Anemia and risk of periprocedural cerebral injury detected by diffusion-weighted magnetic resonance imaging in patients undergoing transcatheter aortic valve replacement
Stella Ng, Qi-feng Zhu, Ju-bo Jiang, Chun-hui Liu, Jia-qi Fan, Ye-ming Xu, Xian-bao Liu, Jian-an Wang
1 Department of Cardiology, the Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou 310009, China
2 Zhejiang University School of Medicine, Hangzhou 310058, China
KEYWORDS: Aortic stenosis; Anemia; Cerebral ischemic lesions; Transcatheter aortic valve replacement
Aortic stenosis is one of the major degenerative valvular heart diseases. The incidence is associated with the increasing age of the population.Surgical aortic valve replacement (SAVR) used to be the only promising treatment option for patients with severe aortic stenosis even without solutions regarding the problems associated with high-risk surgical patients. But, this has changed since the introduction of transcatheter aortic valve replacement (TAVR) in 2002 by Cribier et al.TAVR has rapidly grown to be the preferred treatment for severe aortic stenosis patients who are at increased risk for SAVR.Despite the amelioration in technique and technology of TAVR, questions remain related to the safety and efficacy of this procedure, especially regarding to the complications that follow TAVR.Silent ischemic cerebral infarction is one of the major concerns that have been associated with TAVR. Previous studies reported as high as 58%-91% of new silent ischemic stroke detected by diffusion-weighted magnetic resonance imaging(DW-MRI) occurred following TAVR.Micro-embolic particles arising from the native valve during valve implantation have been thought to be one of the possible causes of ischemic lesions. As mentioned before, with most of the TAVR patients being the elderly, they are usually associated with multiple comorbidities. There was a high prevalence of pre-procedural anemia ranging from 45% to 64% among TAVR candidates.In spite of the high prevalence of anemia, which has been reported to be associated with worse outcomes after TAVR, few studies have evaluated the impact of anemia and new ischemic lesions post TAVR.Therefore, the aim of this single-center study is to evaluate the association of pre-procedural anemia and the risk of periprocedural cerebral injury detected by DW-MRI in patients undergoing TAVR.
This study included 158 patients from TORCH(Transcatheter Aortic Valve Replacement Single-Center Registry in Chinese Population) registry (NCT02803294)who received TAVR from December 2016 to April 2019 based on the evaluation of a multidisciplinary heart team.The data were collected prospectively and informed consent was obtained from all patients for TAVR and the use of data for research purposes. Exclusion criteria were: (1)history of pacemaker implantation; (2) history of stroke or transient ischemic attack (TIA) within the prior six months;(3) absence of DWI-MRI imaging due to other reasons(e.g., in-hospital death, conversion to SAVR, emergency cardiopulmonary bypass during TAVR, and poor clinical condition); (4) refusal of DW-MRI; (5) poor quality imaging.TAVR procedures were performed according to the standards of care.Blood samples were collected and hemoglobin levels were determined before TAVR, after the procedure,and prior to hospital discharge. Diagnosis of anemia was based on the World Health Organization criteria as hemoglobin <12 g/dL in women and <13 g/dL in men.All patients completed one-year follow-up, and complications and endpoints were defined according to Valve Academic Research Consortium-2 criteria.
All patients completed magnetic resonance imaging (MRI)before and within 4-7 days after TAVR, using either a 1.5-Tesla(1.5-T) or 3-Tesla (3-T) MRI system (GE Signa, USA). The imaging protocol consisted of transversal T2-weighted turbo spin echo (TSE); 1.5-T: repetition time (T)/echo time (T)4,800/100 ms; 3-T: T/T3,300/80 ms and transversal fluidattenuated inversion recovery (FLAIR); 1.5-T: T/T6,000/120 ms; 3-T: T/T1,200/140 ms. DW-MRI was performed with a spin-echo echo-planar pulse sequence (1.5-T: T78 ms, T2,921 ms, matrix 128×256, total acquisition time 21.4 s; 3-T:T47 ms, T3,866 ms, matrix 128×256, total acquisition time 46.3 s) with diffusion sensitization b-values of 0 and 1,000 s/mm. Additionally, images were obtained in 5-mm slices with a 1-mm intersection gap in both 1.5-T and 3-T systems. TAVR-associated cerebral infarction was defined as high-intensity areas (HIAs) in DW-MRI and hypointense areas on apparent diffusion coefficient (ADC) maps caused by the restricted diffusion of water.Exclusion of other lesions or artifacts that could mimic stroke was done using T2-weighted and FLAIR sequences. The brain MRI was assessed by two independent authors using MRIcron software and reconfirmed by the neurologists.Classification of vascular territories was done according to a previous study: anterior cerebral artery (ACA), middle cerebral artery (MCA), and posterior cerebral artery (PCA)for each side, respectively. Furthermore, vascular border zones (watershed zones) were defined as the area between ACA and MCA (ACA/MCA), MCA and PCA (MCA/PCA), vertebral artery and basilar artery (VA/BA). Acute periprocedural cerebrovascular events (CVEs) detected by means of DW-MRI were assessed for: (1) the total number of lesions per patient (lesion count); (2) the number of lesions located in the right and the left hemisphere; (3) the location of lesions regarding arterial distribution territories;(4) the total lesion volume per patient, accounted from the sum of all single lesion volumes; (5) the total volume/lesions in described vascular territories.
Depending on the distribution of variables, independentsamples t-test or Mann-Whitney U-test was used to compare continuous variables, and the data were presented as mean±standard deviation (SD) or median (25to 75percentiles). Categorical data were compared using Pearson Chi-squared test or Fisher’s exact test and were presented as frequencies (percentages). To identify the relationship between patient factors and the total volume/lesions in ACA/MCA and MCA zones, linear regression analysis was used. Individual factors were identified using univariate analysis and variables with P-value <0.2 were entered into a multivariate linear regression analysis to determine their independence. A two-sided P-value <0.05 was considered statistically signif icant. All statistical analyses were performed with SPSS software (version 25.0, SPSS Inc., USA).
In total, 158 patients who underwent TAVR procedures from December 2016 to April 2019 were analyzed.The patient selection flow is shown in Figure 1. Table 1 summarized the baseline clinical, laboratory and echocardiographic characteristics of the patients.

Figure 1. Patient selection flow. AS: aortic stenosis; TAVR:transcatheter aortic valve replacement; MRI: magnetic resonance imaging; TIA: transient ischemic attack; SAVR: surgical aortic valve replacement.
The results of the clinical outcome were listed in Table 2. Anemic patients were more likely to have longer hospitalization compared to the non-anemic patients(<0.001). There was no significant difference in the incidence of stroke between the two groups (=0.374).

Table 1. Baseline characteristics of anemic and non-anemic TAVR patients
All patients had a follow-up of DW-MRI 4-7 days post TAVR procedures (Table 3). There was no difference between the two groups for the days of DW-MRI followup (=0.195). Of the 158 patients who underwent TAVR,126 (79.7%) patients had 718 new DW-positive lesions with a mean of 4.54±5.26 lesions per patient. These HIAs were mostly multiple and spread out in both hemispheres. Details of the lesion distribution were: ACA (=97), ACA/MCA(=98), MCA (=218), MCA/PCA (=20), PCA (=143),and VA/BA (=143).
In our univariable linear regression analysis,we identified that NYHA functional class, smoker,dyslipidemia, atrial fibrillation, anemia, INR, activated partial thromboplastin time (APTT), aortic mean gradient,maximum velocity, and procedural time were the predictors of volume/lesions in the ACA/MCA region. Meanwhile,the predictors for the volume/lesions in the MCA region were NYHA functional class, prior history of percutaneous coronary intervention (PCI), anemia, APTT, valve types,and procedural time. However, based on multivariable linear regression analysis, only NYHA functional class(= -14.361, 95% confidence interval [95%] -24.807 to -3.915,=0.007), anemia (=16.796, 95%2.001 to 31.591,=0.026) and aortic mean gradient (=0.218, 95%0.007 to 0.430,=0.043) were independently associated with the volume/lesions in the ACA/MCA region; while,only anemia (=0.020, 95%0.001 to 0.040,=0.041) and valve types (= -0.025, 95%-0.046 to -0.004,=0.022)remained as the independent predictors of the volume/lesions in the MCA zone (Table 4).
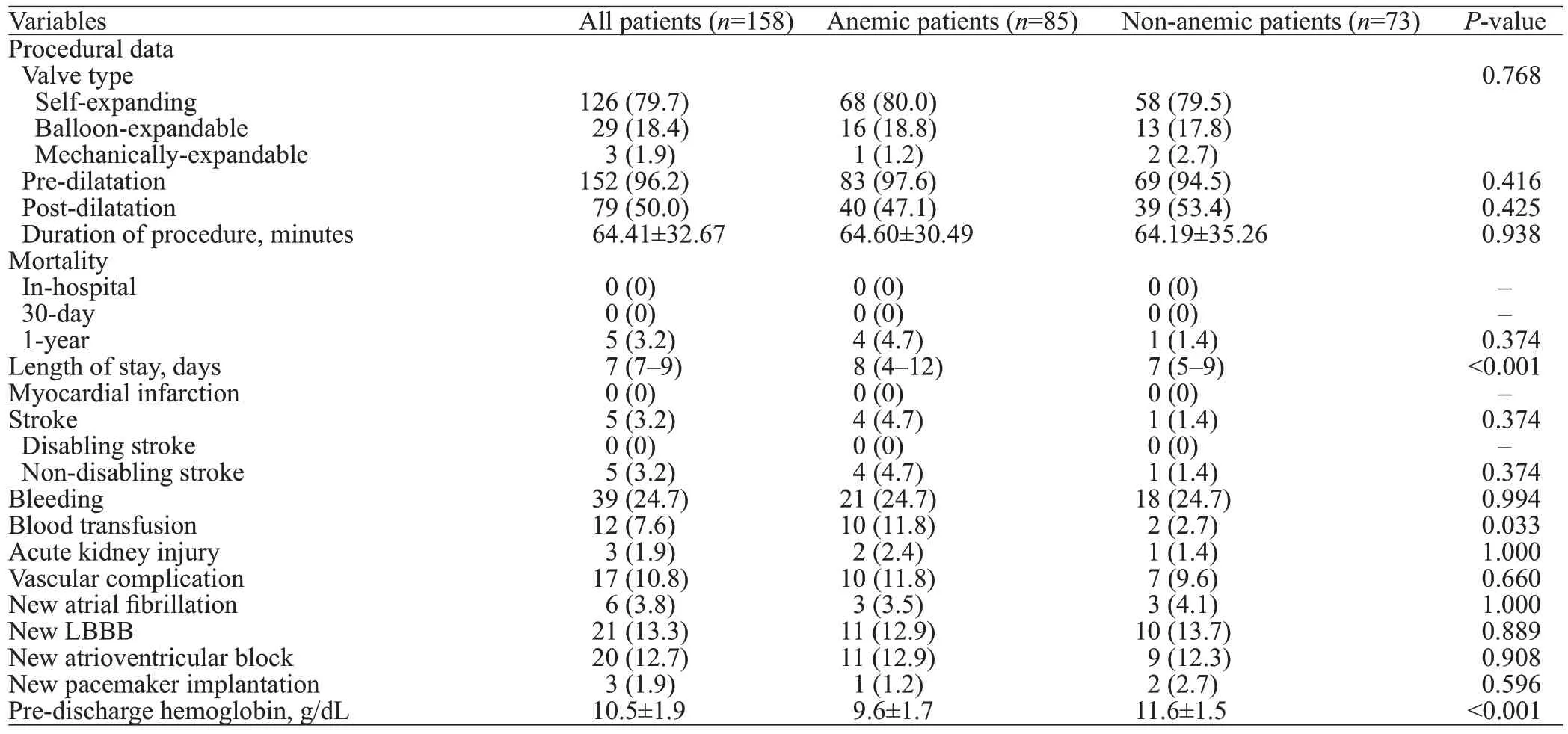
Table 2. Procedural data and periprocedural clinical outcome following TAVR
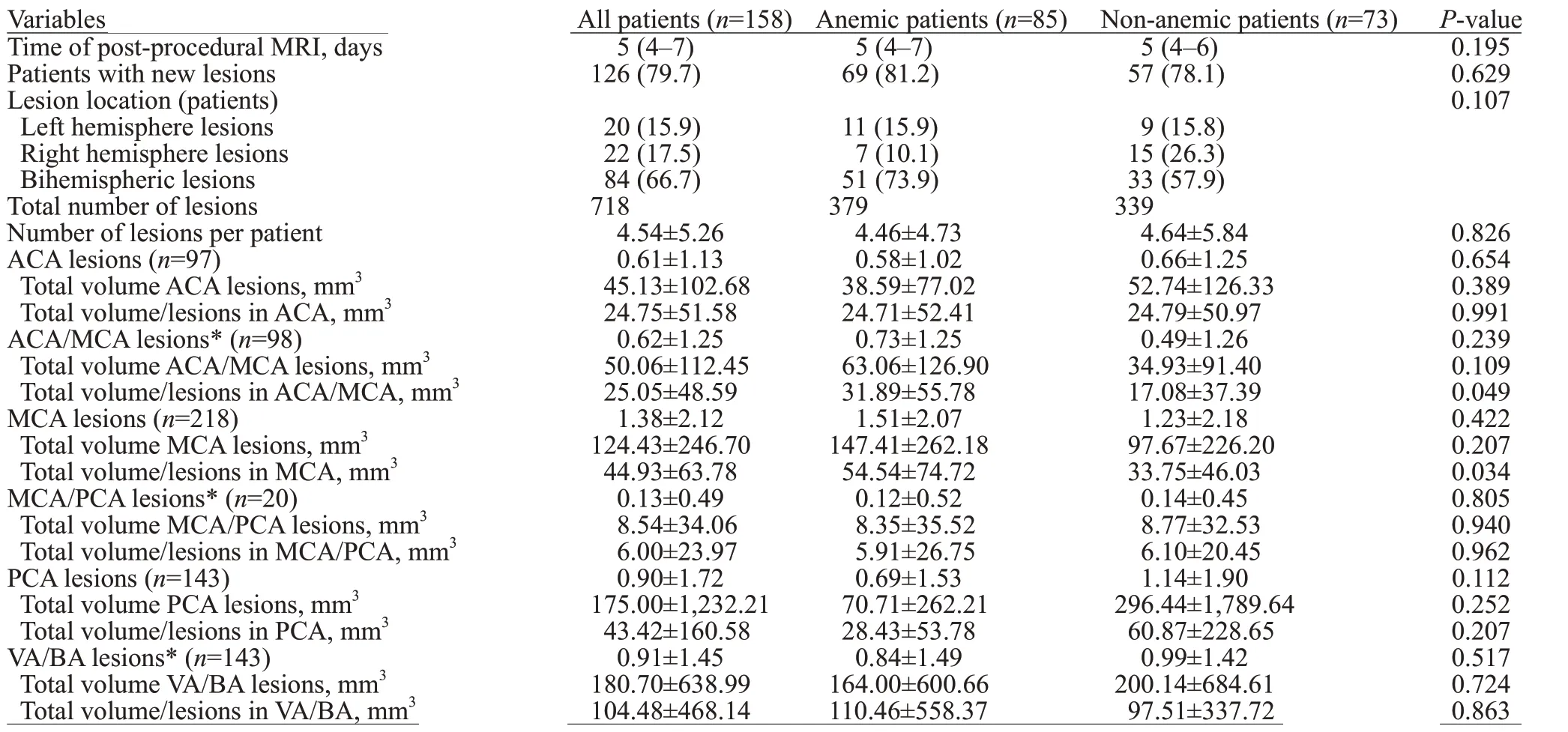
Table 3. Post-procedural DW-MRI f indings

Table 4. Linear regression analysis for the prediction of the volume/lesion in ACA/MCA and MCA region (on post-TAVR DW-MRI)
Despite a known high prevalence of anemia and periprocedural stroke risk among TAVR patients, few studies have evaluated the relationship between anemia and new ischemic lesions detected by DW-MRI. In this study, we revealed that anemia was not directly related to the incidence of new ischemic lesions and did not affect the number of lesions in post TAVR patients. However, patients with anemia were shown to have bigger total volume/lesions in the ACA/MCA and MCA regions compared with the nonanemic group.
The prevalence of pre-procedural anemia among the TAVR candidates has been reported to be higher than that in cardiac surgery candidates, ranging from 45% to 64%in the former and from 16% to 36% in the latter.Although little is known about the causes of anemia in patients with aortic stenosis, the occurrence of recurrent hemorrhage or shear stress-dependent intravascular hemolysis has been reported in up to 25% of patients with severe aortic stenosis.Furthermore, a potentially reversible cause of anemia was detected in almost 90% of patients in one study.Interestingly, more than half of these patients were identif ied to have iron deficiency. In addition, anemia incidence is also known to be associated with the increasing age.As most TAVR procedures are offered to elderly patients, they contribute to the higher proportions of baseline anemia.
Pre-procedural anemia interrelates with worse outcomes after TAVR. Some of the risks that have been reported include a higher risk for acute kidney injury (AKI), blood transfusion, prolonged hospitalizations, and long-term mortality.Furthermore, a study found that a lower predischarge hemoglobin level could predict early readmission.In fact, a high percentage of TAVR patients are anemic to a certain extent at hospital discharge and are at greater risk for readmission. This could be explained by the connection between low hemoglobin levels and heart failure decompensation that happen in anemic patients post-TAVR.
Similar to the previous study,the anemic patients in our current study also demonstrated longer hospitalization time, which might be related to the higher demand for blood transfusion. Correspondingly, Seiffert et alreported an independent association between anemia and in-hospital blood transfusion. Red blood cell (RBC) transfusions have been reported to be associated with a higher risk of oneyear mortality, major cerebrovascular events, and AKI after TAVR.In the current study, the anemic patients were transfused about four times more frequent compared to the non-anemic patients. A reasonable explanation behind this could be the lower hemoglobin level in anemic patients that reaches the threshold for transfusion faster even in the absence of overt bleeding. Despite being transfused,our patients did not show any signs of AKI, which was discordant with a previous study.
Anemia is believed to have a clear relationship with CVEs, as anemia may impair tissue oxygen delivery.As arterial oxygen content is dependent on hemoglobin level, anemia may affect the oxygen delivery to the brain.Even though hypertension and atherosclerosis have been reported to be the main cause of stroke, there are also other less common causes of stroke that includes cardioembolism, hematologic disorders, substance abuse,trauma, dissections, oral contraceptive use, connective tissue disorder, pregnancy, postpartum stage, and migraine.In particular, according to the INTERSTROKE study,ten risk factors were associated with 90% of the risk of stroke.Even though anemia was not listed in the INTERSTROKE study, it is consistently present in patients with acute stroke and TIA, ranging from 15% to 29%.That is why the importance of anemia, particularly the value of hemoglobin and hematocrit is still debatable among the numerous wellknown risk factors for ischemic stroke.
Iron def iciency anemia (IDA) has been suggested as an etiological factor for ischemic stroke and there is signif icant evidence for this in pediatric TIA and ischemic stroke.Even though the evidence that IDA confers risk for ischemic stroke in adults is less well-established, a previous studyhave reported its possible associations with ischemic stroke. Being the top cause of anemia, IDA is a global health problem, as it affects more than 2 billion people worldwide.Its prevalence has been confirmed by the analysis of numerous reports on the burden of disease in 187 countries between 1990 and 2010. The systemic analysis also found out that there was a high rate of iron def iciency in the aging population.
Few mechanisms have been proposed by which anemia, precisely IDA, could contribute to the development of stroke, including thrombocytosis, altered erythrocyte deformability, blood f low, anemic hypoxia, and inf lammation related endothelial dysfunction.In the settings of IDA,erythropoietin (EPO) levels are commonly increased and can lead to secondary thrombocytosis. It is primarily due to the resemblance of EPO and thrombopoietin (TPO) molecules,hence causing the interaction of EPO and TPO receptors on the surface of megakaryocytes. Additionally, the changes in erythrocyte deformability may impact tissue perfusion,either through decreased oxygen capacity or diminution of blood supply. Apart from that, in the case of hypoxic anemia,inflammatory endothelial dysfunction can also lead to ischemic injury of the brain.Despite all previous studies that have evaluated the association of anemia and stroke, in our current study, we did not f ind any signif icant difference in the incidence of stroke between the anemic and nonanemic groups due to the small sample size.
TAVR has been known to be associated with frequent occurrence of cerebral complications. Few studies have reported the incidence of clinically apparent stroke about 3.3%-9.1% in the early postoperative period.Additionally,the perioperative incidence of MRI-defined cerebral infarction ranges from 58% to 91%.Consistently, our present study found that 79.7% of patients presented with new ischemic lesions on post-procedural DW-MRI.
In the ADVANCE trial, using the CoreValves exclusively, about half of the strokes were reported to occur between day 2 and day 30, while the other half occurred on the day of the procedure. This suggests that the risk of stroke is not limited to the procedure itself.Furthermore,in the Claret Embolic Protection and TAVI (CLEANTAVI) randomized clinical trial, numerous materials were captured and removed by the filters that included old and fresh thrombus, endothelium, atheromatous plaque, valve tissue, and calcium.It becomes evident that the causes of cerebral injury are multifactorial and that the embolic risk persists even after a TAVR procedure. With regard to the potential risk factors for the development of new MRIdefined cerebral infarction, several studies have found an association between age and the number of lesions after these procedures.
In our study, patients with anemia were significantly older than the non-anemic patients. Despite statistical insignif icance, the anemic group had more patients with new lesions. Furthermore, the total volume/lesions in ACA/MCA and MCA regions were larger in this group. These findings are conceivable as in healthy awake patients, anterior and middle cerebral arteries, which are the branches of internal carotid arteries, contribute about 70% of the brain’s blood supply. The remaining 30% of cerebral blood f low arises from two vertebral arteries, which supply the posterior cerebral circulation. Therefore, based on the blood flow distribution,two-third of all cardiogenic emboli would be expected in the anterior and middle circulation.This problem can be exacerbated with the presence of anemia, in which there is a reduced blood flow to the brain. In other words, anemia could magnify the effects of microemboli by impairing their washout.
Moreover, the multivariable linear regression analysis revealed that NYHA class, anemia, and mean gradient were the independent predictors for the total volume/lesions in ACA/MCA zone. Meanwhile, anemia and valve types were the only independent predictors for the total volume/lesions in the MCA region. In a previous study,a linear relationship was found between hemoglobin decrease and a larger final infarct volume and greater and faster infarct growth. The causal relationship is conceivable as anemia entails a decrease of oxygen-carrying capacity of blood, which might exert a negative effect on penumbra evolution.In addition, with the presence of worse NYHA class, it means that the heart will have less ability to compensate for procedural stress during TAVR. Meanwhile,a higher mean transaortic gradient usually reflects a more severe aortic valve stenosis, which is often accompanied by a higher degree of aortic valve calcif ication. Similarly, a study by Samim et alrevealed that only peak transaortic gradient was independently associated with post-procedural total infarct volume. Furthermore, with the majority of the patients having self-expandable valves, there are few possible mechanisms that can explain the association of valve types and volume/lesions in the MCA region. Firstly, the slow and stepwise implantation of self-expandable valves might have caused a higher number of HIA in DW-MRI. Secondly,the continuously expanding properties of self-expandable valves for approximately 10 days to best f it the surrounding anatomical structures might have caused the debris from the native valve or surrounding tissue to slough off. Lastly,the larger attachment area of self-expandable valves might impact not only the native valve and surrounding tissue, but also on the aortic root area, which can result in greater debris dissemination to the brain.
Although the consequences of silent ischemic brain injuries are still uncertain, they have been associated with cognitive decline and increased risk of dementia and depression.Therefore, any risk factors that can pose a higher risk to cerebral injury post TAVR should be clearly assessed prior to a TAVR procedure.
This single-center, non-randomized study had several limitations that warranted mention. First and foremost, this study involved only a small number of patients, and therefore needs to be conf irmed with a larger randomized clinical trial.Inter-ethnic differences between Asian and other population might also played as a confounding factor.Secondly,the risk strata of our cohort were heterogeneous, with a mixture of low-risk, intermediate-risk, and high-risk patients.In addition, analysis of the causes of anemia was not systematically performed, so a potentially correctable cause of anemia was not identif ied. Last but not least, this present study still requires longer follow-up to see the result of the negative impact of anemia on mortality, stroke, neurological,and cognitive function assessment.
Patients with anemia may have bigger total volume/lesions in the ACA/MCA and MCA regions compared to the non-anemic patients. Nonetheless, the incidence of new ischemic lesions as well as the number of new ischemic lesions is not statistically different between the two groups. An independent association is found between NYHA functional class, anemia and aortic mean gradient and the volume/lesions in the ACA/MCA region. However, only anemia and valve types are independently associated with the volume/lesions in the MCA zone. Whether the consequences of bigger total volume/lesions impact neurological and cognitive outcomes remains to be investigated.
This study was funded by Zhejiang Province Science and Technology Department Key R&D Program (2018C03084,2021C03097).
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the Second Affiliated Hospital of Zhejiang University School of Medicine.
The authors have no conflicts of interest to declare that are relevant to the content of this article.
SN and QFZ contributed equally to the manuscript.XBL, JAW, and JBJ established the registry, conceived, and designed the study. SN and QFZ drafted the manuscript. CHL and JQF performed the MRI image analysis. SN, YMX, and QFZ analyzed data and interpreted the results. All authors edited and revised the manuscript. All authors read and approved the f inal manuscript. XBL and JAW are the study guarantors, responsible for overall content of the manuscript.
