Severe thrombocytopenia and jaundice associated with Lemierre’s syndrome: A case report
Jian-min Ling, Zhao-hua Wang, Li Yan
Emergency and Intensive Care Unit, Tongji Hospital Affiliated to Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, China
Lemierre’s syndrome was f irst described in detail by André Lemierre in 1936 as infectious thrombophlebitis of the internal jugular vein (IJV).In the current case, the pathogen was confirmed by next-generation sequencing (NGS). After proper use of antibiotics, the patient recovered and was discharged.
A 19-year-old male patient was transferred from a local hospital to our emergency department because of an exacerbated sore throat, fever, and jaundice,although he had been diagnosed with upper respiratory infection and was injected with cefoperazone for 5 days.The patient also complained of pain in the left chest,particularly during coughing. He reported a history of recurrent tonsillitis, rhinorrhagia, smoking, and alcohol consumption for more than 2 years. On examination, he was febrile at 39.3 °C; his blood pressure was 127/82 mmHg (1 mmHg=0.133 kPa), heart rate was 98 beats/minute, and oxygen saturation was 94%; and the color of his skin and sclera was noticeably xanthochromic.In addition, we found symmetric hypertrophy of the bilateral tonsils that were painful on palpation but without exudate.
Thoraco-abdominal computed tomography (CT)revealed bilateral multiple pulmonary nodules of varying size, cavitary lesions of the left lung, and left pleural effusion (Figure 1A). Approximately 3 hours after arriving at our emergency room, the patient developed sudden spontaneous nasal hemorrhage of approximately 600 mL.Accordingly, his heart rate increased to 128 beats/minute,and hemoglobin decreased to 103 g/L from 132 g/L. After nasal cavity packing by an otolaryngologist, the patient was diagnosed with severe sepsis and admitted to the intensive care unit (ICU).
Immediately after admission, blood and bone marrow cultures were performed before the administration of antibiotics, prompt and adequate resuscitation, administration of broad-spectrum antibiotics (carbapenems), monitoring of changes in microcirculation, platelet transfusion, puncture and drainage of left pleural effusion, bone marrow biopsy,and blood and bone marrow for NGS. The laboratory test results are shown in Table 1. NGS demonstrated that the infected microorganism was Fusobacterium necrophorum both in blood ( Figure 1B) and in bone marrow. Ultrasound indicated that the left IJV was filled with thrombus (Figure 1C), but was recanalized 7 days later (Figure 1D). Bone marrow biopsy revealed active hyperplasia, particularly in the granulocyte and megakaryocytic series (Figure 1F). On day 3 after admission, we de-escalated the carbapenems to ceftriaxone and metronidazole.
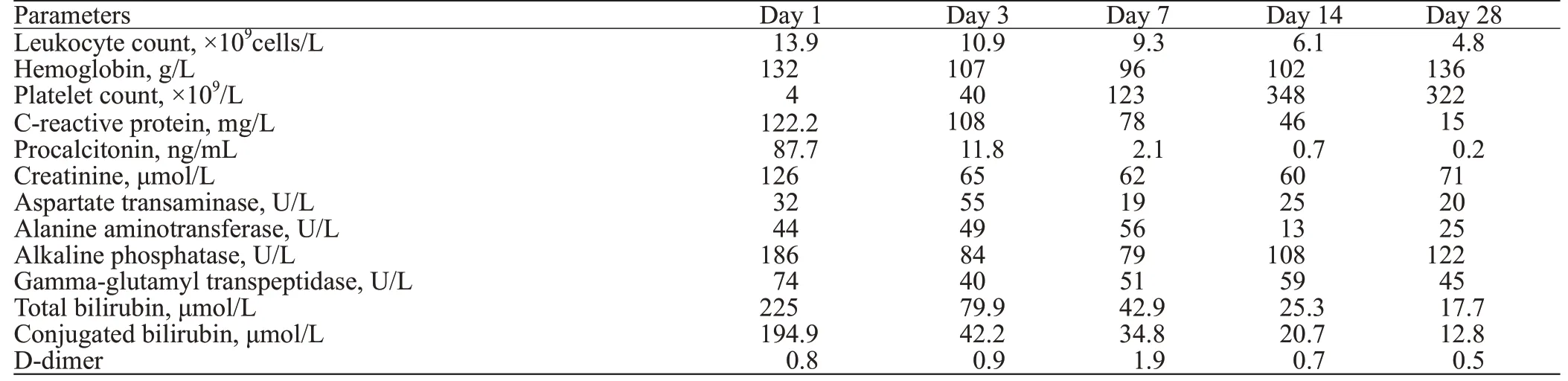
Table 1. Laboratory examination results in different days after admission
He remained in the hospital for 28 days, during which time his clinical symptoms were improved, the size of pulmonary multiple nodules decreased, and the left pleural effusion was absorbed (Figure 1E). He was discharged home with close follow-up in the respiratory department. In the next 3 months of follow-up, the patient had no abnormal performance.
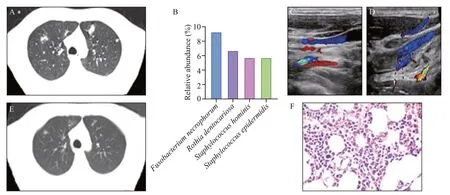
Figure 1. Examination results of the patient. A: computed tomography (CT) shows bilateral multiple pulmonary nodules of varying size, cavitary lesions in the left lung, and left pleural effusion; B: next-generation sequencing (NGS) demonstrated that the infected microorganism was Fusobacterium necrophorum; C: ultrasound indicated that the left internal jugular vein (IJV) contained a thrombus; D: the left IJV was recanalized 7 days later; E: CT of lungs before discharge indicated that the size of pulmonary multiple nodules decreased and the left pleural effusion was absorbed; F: bone marrow biopsy revealed active hyperplasia, particularly in the granulocyte and megakaryocytic series.
Although Lemierre noted the primary presentation when he originally described the disease, the diagnosis of Lemierre’s syndrome is often missed.The most common pathogen associated with Lemierre’s syndrome is, an anaerobic gram-negative bacillis.A new method using NGS has emerged to diagnose pathogens in sepsis.Most antimicrobial regimens include penicillin, metronidazole, cephalosporins, clindamycin,and carbapenem, and >50% of the patients receive three or more different drugs.Riordanreported that antibiotics should be administered for 3-6 weeks. In our case, the patient was diagnosed with severe sepsis on admission, and carbapenem was prescribed to cover all possible bacteria. With further identification of the pathogen, the anti-infection regimen was adjusted accordingly for 4 weeks.
Although Lemierre’s syndrome is characterized by thromboembolic events, the benefit of anticoagulant treatment is still debated.This may be due to major bleeding induced by anticoagulation, particularly in patients with severe thrombocytopenia or hemorrhage.In a retrospective study of invasiveinfections, thrombocytopenia on admission was common among patients diagnosed with Lemierre’s syndrome.In our case, the patient developed massive spontaneous nasal hemorrhage due to severe thrombocytopenia. Although the patient’s platelets gradually returned to normal as the treatment progressed,we did not administer anticoagulant therapy for the following reasons: firstly, the patient had no thrombus anywhere else aside from the jugular vein; secondly,the left IJV was recanalized 7 days later; lastly, both the patient’s basic condition and repeated blood tests suggested that he was at a low risk of venous thrombosis.
The mechanisms of thrombocytopenia in Lemierre’s syndrome are not fully understood. Chaudhary et alstated that thrombocytopenia in Lemierre’s syndrome was due to either sepsis-induced marrow suppression or presumed hemophagocytosis, which was not accurate.In the present case, bone marrow biopsy revealed active hyperplasia, indicating that bone marrow hematopoiesis was increased instead of suppressed; the increase in platelet consumption and destruction may be the main mechanism of thrombocytopenia in Lemierre’s syndrome.
It is uncommon for patients with Lemierre’s syndrome to have jaundice, and only a few cases have been reported.The incidence of jaundice associated with sepsis in the ICU is approximately 40%, and persistent jaundice has been regarded as a marker of patient morbidity and mortality.In our case, the patient showed a significant increase in the direct bilirubin level. After obstruction of the biliary tract was ruled out, the most likely explanation was cholestasis.Several mechanisms must be considered to contribute to jaundice.As for the management of patients with jaundice associated with Lemierre’s syndrome,reasonable antibiotics and anti-inflammatory treatment may be the best option.
The limitation of our case is the relatively short follow-up period. The common complications of Lemierre’s syndrome include in-hospital and long-term complications, such as new venous thromboembolism,neurologic deficits, and debilitating conditions.Although the patient accepted a timely and appropriate treatment and did not experience any complications inhospital or during the 3-month follow-up period, greater attention should be paid to longer follow-up.
Patients with Lemierre’s syndrome, which may be overlooked by clinicians, tend to be young and have a history of recurrent oropharyngeal infection.
Fusobacterium necrophorum is the main pathogen responsible for Lemierre’s syndrome. NGS can confirm infected microorganisms and diagnose Lemierre’s syndrome earlier. Early, reasonable, and adequate antiinfection treatments can significantly improve the prognosis of patients.
None.
Not needed.
A ll authors have no conf lict of interest.
YL designed the study. LJM and WZH drafted the manuscript. All authors have approved the f inal version of the manuscript.
