Effects of oxidants on the degradation of tributyl phosphate under supercritical water oxidation conditions
Qiang Qin· Xiao-Bin Xia · Shi-Bin Li · Shuai Wang · Hong-Jun Ma
Abstract The effects of additional oxidants, such as NaNO3, Na2S2O3, KClO4, and K2Cr2O7, on the supercritical water oxidation (SCWO) of tributyl phosphate (TBP)were studied.The coupling of an ionic oxidant with SCWO can effectively enhance the oxidative degradation ability of the system, thus increasing its organic-matter-removal efficiency at a reduced reaction temperature.Moreover,the addition of NaNO3,KClO4,or K2Cr2O7 could improve this efficiency at a reaction temperature of 500 °C compared with that of the original system at 550 °C. Additionally,based on the conditions adopted in this study, the addition of either of these oxidants could reduce the final total organic carbon (TOC) of the effluent from ~500 to <100 ppm. Concurrently, the ionic oxidants could effectively improve the processing capacity of the SCWO system to reduce the scale of the equipment, as well as the amount of produced wastewater. Compared with KClO4 and Na2S2O3, the addition of 10 mmol/L NaNO3 and K2Cr2O7 to the organic feed could increase the processing capacity of the system from 4 to 10%while maintaining the TOC removal at >99%. The effects of the ionic oxidants on the gas products, including CO2, CO, H2, and CH4, as well as other organic gases,have also been studied.Among these gas products,CO2 accounted for the main gas product with a proportion of more than half. At <500 °C, temperature significantly affected the as products (CO, H2,CH4, and other organic gases). However, the gas product was mainly CO2 when the temperature was increased to≥500 °C. This study initially revealed the enhancement effect of ionic oxidants on SCWO, which still requires further research.
Keywords Supercritical water oxidation · Ionic oxidant ·Organic wastes
1 Introduction
Supercritical water oxidation (SCWO) is the process of degrading an organic matter employing supercritical water(SCW) above the critical temperature (374.3 °C) and pressure (22.1 MPa). SCW exhibits significantly changed physicochemical properties, such as the number of hydrogen bonds, density, thermal conductivity, diffusivity, viscosity, dielectric constant, and ion product [1]. Further,SCW is completely miscible with n-alkanes (C2-C7) and gases,such as N2,CO2,H2,and O2[2].Furthermore,SCW can generate many highly reactive free radicals, including hydroxyl radicals(HO·),which are a strong oxidant with an oxidation-reduction potential (ORP) of 2.8 V and an oxidizing capacity of >502 kJ/mol,which is greater than CX bonds. In theory, SCW can achieve the complete degradation of all organic matters.Additionally,SCW-facilitated reactions are quite selective because of the high electronegativity of HO·. SCWO is a physicochemical process that can be controlled to satisfy the desired requirements;it can even degrade organic pollutants with concentrations of <10-9mol/L at a diffusion rate, K, of >109mol/(L·s). The combination of these properties makes SCW an ideal medium for oxidizing organics. It is also considered one of the most promising waste treatment technologies[3]. SCWO has been listed as the most promising waste treatment technology in ‘‘Energy and Environment’’, as well as one of the six major fields listed in the national key technologies in the United States.Further,a major attribute of SCWO is its ability to rapidly and completely oxidize a wide range of organic compounds in a reaction system while satisfying the concept of a ‘Totally Enclosed Treatment Facility’’ [4, 5].
Through in-depth development, the research on SCWO has expanded from the treatments of rocket propellants and explosive waste liquids in the national defense industry to conventional industries [6-8], cutting across the pesticide,medical, textile, and electronic industries. These studies have accumulated many research data and abundant equipment operation experience. In recent years, SCWO has transcended laboratory research to exhibiting technical applications.
However, several shortcomings, such as salt deposition and equipment corrosion during operation, account for the major obstacles to the industrial application of SCWO.On the one hand, the low solubility of inorganic salts during SCWO operation can cause salt deposition, which can subsequently inhibit heat transfer, as well as block the reactor, thereby affecting the safe operation of the system.On the other hand, the harsh operating conditions of SCWO,such as high temperature,high pressure,and strong oxidation, can severely corrode the equipment body. The development history of SCWO reveals that some SCWO industries have been forced to close because of the severe corrosion of component materials [9]. To alleviate the shortcomings of the SCWO process, researchers have conducted extensive studies on the associating salt deposition and corrosion challenges. Thus far, the addition of interference salts[10],changing of the operating conditions[11,12],and optimization of the reactor structure[13]have effectively alleviated salt deposition and its accompanying issues. Simultaneously, the selection of appropriate structural materials [14, 15], the development of new materials and technologies [16], and the optimization of the reactor design can mitigate the issues of equipment corrosion,thus attracting increased attention toward SCWO.
In recent years, related studies have been conducted on the treatment of radioactive waste [17]. Regarding the treatment and management of radioactive organic wastes,their chemical toxicities and radioactivities must be considered. These wastes generally exhibit complex components, which are flammable, volatile, and susceptible to radiation; moreover, the presence of organic matters in water, soil, etc., accelerates the migration of radionuclides therein. To date, most of such waste can only be stored temporarily. Research was recently conducted on the application of SCWO in the treatment of radioactive organic wastes. Our previous study focused on the application of SCWO in the radioactive spent extraction solvents that were produced via the THROX process, as well as from a Dasset detergent and lubricant VG32/VG46 of the Qinshan nuclear power plant [18-21]. Moreover,>90% removal of organic matter can be achieved within 1 min,and no harmful or radioactive products were detected in the exhaust gas. Furthermore, SCWO has exhibited a great application potential in the field of radioactive organic waste treatment.
However, many shortcomings must still be addressed before the large-scale application of SCWO can be achieved. For example, the SCWO process must induce harsh conditions (a high temperature and high pressure),which results in increased construction and operation requirements for the equipment. Particularly, the SCWO process severely increases the volume of effluent wastewater.Thus far,the highest reported proportion of pure organic matter in the SCWO system was 3-4%[22];it was limited by the oxidation capacity. Therefore, researchers have considered the oxidants of SCWO.Thus far,the Fenton reagent,K2S2O4, KMnO4, and NaNO3have been demonstrated as effective oxidants for SCWO; they can effectively increase the conversion rate of complex organics,shorten the reaction time, and reduce the required reaction temperature. The introduction of Fe2+into the SCWO system would allow Fe2+, H+, and hydrogen peroxide (H2O2) to form a new advanced oxidation environment within a certain period,i.e.,the supercritical Fenton oxidation (SCFO). This could synergistically play the roles of SCWO and Fenton oxidation,thus rapidly oxidizing and degradation of an organic matter at a reduced temperature and shortened times. Compared with SCWO,SCFO can increase the removal rate of the total organic carbon (TOC) from 75 to 90% under the following conditions: temperature = 420 °C, pH 3.0, and 0.3 mg/L Fe2+[23]. Kronholm [24] studied the effect of K2S2O4on the degradation of 4-chloro-3-methylphenol via SCWO and observed that it achieved >90% conversion within 16 s at 40 mmol/L and 390 °C. K2S2O4exhibited a higher oxidation ability than H2O2or O2; it effectively reduced the degradation temperature. Chang [25] reported that KMnO4produced oxygen under SCWO conditions and also promoted the oxidation process during the SCWO degradation of ethyl acetate; regarding the degradation of organic matters, it achieved a 77% degradation efficiency at 400 °C,which was better than those of H2O2and O2.Over the years,the Los Alamos National Laboratory persistently performed the oxidation of nitrates in hydrothermal systems to destroy high-explosive/propellant waste streams and Hanford storage tank waste,and the results revealed that NO3-exhibited good oxidation ability under an SCWO condition [26].The study of the nitrate-based SCWO decomposition of polychlorinated biphenyls (PCBs) [27] revealed that the decomposition efficiency could be effectively increased to>99.95% at 450 °C. The valency of the nitrogen atom exhibited continuous change from + 5 to + 3 to 0. The above studies demonstrated that the addition of extra oxidants to an SCWO system is a feasible strategy for effectively reducing the temperature required for the degradation reaction, as well as the reaction time.
To increase the oxidation capacity of SCWO, reduce wastewater discharge, and concurrently increase the degradation rate of complex organics,this study further shortened the reaction time and lower the required reaction temperature by elucidating the influence of additional oxidants on SCWO.Tributyl phosphate(TBP),which is considered very challenging to degrade in the spent extraction solvent (one of the largest known stocks of radioactive organic waste liquid), was utilized as the model compound. Further, the SCWO degradation of TBP was performed employing NaNO3, Na2S2O3, KClO4, and K2Cr2O7as additional ionic oxidants. Finally, the degradations of TBP under different conditions,as well as the influence of the additional oxidants on the SCWO system, were investigated.
2 Materials and methods
2.1 Apparatus and procedure
Figure 1 shows the SCWO system that was employed in this study [18]. The system was jointly designed by the Shanghai Institute of Applied Physics (China) and Supercritical Fluid Technologies (USA) and manufactured by Supercritical Fluid Technologies. This system mainly comprises a feeding system, preheater, reactor, heat exchanger, pressure control valve, and gas-liquid separator. Among them, the reactor is made of Inconel 625(volume = 200 mL), while the preheater is made of Inconel 625 (volume = 250 mL). The facility is designed to withstand a temperature and pressure of up to 600 °C and 28.4 MPa, respectively. The pressure of the entire system was controlled by a back-pressure valve, and CV was <0.03%. The preheater and reactor were heated by electrical heating wires that were coiled around its outer wall. The feedback controls of the temperature and pressure were achieved with a proportional integral derivative(PID) electric actuator. The feeding system includes two high-pressure metering injection pumps, which were employed to feed the water and organic phases a flow rate ranges of 1-100 and 0-36 mL/min, respectively. Additionally, an online pH probe was set before the gas-liquid separator (the front panels allow access to the pressure vessels, valves, fittings, and electronics). Moreover, two rupture disk assemblies offered mechanical protection against the overpressure of the system as an additional safety precaution (one was incorporated on the pipes between the water pump and preheater, and the other was placed between the cooler and back-pressure regulator).
Before each experiment, the temperatures and pressures of the preheater and reactor were increased to the desired values with deionized water. Thereafter, the fluid comprising H2O and H2O2was first introduced into thepreheater via Pump 1, after which the feed solution was directly introduced into the reactor via Pump 2. The effluent (exiting the top of the reactor) was cooled rapidly after passing through the heat exchanger (cooler) and was depressurized to ambient pressure through the back-pressure regulator. Afterward, the effluent was introduced into the gas-liquid separator. Next, the gas products were transported to a gas chromatograph for composition analysis. The liquid products were sampled with tubes, and each experiment was performed three times within 60 min after 20 min of stable running.
2.2 Materials and analytical methods
TBP(AR,98.5%),H2O2(AR,30%,W/W),NaNO3(AR,99%), Na2S2O3·5H2O (AR, 99%), KClO4(AR, 99.5%),and K2Cr2O7(AR, 99.8%) were purchased from Sinopharm Chemical Reagent Co., China. Deionized water(18.2 MΩ) was prepared employing a Milli-Q ultrapure water purification system with a 0.22 μm filter.
TOC of the liquid effluent was analyzed employing a TOC analyzer (Shimadzu TOC-L CSH, Japan). Three samples were measured, and the average was utilized to calculate the rate of TOC removal. Furthermore, the gas composition was determined by a gas chromatograph(Agilent GC 7890A, Agilent Technologies, Inc. USA),which was equipped with (1). a thermal conductivity detector (TCD) and a G3591-80,013 Q packed column(helium was employed as the carrier gas at a flow rate of 40 mL/min); (2). flame ionization detector (FID) and a G3591-80,013 Q packed column (N2was employed as the carrier gas at a flow rate of 40 mL/min).The column,TCD,and FID temperatures were maintained at 50, 250, and 300 °C, respectively.
The amount of feed added was calculated,following the IAPWS-IF97 guide (http://www.iapws.org/relguide/IF97-Rev.html).
2.3 Conditions
In this study,the feed comprised two phases,namely the water and organic phases. Among them, the water phase was H2O + H2O2in proportion,and the organic phase was the solution, which was prepared, as presented in Table 1.To minimize the influence of high temperature(preheating)on the oxidants and improve the reliability, NaNO3, Na2-S2O3, KClO4, and K2Cr2O7were first added to TBP and stirred continuously for 12 h under sealed conditions at room temperature (25 °C) until they were completely mixed. The composition of the feed is listed in Table 1.
Here, all the experiments were performed at a pressure of 25 MPa (3625 ± 50 psi) and a temperature range of 450-550 °C(± 1 °C).Before each experiment,the SCWO system was fully rinsed with deionized water to eliminate any possible interference.
2.4 Calculation
The residence time (t) is approximately the period from when the feed entered the reactor to when it left.It depends on the feed rate(Q),reaction temperature,and pressure and can be calculated employing Eq. (1):
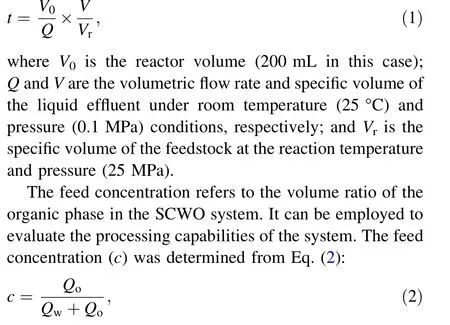
where Qoand Qware the volumetric flow rates of the organic and aqueous phases, respectively.
TOC was determined to evaluate the degradation efficiency of the organics in the SCWO system. The TOC of the liquid effluent was measured by a TOC analyzer, and the TOC removal of the liquid effluent was calculated via Eq. (3):


Table 1 Composition of the feeds
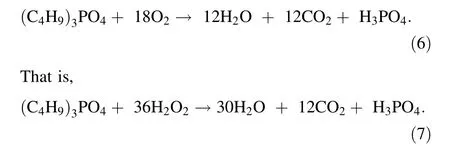
3 Results and discussion
3.1 TOC Removal employing SCWO
In SCWO, the reaction temperature is among the most critical factors that affect the degradation of organic matter.Based on our previous work[19], the degradation efficiencies of the organic matters were studied under the following conditions:a temperature range of 450-550 °C,4%(V/V) TBP, and a residence time of 45 s with/without additional oxidants.The injection volume of H2O2was the theoretical amount required for the complete degradation of TBP according to Eq. (4), and the results are presented in Table 2.
Figure 2 shows the degradation of TBP under different conditions.Figure 2a shows the TOC value of the effluent,and 2b shows the TOC removal of the influent. The figure shows that temperature plays a very crucial role in the degradation of organic matter. TOC of the effluent decreased significantly, TOC decreased rapidly from >7500 to <1500 ppm, and the TOC removal increased from <70% to >90% as the temperature increased,especially from 450 to 500 °C. Thermodynamically, the increase in the temperature would activate more molecules whose energy exceeds the apparent activation energy (Ea),thereby promoting the reactions and causing the further degradation of by-products. Regarding the kinetics, the increase in the temperature causes an increase in the apparent rate constant (Ka), following the Arrhenius equation, and this accelerates the degradation reaction[29,30].Additionally,temperature also causes a change in the density of the reaction medium, which consequently affects the degradation process of organic matters.
Most of the organic matters were degraded when the temperature reached ≥500 °C, and the effect of the temperature on TOC was no longer significant. However, the TOC of the effluent remained roughly stable. It is very likely that some substances, which cannot be readily degraded or exhibit very slow degradation kinetics, have accumulated in the system. Thus, such substances were analyzed via gas chromatography-mass spectrometry(GCMS).Meanwhile,it is possible that the addition of an extra oxidant exerted a certain effect on the removal of the organic matter. At 475 °C, the additional oxidant reduced TOC from ~5500 to ~3000 ppm. Put differently, the addition of NaNO3, Na2S2O3, KClO4, or K2Cr2O7enhanced the degradability of the SCWO system. Namely,under the same degradation effects, the addition of an oxidant can effectively lower the required temperature of the system, thus reducing the energy consumption and the negative impact of high temperature on the equipment.Further, at >500 °C, the addition of NaNO3, KClO4, or K2Cr2O7reduced TOC of the effluent from ~1200 to <500 ppm, obtaining increased degradation efficiency.

Table 2 TOC Removal under conditions of Residence time 45 s, 25 MPa, TBP 4%(V/V)

Fig. 2 Degradation of TBP under different oxidation conditions: a. TOC of the effluent with temperature b. TOC Removal of influent with temperature
3.2 Study of SCWO capacity
Regarding an SCWO system that is coupled with an ionic oxidant,the change in the capacity of the oxidant also requires elucidation, and no relevant study has been conducted to date. To further study the effect of additional oxidants on the SCWO system,the degradation behavior of SCWO at different TBP concentrations was studied under the following conditions: 550 °C, 45 s, and 25 MPa, and the results are presented in Table 3.
Figure 3 shows the degradation of TBP at different feed concentrations. The degradation of each system began to diverge as the TBP feed increased. TOC of the effluent clearly increased sharply from ~550 to ~9500 ppm,and the TOC removal decreased from ~98% to ~85%as the TBP concentration increased from 4 to 10%without an extra oxidant. When the TBP concentration increased to >10%, an insoluble organic matter appeared in the effluent. Compared with Feed 1, TOC decreased from >4000 to <1000 ppm owing to the addition of NaNO3,Na2S2O3, KClO4, or K2Cr2O7by increasing the TBP concentration to 6%, indicating that an additional oxidant could effectively improve the removal of the organic matter at this concentration. However, as the feed concentration increased to 8%, the effect of KClO4on the removal of the organic matter reduced,and TOC increasedto >5000 ppm. Meanwhile, the addition of NaNO3, Na2-S2O3,or K2Cr2O7could still maintain a low level for TOC of the effluent in this situation. When the feed concentration increased to 10%, the additions of NaNO3and K2Cr2O7maintained the TOC of the effluent at <500 ppm and that of Na2S2O3was ~1800 ppm. By increasing the feed grains to 12%,the addition of K2Cr2O7maintained the TOC of the effluent at <1500 ppm, which was ~3000 and 5500 ppm for Na2S2O3and NaNO3, respectively.Thus,it can be reasonably concluded that the addition of an oxidant could effectively increase the oxidation capacity of the original SCWO system.Further,compared with KClO4and Na2S2O3, NaNO3and K2Cr2O7exerted a better effect on SCWO at high feed concentrations (10%).
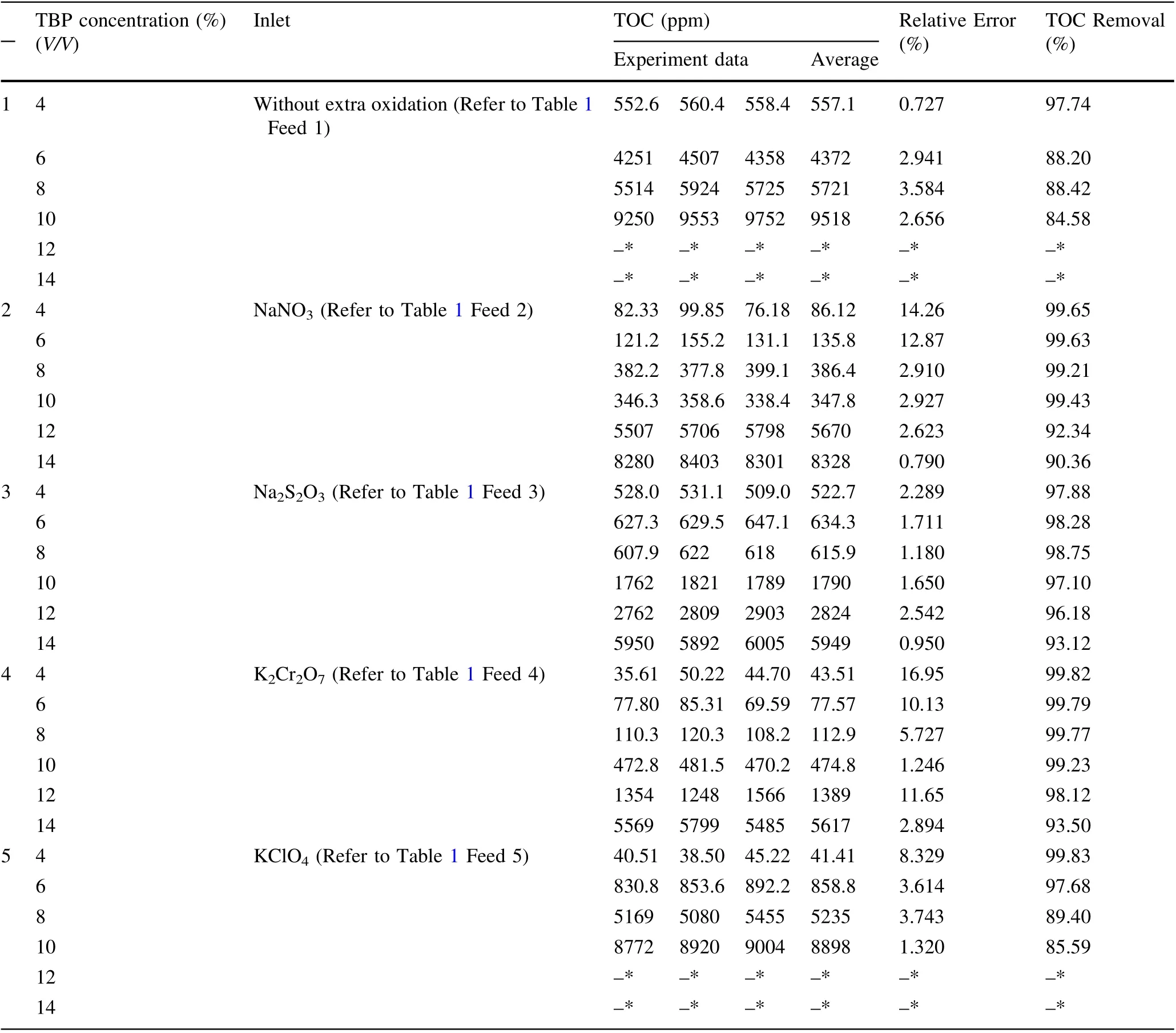
Table 3 TOC removal under the following conditions: residence time, 45 s; temperature, 550 ± 1 °C; pressure, 25 MPa

Fig. 3 TOC at different feed concentrations a TOC of the effluent with the feed concentration b TOC Removal of the influent with the feed concentration
It is inferrable that the addition of a small amount of an ionic oxidant might affect the SCWO system in the following aspects: (1) promote the generation of free radicals in the system, (2) interact with the free radicals in the system to extend their existence, (3) avail other oxidation fragments while participating in the reaction, (4) and increase the ion reaction share in the SCWO system. Thus far,there has been no direct evidence regarding the role of ionic oxidants in it; thus, further research is still required.
3.3 Analyses of the gas products
In the above experiments,the generated gas was directly introduced into the gas chromatograph for online detection.The gas products of each SCWO system are shown in Fig. 4 with the exclusion of O2.
Under the research conditions of this work (residence time = 45 s, pressure = 25 MPa, and the organic feed concentration = 4% (V/V)), the results demonstrated that the main gas products included CO2, CO, CH4, H2, and small amounts of other organic gases. The high temperature and strong oxidizing properties of SCWO availed a favorable environment for the production of these gases.The organic matter underwent different reactions in SCWO, including free-radical, pyrolysis, hydrolysis, and ionic reactions [31]. Water is a reaction medium, which accelerates reaction processes; simultaneously, it also participates in the reaction, supplying a certain amount of H·free radicals and producing a small amount of hydrogen[32]. Notably, a small part of TBP would be carbonized at the feed inlet,and the generated tar/jar/coke would produce CO and H2via the water-gas shift reaction at a high temperature. The reactions that significantly affect the gaseous products mainly include steam reforming, watergas shift, methanation, and minor hydrogenation reactions(see Eqs. (8)-(13))[33-35].


Fig. 4 Effects of the reaction temperatures on the gas products
Figure 4 reveals that the reaction temperature significantly affected the composition of the gas-phase products,especially at 500 °C. As the temperature increased from 450 to 500 °C, the CO2content of the product also increased significantly along with other incompletely oxidized gases, such as CO, CH4, H2, and the other organic gases, which gradually decreased. Namely, as the temperature increased, these non-CO2gases (incompletely oxidized gases) were further oxidized. When the temperature reached 500 °C,CO2was the main product in the gas,and its content reached >99%; the remaining component of the gas was CO, which was <1%. In addition to CO2,most of the remaining gaseous products were CO. At 450 °C, the proportion exceeded 30%. Similarly, as the proportion decreased rapidly, the temperature increased.When the temperature increased to 500 °C, the proportion of CO decreased sharply to <1% probably because of the following two reasons: (1). at high temperatures, organic matters tend to be completely oxidized and converted into CO2rather than other incomplete gas products; 2. CO was further oxidized into CO2, thereby reducing the CO content.Compared with CO2and CO,the contents of CH4,H2,and other organic gases were relatively low under all the conditions employed in this study.The proportions of CH4,H2,and the total amount of the other gases were <1,<5,and <2%.As the temperature changed,their changes were largely the same, i.e., they decreased with the increasing temperature until they became undetectable. Notably, relative to the temperature dependence of the production of the other gases, that of CH4production did not exhibit much dependence on the temperature probably because the temperature was related to the low content of CH4and the generation process of CH4, as well as the subsequent oxidation process.
The additions of the oxidants(NaNO3,Na2S2O3,KClO4,and K2Cr2O7) to the SCWO system also exerted a certain effect on the gas products.At <500 °C,the addition of the oxidants increased the non-CO2components significantly.Compared with the TOC in the liquid effluent (previous chapter), the SCWO system increased the degradation rate of the organic matters via the addition of the oxidants.Therefore, more gases were produced under the same conditions; these gas products were not further oxidized,and this increased their proportion. When the temperature increased to ≥500 °C, the proportion of CO2increased rapidly to >90%, and those of the other gases decrease sharply until they were below the detection limit, indicating that temperature significantly affected the gas product at ≥500 °C under the conditions of this study.
Figure 5 shows the effects of the feed concentration on the gas products.This part of the study was conducted at a reaction temperature of 550 °C,the residence time of 45 s,and pressure of 25 MPa. As observed, CO2was still the main gas product, and its proportion was >50%. As expected, the non-CO2components increased with the increasing feed concentration. During the degradation process, an increasing number of non-CO2gaseous products were generated as the organic matters in the SCWO system increased.Figure 5 shows that the proportion of CO increased rapidly and the TBP feed in the system increased.The highest proportion of CO was >38.25%. However,the proportion of CH4also increased slightly. The CH4concentration was <2%.In the SCWO process,the carbon in the organic matter was more likely to be converted into CO2and CO than into CH4. Moreover,increasing the feed concentration benefitted the production of CO. Under the experimental conditions employed in this study,the highest proportions of CH4, H2, and the other organic gases were 1.99, 5.26, and 6.54%, respectively.
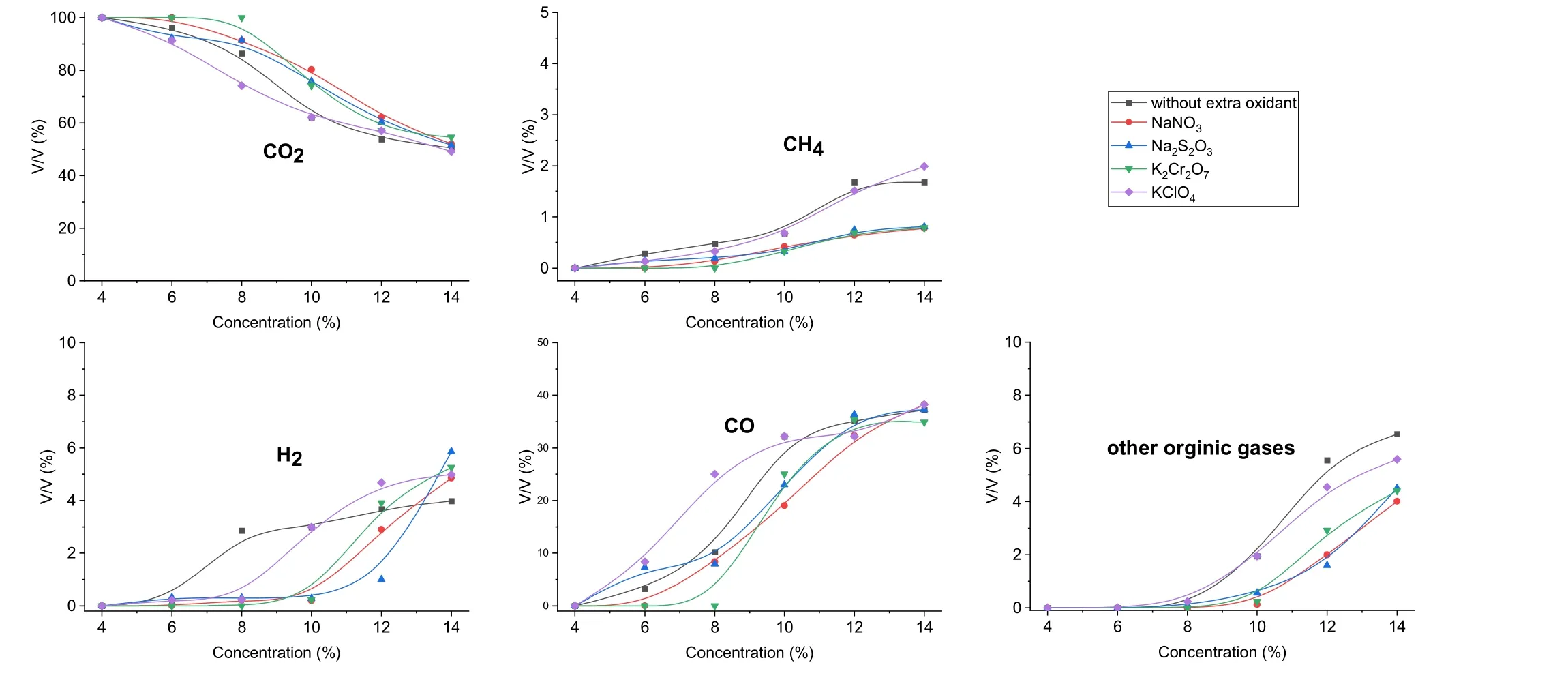
Fig. 5 Effects of the feed concentration on the gas products
Notably, the addition of ionic oxidants, especially KClO4, exerted an inhibiting effect on the gas products,although its impact on the gas products was almost negligible. As the concentrations of the organic matter in the system increased, their influence on the gas products became increasingly negligible. Meanwhile, the additions of NaNO3, Na2S2O3, and K2Cr2O7exerted a certain inhibitory effect on the productions of CH4and H2. Particularly, when the concentration of organic matter in the system was <10%, the additions of the oxidants would effectively reduce the productions of H2and CH4.
4 Conclusion
This work preliminarily revealed the degradation behaviors of organic compounds in an SCWO system,which was coupled with ionic oxidants.The degradation of TBP in SCWO that was coupled with additional oxidants(NaNO3, Na2S2O3, KClO4, and K2Cr2O7) was studied.
(1) The addition of an ionic oxidant could effectively improve the removal of organic matters. Under the conditions employed here, NaNO3, KClO4, and K2Cr2O7exerted higher degradation effects on SCWO than Na2S2O3.
(2) The addition of NaNO3, KClO4, K2Cr2O7, or Na2S2O3could effectively reduce the TOC content of the effluent. Further, NaNO3, KClO4, and K2Cr2O7could reduce TOC from ~550 to <100 ppm at 550 °C and a feed concentration of 4%(V/V).
(3) The addition of ionic oxidants could effectively increase the processing capacity of the SCWO system. Compared with KClO4and Na2S2O3, the additions of 10 mmol/L NaNO3and K2Cr2O7maintained the TOC removal of >99% after the feed concentration of the organic matter was increased from 4 to 10%.
(4) During the SCWO degradation of TBP, CO2and CO accounted for the most significant gas components. At <500 °C, the addition of the ionic oxidants to the SCWO system promoted the conversion of the organic matter into gas-phase products in the system, thus producing additional non-CO2components.
Overall, this paper reports a preliminary study on the performance of ionic oxidants in SCWO, and the results demonstrated that the addition of a very small amount of an oxidant could effectively enhance the oxidation capacity of SCWO. Additionally, the introduction of heteroatoms into the system might result in corrosion issues and introduce harmful inorganic chemicals into the effluent. Therefore,the disadvantages that might be caused by the addition of ionic oxidants must also be studied. Thus, in-depth research on how these oxidants function and how much the reaction accounts for is worthwhile.
Author contributionsAll authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Qiang Qin, Xiao-Bin Xia, Shi-Bin Li, Shuai Wang,and Hong-Jun Ma.The first draft of the manuscript was written by Qiang Qin,and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
 Nuclear Science and Techniques2022年3期
Nuclear Science and Techniques2022年3期
- Nuclear Science and Techniques的其它文章
- Design and tests of the prototype a beam monitor of the CSR external target experiment
- Development of a wide-range and fast-response digitizing pulse signal acquisition and processing system for neutron flux monitoring on EAST
- On the viability of wearing evaluation by Thin Layer Activation in the presence of non-occupationally exposed individuals
- Enhancement in optical absorption of CsI(Na)
- Research on tune feedback of the Hefei Light Source II based on machine learning
- Development of a subchannel code for blockage accidents of LMFRs based on the 3D fuel rod model
