Interrogating the interplay of angiogenesis and immunity in metastatic colorectal cancer
Katerina Kampoli, Periklis G Foukas, Anastasios Ntavatzikos, Nikolaos Arkadopoulos, Anna Koumarianou
Katerina Kampoli, Anastasios Ntavatzikos, Anna Koumarianou, Hematology Oncology Unit, The Fourth Department of Internal Medicine, School of Medicine, National and Kapodistrian University of Athens, Attikon University Hospital, Haidari 12462, Athens, Greece
Periklis G Foukas, The Second Department of Pathology, School of Medicine, National and Kapodistrian University of Athens, Attikon University Hospital, Haidari 12462, Athens, Greece
Nikolaos Arkadopoulos, The Fourth Surgical Clinic, School of Medicine, National and Kapodistrian University of Athens, Attikon University Hospital, Haidari 12462, Athens, Greece
Abstract Colon cancer is the third most common malignancy and the fifth most frequent cause of death from neoplastic disease worldwide. At the time of diagnosis, more than 20% of patients already have metastatic disease. In the last 20 years, the natural course of the disease has changed due to major changes in the management of metastatic disease such as the advent of novel surgical and local therapy approaches as well as the introduction of novel chemotherapy drugs and targeted agents such as anti-epidermal growth factor receptor, anti-BRAF and antiangiogenics. Angiogenesis is a complex biological process of new vessel formation from existing ones and is an integral component of tumor progression supporting cancer cells to grow, proliferate and metastasize. Many molecules are involved in this proangiogenic process, such as vascular endothelial growth factor and its receptors on endothelial cells. A well-standardized methodology that is applied to assess angiogenesis in the tumor microenvironment is microvascular density by using immunohistochemistry with antibodies against endothelial CD31, CD34 and CD105 antigens. Even smaller molecules, such as the micro-RNAs, which are small non-coding RNAs, are being studied for their usefulness as surrogate biomarkers of angiogenesis and prognosis. In this review, we will discuss recent advances regarding the investigation of angiogenesis, the crosstalk between elements of the immune microenvironment and angiogenesis and how a disorganized tumor vessel network affects the trafficking of CD8+ T cells in the tumor bed. Furthermore, we will present recent data from clinical trials that combine antiangiogenic therapies with immune checkpoint inhibitors in colorectal cancer.
Key Words: Vascular endothelial growth factor; Circulating tumor cells; Colorectal cancer;MicroRNAs; Microvascular density; Crosstalk; Angiogenesis; Immunity
INTRODUCTION
Colorectal cancer (CRC), the third most common cancer in both genders, accounts for 9% of new cancer diagnoses in men and 8% in women and is the third leading cause of cancer death in both sexes. Although it is more common in those over the age of 70, a significant proportion of patients are of middle age. The 5-year survival rate of patients with localized disease CRC is 90%. However, this rate is significantly lower in patients with metastatic disease, reaching 14% and 15% in those with colon and rectal cancer, respectively[1].
Due to the poor prognosis of metastatic CRC (mCRC), the need for novel therapeutic approaches in these patients is urgently needed. A step towards this direction was made possible by the introduction of antiangiogenetic agents, but there are several unmet needs to better define patient profiles benefiting from such an approach.Moreover, despite this and other new treatments, the prognosis of patients with mCRC is still poor, and research is focusing on biomarkers with predictive and prognostic value (Table 1). Despite extensive research, in everyday practice only mutations inRAS,BRAF,NTRKandHER2genes as well as the level of microsatellite instability have found application in the targeted therapy of mCRC[2,3]. As the complex process of carcinogenesis and metastasis is continuously defined, this knowledge is expected to lead to the discovery of new therapies.
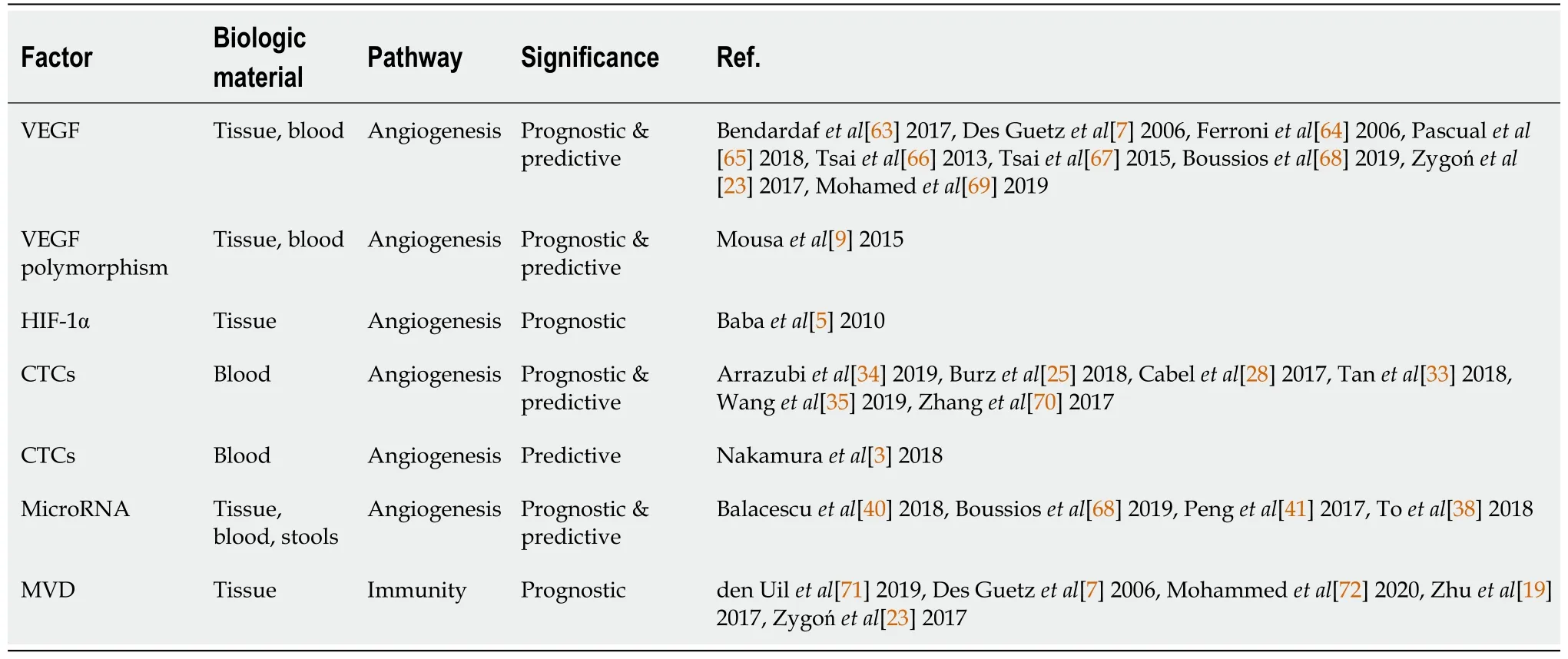
Table 1 Factors related to angiogenesis and immunity and studied as biomarkers in colorectal cancer
In this review, we will discuss recent advances in CRC regarding the investigation of angiogenesis, the crosstalk between the immune microenvironment and angiogenesis and the ways through which cancer cells escape the host immune system.Furthermore, we will present recent data from clinical trials that combine antiangiogenic agents with immune checkpoint inhibitors.
ANGIOGENESIS
Vascular endothelial growth factor
Angiogenesis is a complex mechanism of new vessel production that the cancer cell uses to ensure the supply of oxygen and nutrients and thus to multiply and generate evolving solid tumors with distant metastases[4].
There are two main regulators of angiogenesis that are essential for the development of CRC, hypoxia factor-1α and vascular endothelial growth factor (VEGF).Hypoxia factor-1α is a proangiogenic factor and is found in the tumor microenvironment. It is secreted by the cancer cell under hypoxic conditions and affects a widevariety of signaling pathways, including the upregulation of the VEGF cascade[4-6].VEGF has several important functions, the most important one being the increase of vascular permeability and the induction of new blood vessels through its binding to endothelial cells and by promoting their proliferation[7,8].
VEGF comprises a group of glycoproteins that, together with placental growth factor, interact with three VEGF receptors (VEGFR1, VEGFR2, VEGFR3) and two neuropilin co-receptors (NRP1, NRP2). VEGFRs are tyrosine kinase receptors found in endothelial vascular cells. The binding of the glycoprotein to its receptor results in the initiation of a sequence of events that ultimately result in the formation of new vessels[3].The ligation of VEGF-A with VEGFR-2 is the most important step in the activation of angiogenesis in CRC[9].
Bevacizumab is a monoclonal antibody targeting VEGF-A and the first antiangiogenic agent to be used against metastatic cancer. Bevacizumab was approved in 2004 in the United States and in 2005 in Europe for use in patients with mCRC. Its mechanism of action is mediated through the inhibition of the interaction of VEGF-A with VEGFR, and thus bevacizumab inhibits the signaling pathway that promotes neovascularization[10]. Finding biomarkers that could predict the response to antiangiogenic therapy so that it could be used only in patients who would benefit from its administration is a currently unmet need.
Due to the dominant role of VEGF in angiogenesis, researchers investigated whether the expression of this factor could be a predictive biomarker for patients receiving antiangiogenic therapy. One study indicated that high VEGF baseline levels associated with worse response to bevacizumab treatment and progression-free survival[11]. In 2013 Hegdeet al[12] showed that there is no statistically significant relationship between plasma VEGF-A levels and the clinical response to bevacizumab.Therefore, it has no predictive value in metastatic colon cancer. Another exploratory analysis investigating epithelial and stromal VEGF expression, assessed by in situ hybridization and immunohistochemistry on tissue microarrays and whole tumor tissue sections, suggested that in patients with mCRC the addition of bevacizumab to chemotherapy improves survival regardless of the level of VEGF expression[13].Mavericc was the first prospective mCRC study using gene expression data from blood (plasma VEGF-A protein levels) to evaluate the efficacy of mCRC chemotherapy regimens indicating that high plasma VEGF levels were associated with shorter treatment duration of response and progression-free survival[14]. More interesting,VEGF polymorphisms have also been studied, and it appears that they could possibly be used as predictive agents in mCRC in patients treated with irinotecan and bevacizumab[15]. In another study, VEGF-A (c.*237C>T) was associated with a significantly better time to treatment failure[16]. Another study investigating the predictive role of VEGF-A indicated a significant association of rs833061 single nucleotide polymorphism with the overall response rate in advanced CRC patients treated with cytotoxic chemotherapy plus bevacizumab[17].
Microvascular density
An important indicator used in translational studies to assess the degree of neovascularization of the tumor is the microvascular density (MVD). MVD appears to increase as it progresses from normal mucosa to adenoma and from adenoma to cancer, and this is explained by the intense angiogenesis that aims to meet the neoplastic cells need for oxygen[18]. MVD was found to be higher in primary tumors than in metastases[5,18], while its levels within the tumor were associated with an increased risk of distant metastases[19]. The assessment of MVD includes pan-endothelial cell markers, also expressed in normal tissues, such as CD31 and CD34, as well as endothelial markers expressed on the surface of proliferating endothelial cells, such as CD105[18,20].Endoglin is expressed mainly in vascular endothelial cells during active angiogenesis,while it is only weakly expressed or absent in pre-existing vascular endothelial cells,making this marker an important indicator of neoangiogenesis[18].
A systematic review and meta-analysis have indicated that increased VEGF and MVD expression markers are associated with an increased incidence of metastasis in CRC patients treated with surgery and chemotherapy[21]. An attempt was also made to correlate MVD with clinicopathologic features, such as sex, age, location, grade of differentiation, infiltrated lymph nodes and distant metastases, but with contradictory results. A negative correlation was found in two studies that investigated MVD in relation to the above variables[22,23], but in two other studies MVD staining was positively associated with tumor invasion, lymph node metastases[18] and distant metastases[19].
Since MVD is a biomarker for the quantification of angiogenesis, the question arises whether it can be used as a predictor of the treatment outcome with the antiangiogenic agent bevacizumab.
In 2006, Jubbet al[13], reported a clinical study of 813 patients with mCRC and found no association between elevated MVD or VEGF expression and the clinical outcome in relation to bevacizumab treatment. Although the predictive value of MVD in relation to bevacizumab response has been recognized in other cancers such as advanced ovarian cancer[24], in mCRC this has not yet been demonstrated.
CIRCULATING TUMOR CELLS
It has been postulated that cancer cells circulate in the peripheral blood of patients with metastatic disease[25,26]. It is reasonable to expect that the isolation and study of these cells can provide information about the metastatic potential of primary disease and an assessment of their value as prognostic and predictive biomarkers[25].
The mechanism by which cancer cells enter the circulation and acquire the ability to metastasize is not fully understood. However, this process appears to be activated by tumor hypoxia, which also activates angiogenesis[27].
It has been estimated that the frequency of circulating tumor cells (CTC) is about 1 per 1 mL of peripheral blood[28] or otherwise 1 g of tumor releases 106cells into the bloodstream[25]. Despite the large number of cells released into the bloodstream daily,a small number can be detected and isolated. This is partly due to the fact that these cells are covered by platelets and coagulation factors[29]. However, with the advent of new methods, it is now more feasible to isolate circulating cancer cells and study them[30]. Liquid biopsy, the isolation of CTCs or tumor cell-free DNA from peripheral blood is only minimally invasive compared to tumor biopsy and can be repeated many times for the monitoring of genomic changes that contribute to cancer progression and/or resistance to chemotherapy[31].
Although CTCs have been isolated in the blood of patients with polyps of the colon,the number of CTCs measured in the blood of patients with colon cancer is statistically significantly higher[28]. Furthermore, a smaller number of CTCs is detected in welldifferentiated tumors compared to the less differentiated counterparts. The number of CTCs does not seem to be related to the tumoral histologic subtype, whereas it seems to be related to the anatomical location, being higher in cancer of the rectum and sigmoid colon compared to other sites[32]. Circulating cancer cells is an independent prognostic factor for the survival of patients with CRC[33]. In patients with mCRC and liver secondaries treated with complete resection of the primary tumor site and liver metastases, the presence of two or more CTCs/7.5 mL of blood preoperatively was an indicator of poor disease outcome and low survival[34]. Furthermore, according to another recent study, the CTC-positivity rate was an independent predictive factor of progression-free survival and overall survival in patients with advanced disease treated with chemotherapy. In addition, the CTC concentration was related to the pathological stage of the disease, the presence of metastatic disease, the depth of tumor invasion, the presence of lymphatic invasion and high serum carcinoembryonic antigen levels[35].
MicroRNAs
In recent years microRNAs, have been studied as biomarkers for diagnosis, prognosis and treatment resistance in patients with CRC. MicroRNAs are small non-coding molecules consisting of 18 to 25 nucleotides that control the expression of many target genes, either by inhibiting their expression or by stimulating it. Thus, by affecting the expression of oncogenes it is possible to either inhibit or promote oncogenesis[36].These molecules can be detected not only in tissues but also in the serum and feces of cancer patients. They are found extracellularly either as a result of cancer cell death or due to extracellular secretion by cancer cells[37]. MicroRNAs target the 3’ untranslated region of target genes, thereby degrading and controlling their expression[36].MicroRNA interaction with target genes and their mRNA is affected by single nucleotide polymorphisms in the 3’ untranslated region of these target genes, which also affect their expression. These polymorphisms have been studied to predict treatment outcomes, such as resistance to chemotherapy[38].
MicroRNAs are extremely stable molecules because they are stored in extracellular structures or bound to lipoproteins[38]. This feature and the fact that they do not require invasive methods for their detection make them potential ideal diagnostic and prognostic biomarkers.
The association of microRNAs with CRC was first described by Michaelet al[39] in 2003. In this study, the authors showed that microRNA-143 and microRNA-145 levels were reduced in precancerous adenomatous lesions and CRC compared with normal mucosa. Since then, several research studies and meta-analyses have been published,emphasizing the importance of microRNAs in cancer[40].
In addition to oncogenesis, there are microRNAs that target regulatory molecules that lead to angiogenesis. These molecules, known as “angiomiRs,” either promote or suppress angiogenesis, thereby indirectly affecting tumor formation and metastasis.
MicroRNA-21 is the most representative of neoangiogenesis as it has been studied in many types of cancer and by several researchers. In a meta-analysis published in 2017, Penget al[41] analyzed data from 57 studies and concluded that microRNA-21 has a diagnostic sensitivity of 64% and a specificity of 85%, making it a potential prognostic indicator for patient survival. According to this study, peripheral blood microRNA-21 levels can be used as an indicator of CRC detection, and tissue levels can be an indicator to predict patient survival.
In addition to microRNA-21, there are many other microRNAs that target regulatory molecules leading to angiogenesis. Such molecules are microRNA-126,microRNA-30, microRNA-182, microRNA-194, microRNA-23b, microRNA-27a,microRNA-27b, microRNA-29b, microRNA-143, microRNA-145 and the complexes microRNA17-92, microRNA15a/16-1, microRNA-885-3p and microRNA885-3p[42].
MicroRNAs in the stool are the least studied but have been proven stable enough to correlate with the stage of the disease and have a high sensitivity and specificity in distinguishing patients from healthy individuals[38].
Long non-coding RNAs are made up of about 200 nucleotides and have also been studied as prognostic biomarkers. Although not translated into proteins, they act competitively by binding to common microRNA binding sequences and trapping them to alter the expression of their target genes. Available data suggest that long noncoding RNAs play a role not only in CRC development but also in metastasis[43].
THE CROSSTALK BETWEEN ANGIOGENESIS AND IMMUNITY
Tumor development and progression are highly dependent on the vascular network that penetrates the tumor bed and supplies proliferating malignant cells with oxygen and nutrients[44]. Although several mechanisms contribute to the constant development of the new vascular network,i.e.,neoangiogenesis, most new vessels are considered to be formed by the sprouting from parental ones[45]. The process of neoangiogenesis is triggered by hypoxia and deprivation of nutrients and is regulated by many proangiogenic and antiangiogenic factors such as VEGF-A, fibroblast growth factor, platelet-derived growth factor, transforming growth factor and others[45-47].Compared to normal tissue vasculature, tumor neoangiogenesis is characterized by abnormalities in structure and function, driven by the imbalance between proangiogenic, mainly VEGF, and antiangiogenic factors in the tumor microenvironment[48]. The abnormal structure and function of the tumor vasculature significantly affect the anti-tumor immunity, facilitating immune evasion in many different aspects(Figure 1). Overexpression of VEGF, produced by tumor cells, platelets and inflammatory cells such as neutrophils and monocytes, promotes the formation of an immature vascular network with increased leakiness, which in combination with the increased physical compression in the tumor bed leads to impaired blood perfusion and reduction of delivering oxygen and cytotoxic T cells in the tumor area[8,49].Moreover, hypoxia/acidosis induced growth factors and cytokines such as transforming growth factor-β and VEGF suppress the activity of cytotoxic T cells,suppress the antigen presenting capacity of dendritic cells, reprogram macrophages into a protumorigenic phenotype and upregulate the expression of programmed cell death-ligand 1 by tumor cells, myeloid-derived suppressor cells and dendritic cells and macrophages, further increasing immune evasion in the tumor microenvironment[8,50-52]. Of note, hypoxia-induced chemokines such as C-C motif chemokine ligand 2,C-C motif chemokine ligand 22, C-C motif chemokine ligand 28, C-X-C motif chemokine ligand 8 and C-X-C motif chemokine ligand 12 recruit immunosuppressive cells in the tumor microenvironment such as myeloid-derived suppressor cells,regulatory T cells and M2 macrophages[53] (Figure 1). In addition, tumor endothelial cells, in contrast to normal vasculature, express FasL and acquire the ability to kill effector CD8+ T cells but not regulatory T cells[54,55].
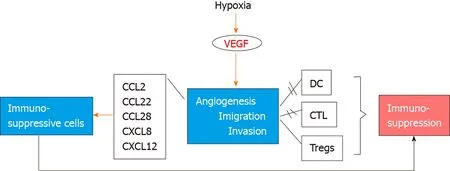
Figure 1 Τhe sequence of events following hypoxia and vascular endothelial growth factor secretion leading to immune system escape and carcinogenesis. VEGF: Vascular endothelial growth factor; CCL: C-C motif chemokine ligand; CXCL12: C-X-C motif chemokine ligand 12; DC: Dendritic cells; CTL: Cytotoxic T lymphocytes; Tregs: Regulatory T cells.
Immunotherapy is now a key therapeutic weapon in the treatment of many cancers,such as melanoma, lung and urothelial cancer and has significantly improved patients’prognosis. Immunotherapies target immune checkpoints that are abnormally expressed in many patients and aim to kill the tumor indirectly by boosting the antitumor immune responses. Cytotoxic T-lymphocyte-associated protein 4 and programmed cell death protein 1 with its ligand programmed cell death-ligand 1 are primarily involved in inhibitory immune signaling and are essential regulators of cancer immune evasion. Current clinical practice includes mainly two types of immune checkpoint inhibitors such as anti-cytotoxic T-lymphocyte-associated protein 4 (ipilimumab and tremelimumab) and anti-programmed cell death protein 1/programmed cell death-ligand 1 (nivolumab, atezolizumab, pembrolizumab)monoclonal antibodies[56]. However, in CRC these therapies have not proved to mediate similar effects, except in tumors with microsatellite instability[57].
As the immunosuppressive tumor microenvironment is additionally induced in part by the dysfunctional vascular network, a window for therapeutic application opens for the combination of immunotherapies and antiangiogenics. This strategy has been exploited in several clinical trials for different tumor types[51], such as non-small cell lung cancer (atezolizumab and bevacizumab)[58], renal cell carcinoma (axitinib and pembrolizumab or cabozantinib and nivolumab)[59,60], endometrial cancer (lenvatinib and pembrolizumab)[61] and hepatocellular carcinoma (atezolizumab and bevacizumab)[62].
Regarding CRC, ongoing clinical studies (Table 2) are investigating the effectiveness of combinations of antiangiogenic agents and immune checkpoint inhibitors. It is possible that such combinations could be applied in the future treatment of mCRC.
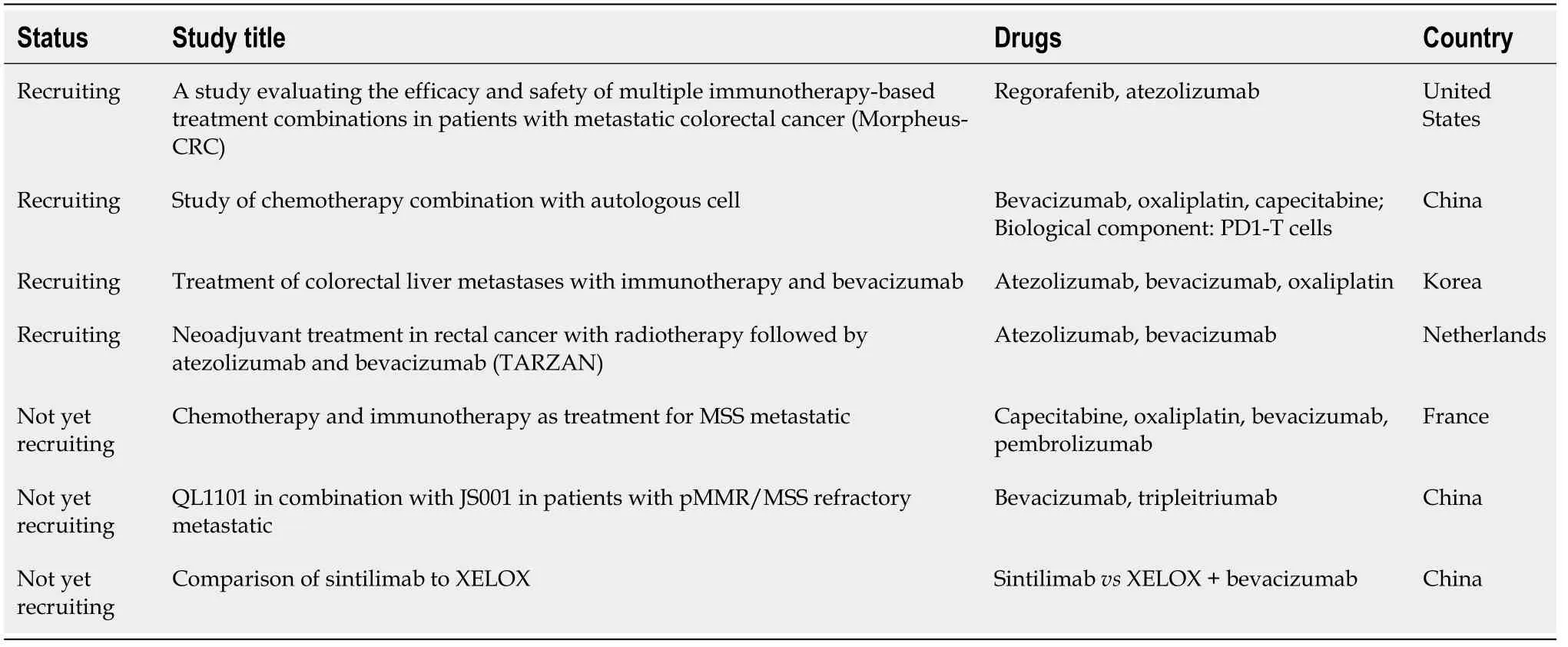
Table 2 Clinical trials related to antiangiogenic agent therapy and immunotherapy in colorectal cancer
CONCLUSION
Due to the poor prognosis of patients with mCRC, research has focused not only on finding prognostic and predictive factors but also on new therapeutic combinations.Immunohistochemistry methods have been instrumental in finding molecules that could be used as predictors, but molecular biology and immunology have been most informative in dissecting the mechanisms by which the cancer cell survives and spreads. Understanding how the immune and vascular microenvironments interact has opened new horizons in cancer treatment. Although such combination therapies for CRC have not yet been approved, the results of clinical trials are eagerly awaited.
Finding new molecular targets for different approaches including immunotherapy may enrich treatment options for CRC in the future.
ACKNOWLEDGEMENTS
Part of the expenses and materials for this narrative review were provided by the Hellenic Society of Medical Oncology and the Hellenic Study Group of Psychoneuroimmunology in Cancer. The authors express their gratitude to Professor Dimitrios T Boumpas for expert revision of the manuscript.
 World Journal of Methodology2022年1期
World Journal of Methodology2022年1期
- World Journal of Methodology的其它文章
- Ophthalmological instruments of Al-Halabi fill in a gap in the biomedical engineering history
- Severe acute respiratory syndrome coronavirus 2 pandemic related morbidity and mortality in patients with pediatric surgical diseases:A concerning challenge
- Liver transplant allocation policies and outcomes in United States: A comprehensive review
- Phenomenology of obsessive-compulsive disorder in children and adolescents: Sample from a tertiary care center in Istanbul, Turkey
