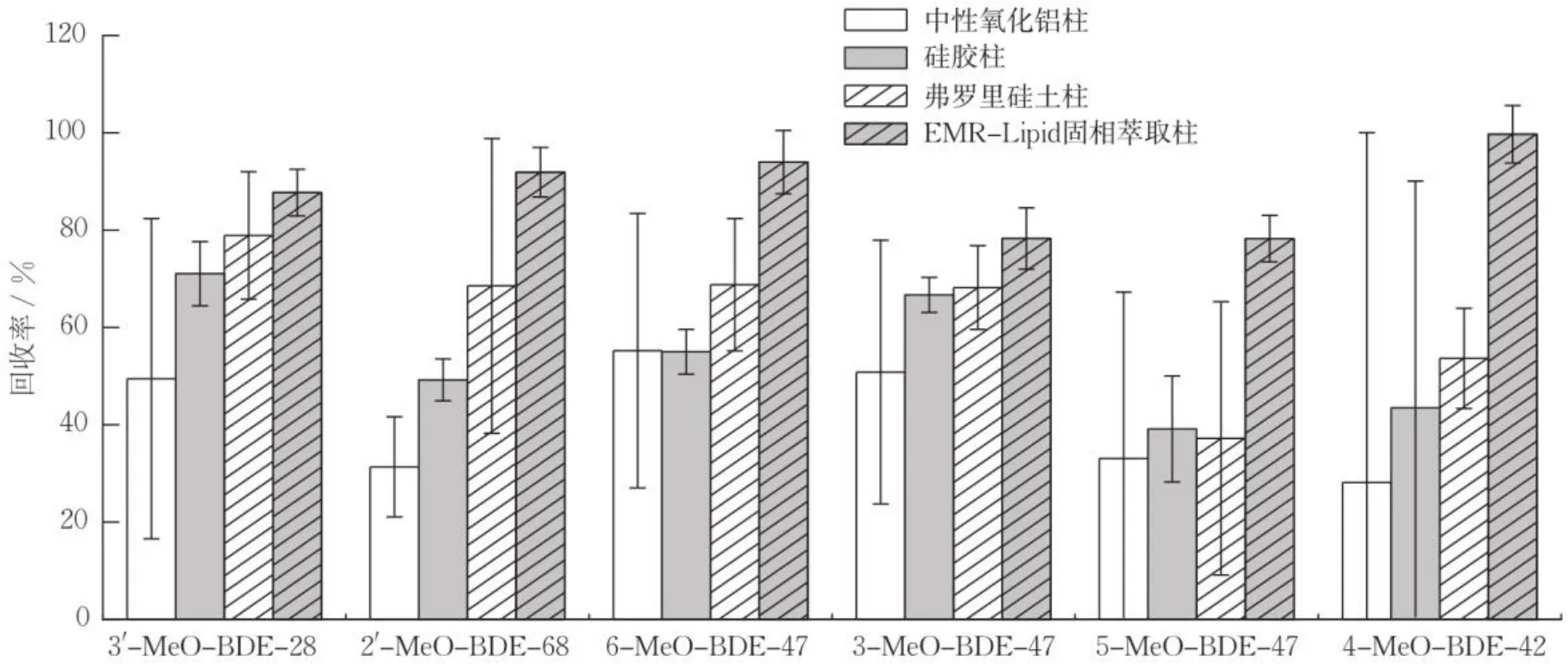
图3 不同固相萃取柱对生物样品中MeO-PBDEs回收率的影响(n=3)Fig.3 Recoveries of MeO-PBDEs in marine organisms samples by different solid phase extraction column (n=3)
2.3.2 酸化时间的确定
用二氯甲烷/正己烷(体积比为1∶1)提取样品,用硫酸酸化,结合EMR-Lipid固相萃取柱,以达到去除样品中脂肪、色素等杂质干扰的目的,但若酸化时间过短,则影响杂质的去除;若酸化时间过长,则影响目标化合物的回收率。选取鱼类、甲壳类和藻类样品,加入10 μg∙L-1的MeO-PBDEs标准品,每组设置3个平行样,酸化时间分别设为0,2,4,8和10 h,实验结果如图4所示。可知,当酸化时间为0时,样品回收率为73.9%~89.6%,且基质干扰效应较强;当酸化时间为2 h和4 h时,样品回收率分别为88.0%~108.9%和77.5%~100.4%。当酸化时间为8 h和10 h时,样品回收率分别为62.5%~93.5%和59.8%~90.7%。可知,采用2 h酸化处理能有效提高样品回收率,且降低基质的干扰效应,因此,选择2 h作为硫酸酸化时间。
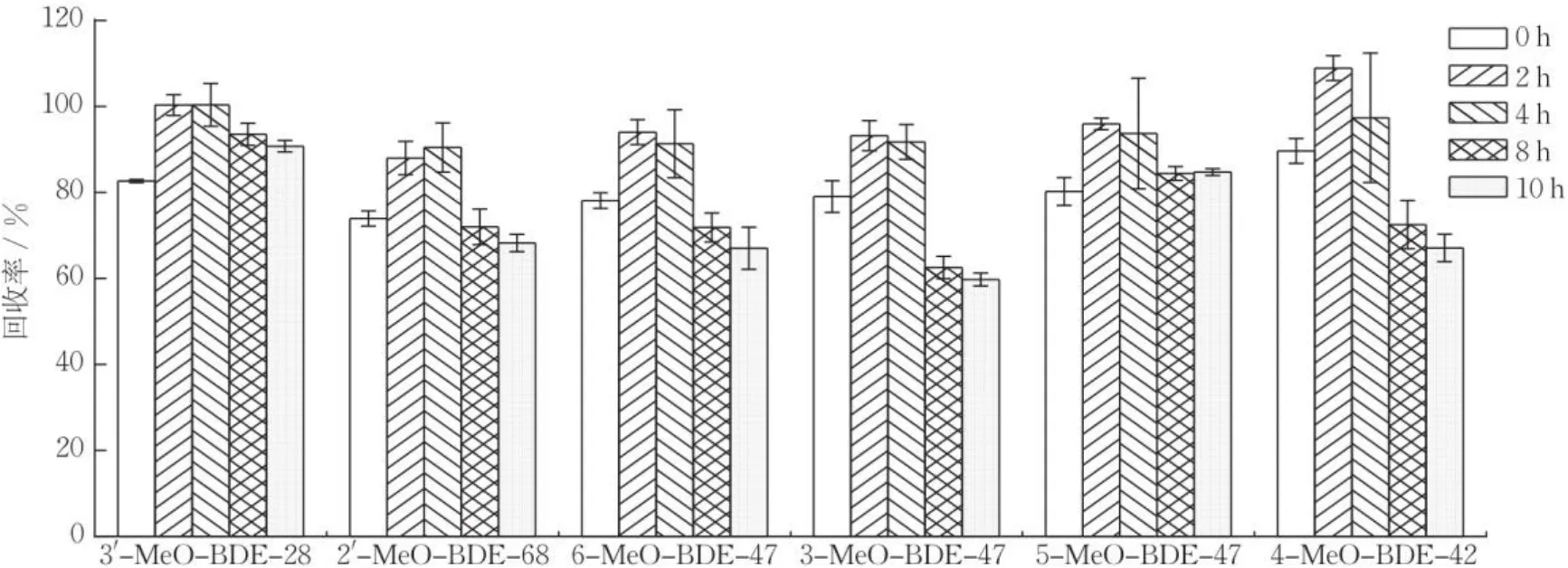
图4 不同酸化时间对生物样品中MeO-PBDEs回收率的影响(n=3)Fig.4 Recoveries of MeO-PBDEs in marine environment by different acidification time (n=3)
2.4 线性相关系数、检出限和定量限
取已配制的MeO-PBDEs混合标准工作溶液,并按上述条件进行测定,以3倍信噪比计算检出限,以10倍信噪比计算定量限。MeO-PBDEs的线性相关系数、检出限和定量限如表2所示。可知,MeO-PBDEs的响应在考查范围内线性关系良好(R²gt;0.999),检出限为0.13~0.22 μg∙kg-1,定量限为0.42~0.72 μg∙kg-1,灵敏度高。
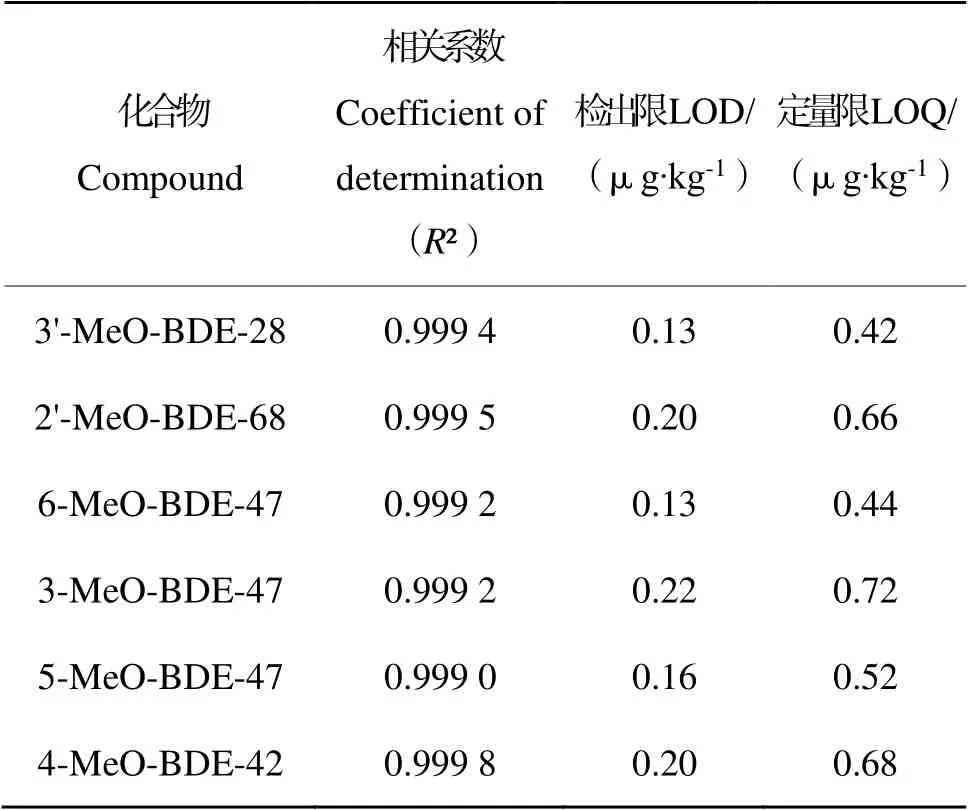
表2 线性相关系数、检出限和定量限Table 2 Linearity range,correlation coefficient, detection limit and quantitative limits
2.5 准确度与精密度
分别以加标回收率和RSD衡量准确度和精密度。在4种生物样品中分别添加浓度为1,10,20 μg∙L-1的MeO-PBDEs混合标准工作溶液,计算不同加标水平下MeO-PBDEs的加标回收率与RSD,结果如表3所示。可知,不同加标水平下MeO-PBDEs在4种生物样品中的加标回收率为76.2%~116.2%,RSD为0.2%~9.7%,准确度和精密度满足实际样品分析要求。
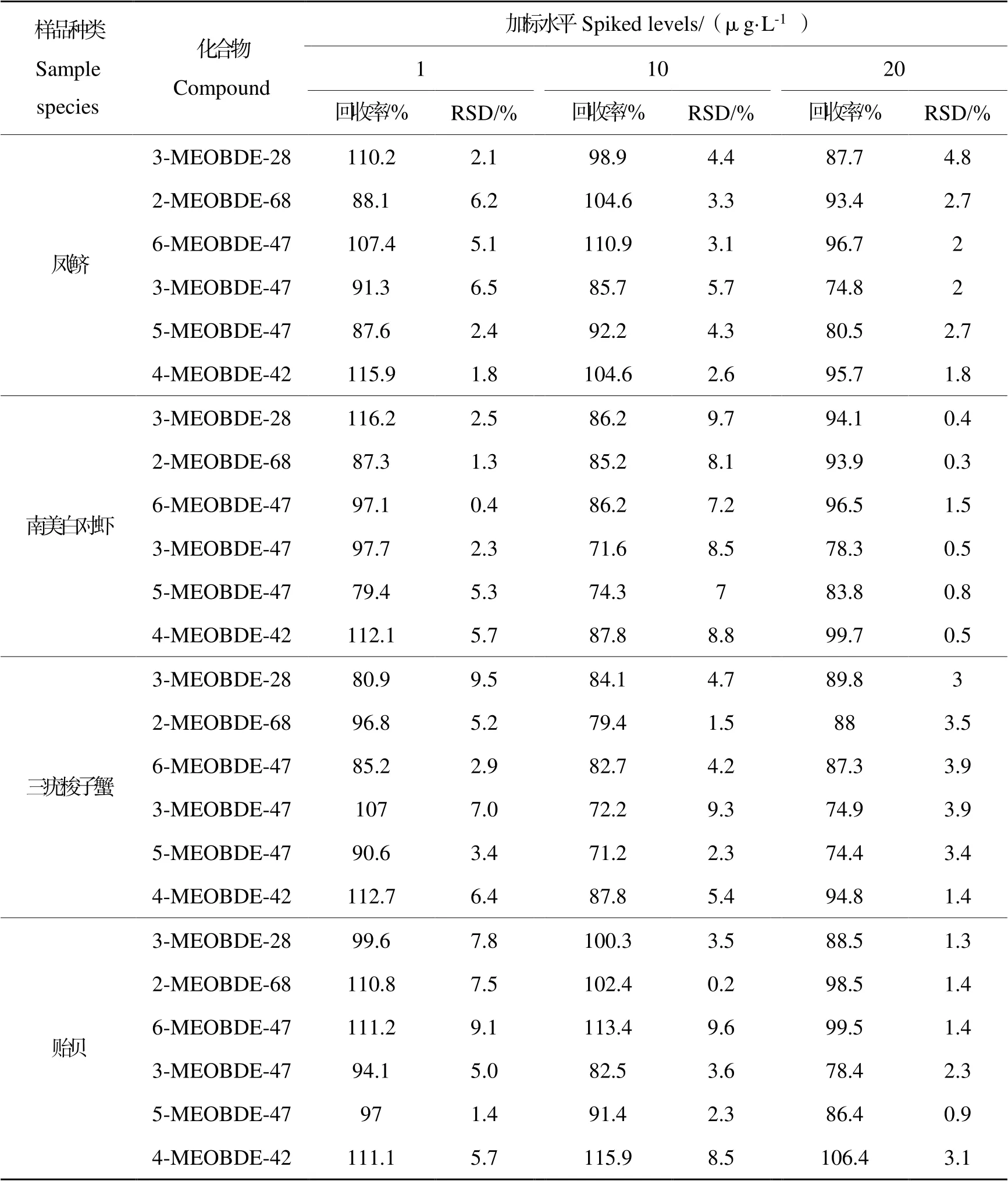
表3 不同加标水平下MeO-PBDEs在4种生物样品中的加标回收率与RSDTable 3 Recoveries and RSD of MeO-PBDEs in four marine organism samples at different spiked levels
2.6 样品的测定
在20种共40份鱼类样品中,16份样品检出了MeO-PBDEs,检出率为40.0%,平均检出浓度为1.23 μg∙kg-1。6-MeO-BDE-47的检出率为40.0%,其在中华海鲶中的检出浓度最高,为2.04 μg∙kg-1;在凤鲫和红狼牙虾虎鱼中检出了 2apos;-MeO-BDE-68,检出浓度分别为0.93 μg∙kg-1和0.28 μg∙kg-1,如图5所示。
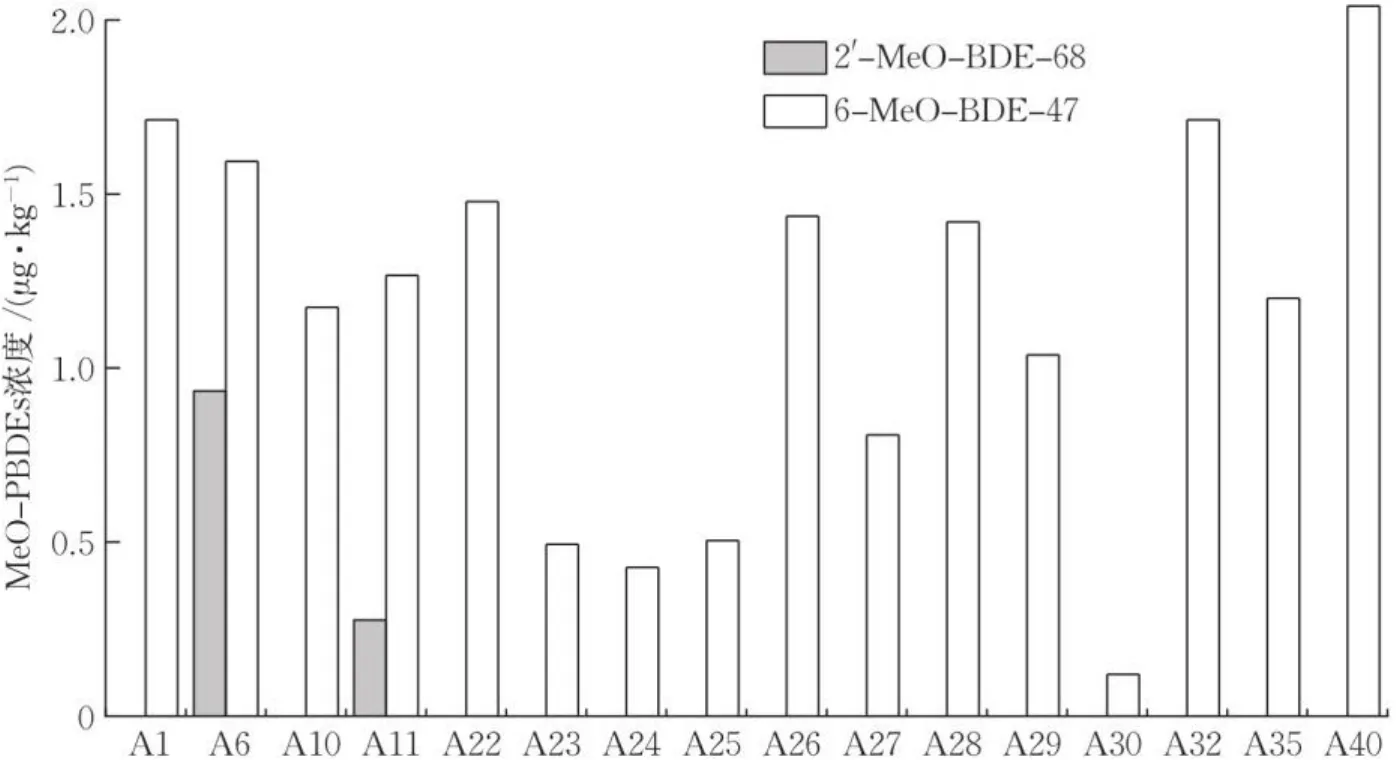
图5 象山海域鱼类样品中检出的MeO-PBDEs浓度Fig.5 Concentration of MeO-PBDEs in fishes samples from Xiangshan sea area
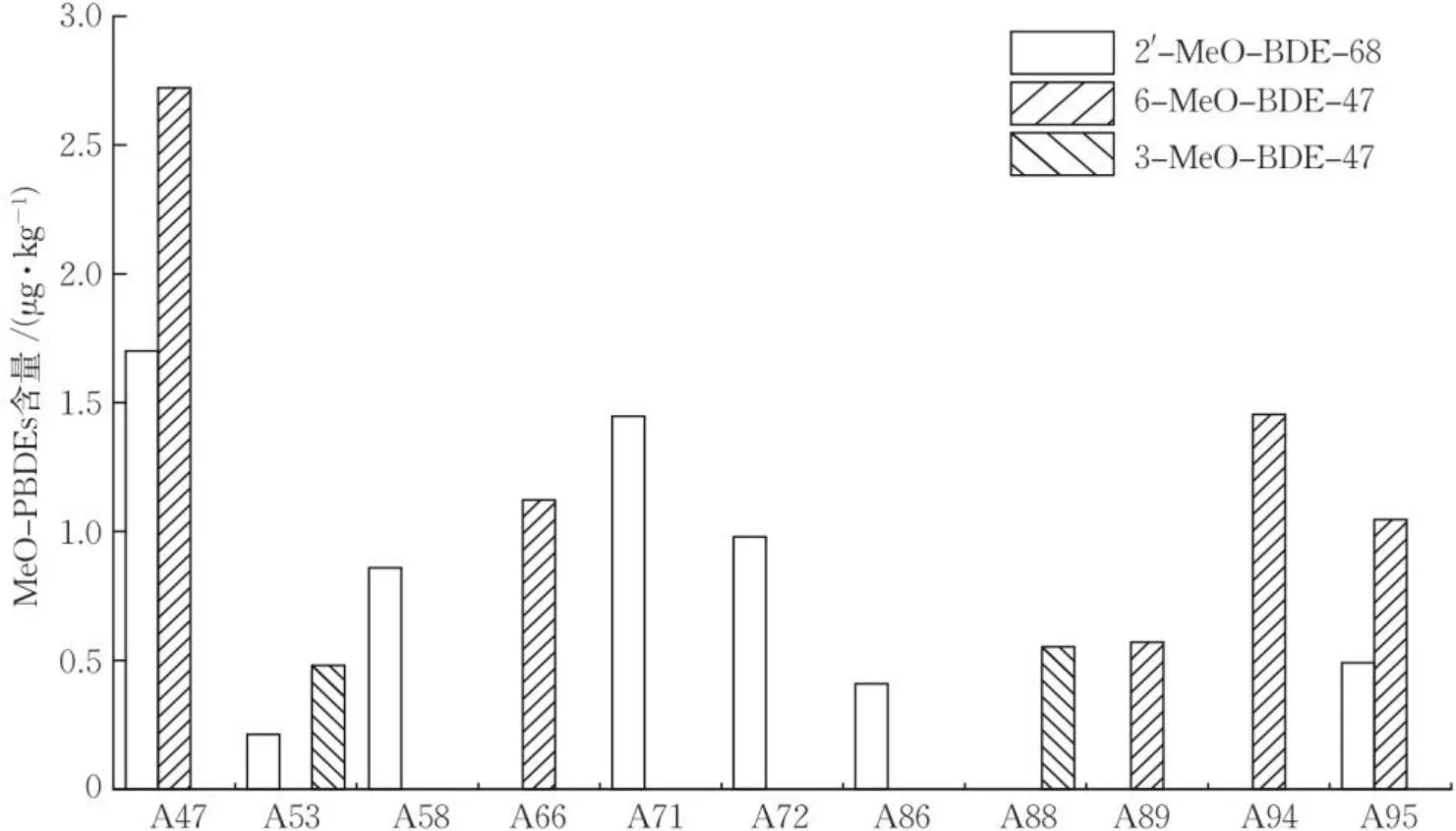
图6 象山海域甲壳类样品中检出的MeO-PBDEs浓度Fig.6 Concentration of MeO-PBDEs in curstaceans samples from Xiangshan sea area
在甲壳类样品中,共有5份虾样品检出了MeO-PBDEs,检出率为16.1%,平均浓度为1.71 μg∙kg-1。2apos;-MeO-BDE-68和6-MeO-BDE-47最高浓度均检出于口虾姑A47样品,分别为1.70和2.72 μg∙kg-1;3-MeO-BDE-47检出于青尖虾A53样品,浓度为0.48 μg∙kg-1。蟹样品中的MeO-PBDEs检出率为27.8%,平均浓度为0.90 μg∙kg-1,最高浓度为1.45 μg∙kg-1,且检出样品均为三疣梭子蟹。其中样品A89,A94,A95中检出了6-MeO-BDE-47,浓度为0.57~1.45 μg∙kg-1;样品A86,A95中检出了2apos;-MeO-BDE-68,浓度分别为0.41和0.49 μg∙kg-1;样品A88中检出了3-MeO-BDE-47,浓度为0.55 μg∙kg-1,如图6所示。此外,在龟足样品A72中检出了2apos;-MeO-BDE-68,浓度为0.98 μg∙kg-1。
头足类和腹足类样品中MeO-PBDEs的检出率为33.3%,在长蛸和荔枝螺中均检出了6-MeO-BDE-47,浓度分别为0.81和0.72 μg∙kg-1。藻类样品,仅在石莼中检出了少量6-MeO-BDE-47。
20份沉积物样品中均未检出MeO-PBDEs。采用索氏提取和加速溶剂萃取方式对结果进行验证,同样未检出MeO-PBDEs。
根据MeO-PBDEs的检出情况,象山海域内洄游种生物体样品中MeO-PBDEs的检出率远高于定居种海洋生物体样品,藻类被认为是MeO-PBDEs的主要生产者,但本实验仅在石莼中检出了少量6-MeO-BDE-47。结合沉积物样品中未检出MeO-PBDEs,推测象山海域MeO-PBDEs的直接来源不多,可能存在外源输入或生物转化。象山海域内海洋生物体样品中MeO-PBDEs的检出率达29.4%,且在所有检出的30份生物体样品中,有23份样品涉及10种生物均为经济价值较高且常见的海鲜消费品。因此,应持续关注象山海域MeO-PBDEs的污染状况。
4 结论与展望
海洋生物体中MeO-PBDEs浓度一般在μg∙kg-1水平,对样品分析提出了较高要求。采用气相色谱-负化学源质谱法,辅以固相萃取净化样品,建立了海洋环境中MeO-PBDEs的检测方法。通过前处理及优化,方法的基质干扰低、灵敏度较高、重现性好、回收率稳定,能够满足近岸海洋环境中MeO-PBDEs的检测要求。研究发现,象山海域海洋生物体中已普遍存在MeO-PBDEs,其中,6-MeOBDE-47、3-MeO-BDE-47和2apos;-MeO-BDE-68被检出,未来需进一步探讨MeO-PBDEs的富集机制及来源途径。
[1]WANG S, WANG S W,SHAH S H, et al. A density functional theory/time-dependent density functional theory study of the structure-related photochemical properties of hydroxylated polybrominated diphenyl ethers and methoxylated polybrominated diphenyl ethers and metal ion effects[J]. Environmental Science and Pollution Research, 2020,27(9): 9297-9306. DOI:10. 1007/s11356-019-07538-0
[2]HAGLUND P S, ZOOK D R,BUSER H-R, et al. Identification and quantification of polybrominated diphenyl ethers and methoxy-polybrominated diphenyl ethers in Baltic biota[J]. Environmental Science amp; Technology, 1997,31(11):3281-3287. DOI:10. 1021/es9702834
[3]ZHANG X L, CHENG X M,YU Y L, et al. Insight into the transplacental transport mechanism of methoxylated polybrominated diphenyl ethers using a BeWo cell monolayer model[J]. Environmental Pollution, 2020,265(A): 114836. DOI:10.1016/j.envpol.2020.114836
[4]DAHLGREN E, LINDQVIST D,DAHLGREN H, et al. Trophic transfer of naturally produced brominated aromatic compounds in a Baltic Sea food chain[J]. Chemosphere,2016, 144:1597-1604. DOI:10.1016/j.chemosphere.2015.10.024
[5]OCHIAI M, NOMIYAMA K,ISOBE T, et al. Polybrominated diphenyl ethers (PBDEs)and their hydroxylated and methoxylated analogues in the blood of harbor,Dallapos;s and finless porpoises from the Japanese coastal waters[J]. Marine Environmental Research, 2017,128: 124-132. DOI:10.1016/j.marenvres.2016.11.004
[6]张帆,余应新,张东平,等. 溴系阻燃剂在环境及人体中的存在和代谢转化[J]. 化学进展, 2009,21(6): 1364-1372.
ZHANG F, YU Y X,ZHANG D P, et al. Metabolism and transformation of brominated flame retardants existing in environment and human body[J]. Progress in Chemical, 2009, 21(6):1364-1372.
[7]CRUZ R, MENDES E,MAULVAULT A L, et al. Bioaccessibility of polybrominated diphenyl ethers and their methoxylated metabolites in cooked seafood after using a multi-compartment in vitro digestion model[J]. Chemosphere,2020, 252:126462. DOI:10.1016/j.chemosphere.2020.126462
[8]ERIKSSON P, FISCHER C,WALLIN M, et al. Impaired behaviour,learning and memory, in adult mice neonatally exposed to hexabromocyclododecane(HBCDD)[J]. Environmental Toxicology and Pharmacology, 2006, 21(3):317-322. DOI:10. 1016/j.etap.2005.10.001
[9]FERNIE K J, MAYNE G,SHUTT J L, et al. Evidence of immunomodulation in nestling American kestrels (Falco sparverius)exposed to environmentally relevant PBDEs[J]. Environmental Pollution, 2005,138(3): 485-493. DOI:10.1016/j.envpol.2005.04.008
[10]VARSHAVSKY J, SMITH A,WANG A, et al. Heightened susceptibility:A review of how pregnancy and chemical exposures influence maternal health[J]. Reproductive Toxicology, 2020, 92:14-56. DOI:10. 1016/j.reprotox.2019.04.004
[11]SUN J T, LIU J Y,LIU Y W, et al. Hydroxylated and methoxylated polybrominated diphenyl ethers in mollusks from Chinese coastal areas[J]. Chemosphere,2013, 92(3):322-328. DOI:10. 1016/j.chemosphere.2013.03.042
通过信息化系统,提供了项目考核与绩效测量的基准所需要的工作内容、工作量、完成时间、完成效果等关键要素的数据,有助于军工科研单位建立一套以贡献为导向、以执行率为参考、以效果为考核的评价体系,实现合理有效的评价机制,充分发挥人员工作积极性。
[12]LIU Y W, LIU J Y,YU M, et al. Hydroxylated and methoxylated polybrominated diphenyl ethers in a marine food web of Chinese Bohai Sea and their human dietary exposure[J]. Environmental Pollution, 2018,233: 604-611. DOI:10.1016/j.envpol.2017.10.105
[13]ZHANG K, WAN Y, AN L H, et al. Trophodynamics of polybrominated diphenyl ethers and methoxylated polybrominated diphenyl ethers in a marine food web[J]. Environmental Toxicology and Chemistry, 2010,29(12):2792-2799. DOI:10. 1002/etc.334
[14]ZHENG S C, WANG P,SUN H Z, et al. Tissue distribution and maternal transfer of persistent organic pollutants in Kentish Plovers (Charadrius alexandrines)from Cangzhou Wetland, Bohai Bay,China[J]. Science of the Total Environment,2018, 612:1105-1113. DOI:10.1016/j.scitotenv.2017. 08.323
[15]NORDLÖF U, HELANDER B,BIGNERT A, et al. Levels of brominated flame retardants and methoxylated polybrominated diphenyl ethers in eggs of white-tailed sea eagles breeding in different regions of Sweden[J]. Science of the Total Environment, 2010,409: 238-246. DOI:10.1016/j.scitotenv.2010.09.042
[16]WEIJS L, LOSADA S,DAS K, et al. Biomagnification of naturally-produced methoxylated polybrominated diphenyl ethers (MeO-PBDEs)in harbour seals and harbour porpoises from the Southern North Sea[J]. Environment International, 2009,35(6): 893-899. DOI:10.1016/j.envint.2009.03.006
[17]WEIJS L, COVACI A,STEVENSON G, et al. Concentrations of some legacy pollutants have increased in South Australian bottlenose dolphins from 1989 to 2014[J]. Environmental Research,2020, 189:109834. DOI:10.1016/j.envres.2020.109834
[18]SUN H Z, LI Y M,HAO Y F, et al. Bioaccumulation and trophic transfer of polybrominated diphenyl ethers and their hydroxylated and methoxylated analogues in polar marine food webs[J]. Environmental Science amp; Technology, 2020,54(23):15086-15096. DOI:10. 1021/acs.est.0c05427
[19]SCHULTZ I R, KUO L J,CULLINAN V, et al. Occupational and dietary differences in hydroxylated and methoxylated PBDEs and metals in plasma from Puget Sound, Washington,USA region volunteers[J]. Science of the Total Environment, 2020,714: 136566. DOI:10.1016/j.scitotenv.2020.136566
[20]CADE S E, KUO L J,SCHULTZ L R. Polybrominated diphenyl ethers and their hydroxylated and methoxylated derivatives in seafood obtained from Puget Sound,WA[J]. Science of the Total Environment,2018, 630:1149-1154. DOI:10.1016/j.scitotenv.2018.02.301
[21]BUTRYN D M, GROSS M S,CHI L H, et al. “One-shot”analysis of polybrominated diphenyl ethers and their hydroxylated and methoxylated analogs in human breast milk and serum using gas chromatography-tandem mass spectrometry[J]. Analytica Chimica Acta, 2015,892: 140-147. DOI:10.1016/j.aca.2015.08.026
[22]季洁云,冯超,卢大胜,等. 生物样品中多溴联苯醚及其代谢物检测方法的研究进展[J]. 环境与职业医学, 2018,35(3): 246-252. DOI:10.13213/j.cnki.jeom.2018.17543
JI J Y, FENG C,LU D S, et al. Research progress on detection methods for polybrominated diphenyl ethers and their metabolites in biological samples[J]. Journal of Environmental and Occupational Medicine, 2018,35 (3): 246-252. DOI:10.13213/j.cnki.jeom.2018.17543
[23]刘秀娟,周志明,王巧玲,等. MSPD-GC-ECD测定血液中甲氧基多溴联苯醚[J]. 分析试验室,2019,38(11):1268-1272. DOI:10.13595/j.cnki.issn1000-0720.2019.011901
LIU X J, ZHOU Z M,WANG Q L, et al. Determination of trace methoxylated polybrominated diphenyl ethers in blood by MSPD-GC-ECD[J]. Chinese Journal of Analysis Laboratory, 2019,38(11):1268-1272.DOI:10.13595/j.cnki.issn1000-0720.2019.011901
[24]叶茂盛. 水产品中甲氧基、羟基多溴联苯醚的高效质谱分析技术研究[D]. 舟山:浙江海洋大学, 2019.
YE M S. Study on High Performance Mass Spectrometry of Methoxy and Hydroxy Polybrominated Diphenyl Ethers in Aquatic Products[D]. Zhoushan: Zhejiang Ocean University,2019.
[25]都烨. 多溴联苯醚在不同区域沉积物中的分布特征[J]. 蚌埠学院学报, 2019,8(5): 26-32.
DU Y. Temporal trends of polybrominated diphenyl ethers in the sediment cores from different areas[J]. Journal of Bengbu University, 2019,8(5): 26-32.
[26]张付海,陈鑫,田丙正,等. 加速溶剂同时萃取和净化-气相色谱-三重四极杆串联质谱测定土壤和沉积物中8种多溴联苯醚[J]. 中国环境监测,2019, 35(4):141-148. DOI:10.19316/j.issn.1002-6002. 2019.04.17
ZHANG F H, CHEN X,TIAN B Z, et al. Determination of 8 PBDEs in soil and sediment by accelerated solvent simultaneous extraction and clean-up with gas chromatography-triple quadrupole mass spectrometry[J]. Environmental Monitoring in China, 2019,35(4): 141-148.DOI:10.19316/j.issn.1002-6002.2019.04.17
Determination of methoxypolybrominated diphenyl ethers in the coastal marine environment using solid-phase extraction and gas chromatography coupled with negative chemical ionization mass spectrometry
GAO Wen1,2, SUN Xiumei2, JIN Yanjian2, HAO Qing2, FU Yu1,2, YE Maosheng1,2, YANG Chenghu2, GUO Yuanming2
(1. Institute of Marine and Fisheries,Zhejiang Ocean University,Zhoushan316021,Zhejiang Province,China;2. Key Laboratory of Sustainable Utilization of Technology Research for Fishery Resource of Zhejiang Province,Zhejiang Marine Fisheries Research Institute,Zhoushan316021,Zhejiang Province,China)
Methoxypolybrominated diphenyl ethers (MeO-PBDEs) were widely present in marine organisms and the marine environment. An analytical method of six methoxypolybrominated diphenyl ethers for biological samples and sediment samples from Xiangshan Sea area using solid-phase extraction and gas chromatography coupled with negative chemical ionization mass spectrometry was developed and optimized. The developed method exhibited satisfying linearity in the range of 0.1-20.0 μg∙L-¹ (R2gt;0.999). The detection limit (LOD) and limit of quantitation (LOQ) for MeO-PBDEs were 0.13-0.22 μg∙kg-1and 0.42-0.72 μg∙kg-1respectively. The spiked recovery was 71.2%-116.2%. The method was applied to the analysis of marine organisms and sediments collected from Xiangshan sea area. All kinds of MeO-PBDEs were not detected in sediment samples. Only 6-MeO-BDE-47 was detected in algae samples with low concentration. Three MeO-PBDEs were detected in other biological samples, and the ratio of detection was 31.3% with a concentration range of 0.21-2.72 μg∙kg-1MeO-PBDEs were not detected in sediment samples.
methoxypolybrominated diphenyl ethers; gas chromatography-negative chemical ionization mass spectrometry; solid-phase extraction; marine environment
X 834
A
1008⁃9497(2022)03⁃344⁃10
10.3785/j.issn.1008-9497.2022.03.012
2021⁃01⁃21.
国家自然科学基金资助项目(21407127);浙江省科技厅项目(2018C37024,LGF22B070004).
郜文(1995—),ORCID:https://orcid.org/0000-0001-6354-8645,女,硕士研究生,主要从事水域环境管理与评估研究.
通信作者,ORCID:https://orcid.org/0000-0002-9547-6452,E-mail:sunxiumei82@sina.com.

