Tranexamic acid for major trauma patients in Ireland
Kieran Walsh, Francis O’Keeffe , Louise Brent, Biswadev Mitra
1 National Trauma Research Institute, the Alfred Hospital, Melbourne 3004, Australia
2 Critical Care Research, School of Public Health and Preventive Medicine, Monash University, Melbourne 3004, Australia
3 Emergency & Trauma Centre, Alfred Health, Melbourne 3004, Australia
4 Emergency Department, Mater Misericordiae University Hospital, Dublin D07 R2WY, Ireland
5 National Office for Clinical Audit, Ardilaun House, Dublin D02 VN51, Ireland
KEYWORDS: Tranexamic acid; Shock; Ireland; Hemorrhage
Trauma is the leading cause of death for people aged under 45 years in developed nations.Among injured patients, hemorrhage is the second leading cause of death after central nervous system (CNS) injury and the number one preventable cause of death.Hemorrhage is complicated in 25%-30% of patients by trauma-induced coagulopathy (TIC).TIC is a complex,multifactorial, and pathological process characterized by hypocoagulability and hyperfibrinolysis.TIC carries a poor prognosis, and trauma patients with TIC are at a four-fold risk of mortality compared to trauma patients without TIC.Tranexamic acid (TXA) is an antifibrinolytic agent, which has been hypothesized to reduce mortality in hemorrhagic trauma patients by retarding the hyperf ibrinolysis seen in TIC.
The Clinical Randomisation of an Anti-fibrinolytic in Significant Hemorrhage-2 (CRASH-2) is the largest randomized control trial (RCT) examining circulatory resuscitation for trauma patients to date and concludes a statistically significant reduction in all-cause mortality in the TXA group (14.5%) compared to the placebo group (16.0%), with a relative risk () of 0.91 (95%confidence interval [] 0.85-0.97,=0.0035).The benefits were restricted to patients receiving TXA within 3 hours of injury, and the trial concluded that TXA administration within 3 hours of injury was safe and cost-effective and may result in a mortality benefit to injured patients.Based on these conclusions, many nations around the world have implemented TXA into their massive transfusion protocols, and recommended routine use of TXA for all trauma patients at risk of hemorrhage.
Implementation of TXA post-CRASH-2 has been enthusiastic within the UK. However, this uptake has not been universal in other nations, and currently there exists significant geographical variance in the use of TXA for trauma patients.A postulated reason for this variation relates to generalisability with many patients in CRASH-2 enrolled from developing nations, which commonly lack ready access to advanced processes and interventions in mature trauma systems.
Geographically, Ireland is the closest nation to the UK. Both countries collect clinical audit data on major trauma in the Trauma Audit and Research Network(TARN) registry. The Irish arm of the audit is governed by the National Office of Clinical Audit (NOCA) and called the Major Trauma Audit (MTA). Furthermore,Ireland is currently in the midst of developing a coordinated national trauma network similar to the recently introduced and successful UK model.
The aim of this study was to assess TXA use for severe trauma patients with hemorrhagic shock in Ireland after publication of CRASH-2 trial results and examine the association between TXA administration and mortality. We hypothesized that TXA administration, after adjustment for potential known confounders, would be associated with reduced mortality at hospital discharge.
A retrospective cohort study was conducted using data derived from the MTA via the NOCA.
TARN is an academically led, independent,and prospective trauma registry for injured patients presenting to hospitals in the UK and Ireland. At present,TARN is the largest trauma registry in Europe capturing data from all injured patients in the UK and Ireland.
NOCA is an Irish clinical audit program established in 2012 designed to be an ongoing measure of clinical practice in Ireland.NOCA is funded by the Health Service Executive Improvement Division and is governed by an independent voluntary board and operationally supported by the Royal College of Surgeons in Ireland.Upon establishment, NOCA engaged the services of TARN as a data registry for Irish trauma patients entitled the MTA. Currently, 26 trauma-receiving hospitals in Ireland give feedback data to TARN.NOCA publishes an annual MTA and has shown a continued increase in the uptake of the TARN database each year, as well as increased robustness of the data collected.
NOCA supports research within an ethical framework. It distributes data for high-quality research,internally to primary NOCA based researchers, and externally to collaborative national and international research partners.
All injured patients presenting to one of the 26 trauma-receiving hospitals in Ireland between January 2013 and December 2018 and evidence of hemorrhagic shock on presentation were included in this study.Hemorrhagic shock was def ined as clinically signif icant hypotension and blood transfusion. The CRASH-2 trial enrolled patients with systolic blood pressure (SBP)<90 mmHg (1 mmHg=0.133 kPa) or heart rate >110 beats/minute or clinically adjudged risk of significant hemorrhage. TARN defines clinically significant hypotension as SBP <110 mmHg and thus indicates TXA for all patients with SBP <110 mmHg. This is due to previous studies showing that trauma patients with SBP <110 mmHg have a two-fold increase in mortality.Although all patients with SBP are indicated TXA as per the TARN protocol and key performance indicators (KPIs), we defined clinically significant hypotension as SBP <100 mmHg to ensure a signif icant population sample size while also making the results of our examination more generalizable to the original CRASH-2 study population. For patients who had an initial SBP missing in the ED, we imputed the prehospital SBP. Blood product transfusion is recognized as the gold standard of management for hemorrhagic trauma patients.In CRASH-2, only half of the patients enrolled actually received blood product transfusion or damage control surgery. This has led some experts to question if all of the CRASH-2 trial patients were actually suffering clinically signif icant hemorrhage. This may have resulted in an underestimation of the benefits of TXA in the CRASH-2 trial as some patients enrolled may have had no chance of benefit. For this study,to ensure the selection of patients with confirmed hemorrhage, the requirement of blood transfusion was added as an inclusion criterion.
We excluded patients for whom there was no recorded data on whether TXA had been administered or not.
Patients with SBP <100 mmHg, receiving transfusion with blood products and confirmed data on TXA status, were sub-grouped into two cohorts based on TXA administration. Variables intended for multivariable analysis initially were: shock index (SI),age, sex, temperature, respiratory rate (RR), heart rate(HR), Injury Severity Score (ISS), Glasgow Coma Scale(GCS), mechanism of injury (categorical description)and mechanism of injury (binary description - blunt or penetrating). These variables were chosen as they had previously been shown to correlate with shock and/or mortality outcomes.Temperature and RR were excluded from multivariable analysis as no RR in ED or temperature was recorded in the TARN database.Arrival mode was added as a variable for analysis as it was reliably recorded, and we believed that it was f itting in a study examining care prior to implementation of a nationwide trauma system.
Variables were summarized using mean and standard deviation (SD) if continuously and normally or nearnormally distributed. Median and interquartile range(IQR) were used if the data were skewed. Ordinal and discrete variables were summarized using median and IQR. Categorical data such as the mechanism of injury were summarized using count and proportion.
Means were compared using Student’s t-test,medians were compared using the Wilcoxon Rank Sum test, and the Chi-square test was used to compare proportions, unless the count in a cell was <5, in which case, Fisher’s exact test was used. A P-value of <0.05 was considered to be statistically signif icant.
Baseline variables of both cohorts were summarized and analyzed for comparability.
All variables demonstrating an association with mortality (P<0.10) as listed in Table 1 were entered into a multivariable regression model to determine independent association with mortality. Results were presented using adjusted odds ratio (OR) and 95% conf idence interval (CI).Additional stepwise regression to arrive at a reduced model was not performed due to the potential absence of important confounders.
The discriminative ability of the model was assessed using the area under receiving operator curve (AUROC).The Hosmer-Lemeshow test was reported for goodness of f it (GOF). Multi-collinearity among included variables was assessed using the variance inflation factor. Age,GCS, and ISS were grouped and analyzed as ordinal variables. Data analysis was performed using Stata Version 15.1 (College Station, USA).
This study was conducted in compliance with the governing legislation of the Health Research Board of Ireland, complying with international European Union standards as well as the General Data Protection Regulation (GDPR) act of 2018. Further GDPR training was undertaken in conjunction with the Mater Misericordiae University Hospitals (MMUH) GDPR officer to ensure compliance with all privacy regulations.This training involved completion of online modules relating to GDPR and the ascertainment of a certificate on the fundamentals of GDPR. Approval by the MMUH ethics board with reference to the Health Service Executive National Consent Protocol was awarded before a data request was submitted to NOCA.Once submitted, the data request was approved by the MTA governance committee.
A total of 21,745 patients were included in the TARN trauma registry from Ireland between January 2013 and December 2018. Among them, 18,211 (83.7%) patients had an initial ED SBP ≥100 mmHg, and therefore, were excluded from this study. For 2,538 (11.7%) patients,the initial ED SBP was not recorded. Of these 2,538 patients, 12 had an initial pre-hospital SBP recorded that was <100 mmHg and were therefore included in the study. This resulted in a total of 1,008 (4.6%) patients presenting to an Irish ED between 2013 and 2018 with an initial SBP <100 mmHg. Of the 1,008 patients, three data entries did not include information relating to TXA.Of the 1,005 patients, 771 did not receive blood products and were thus excluded. This resulted in a final total of 234 (1.1%) patients. Of the patients that met our final inclusion criteria, 133 (56.8%; 95% CI 50.2%-63.3%)received TXA (Figure 1).
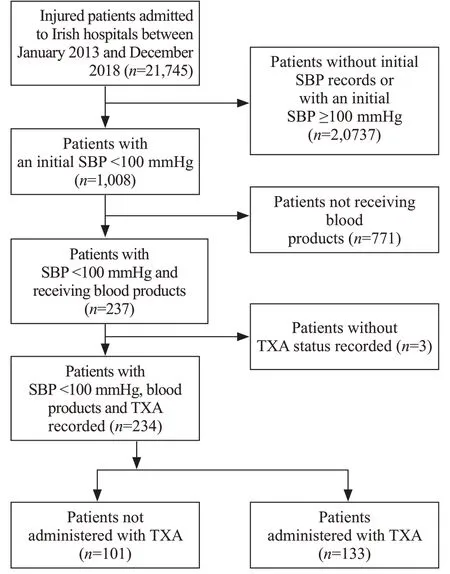
Figure 1. Inclusion and exclusion of patients.
Differences among variables sub-grouped by the primary exposure variables of interest are presented in Table 1.
There were 66 (28.2%; 95% CI 22.5%-34.4%)deaths at hospital discharge. Potential confounders are listed in Table 2 and after adjustment, TXA was not significantly associated with lower mortality (adjusted0.86; 95%0.31-2.38). Variables associated with mortality at hospital discharge were male sex, lower initial systolic BP, and higher injury severity.
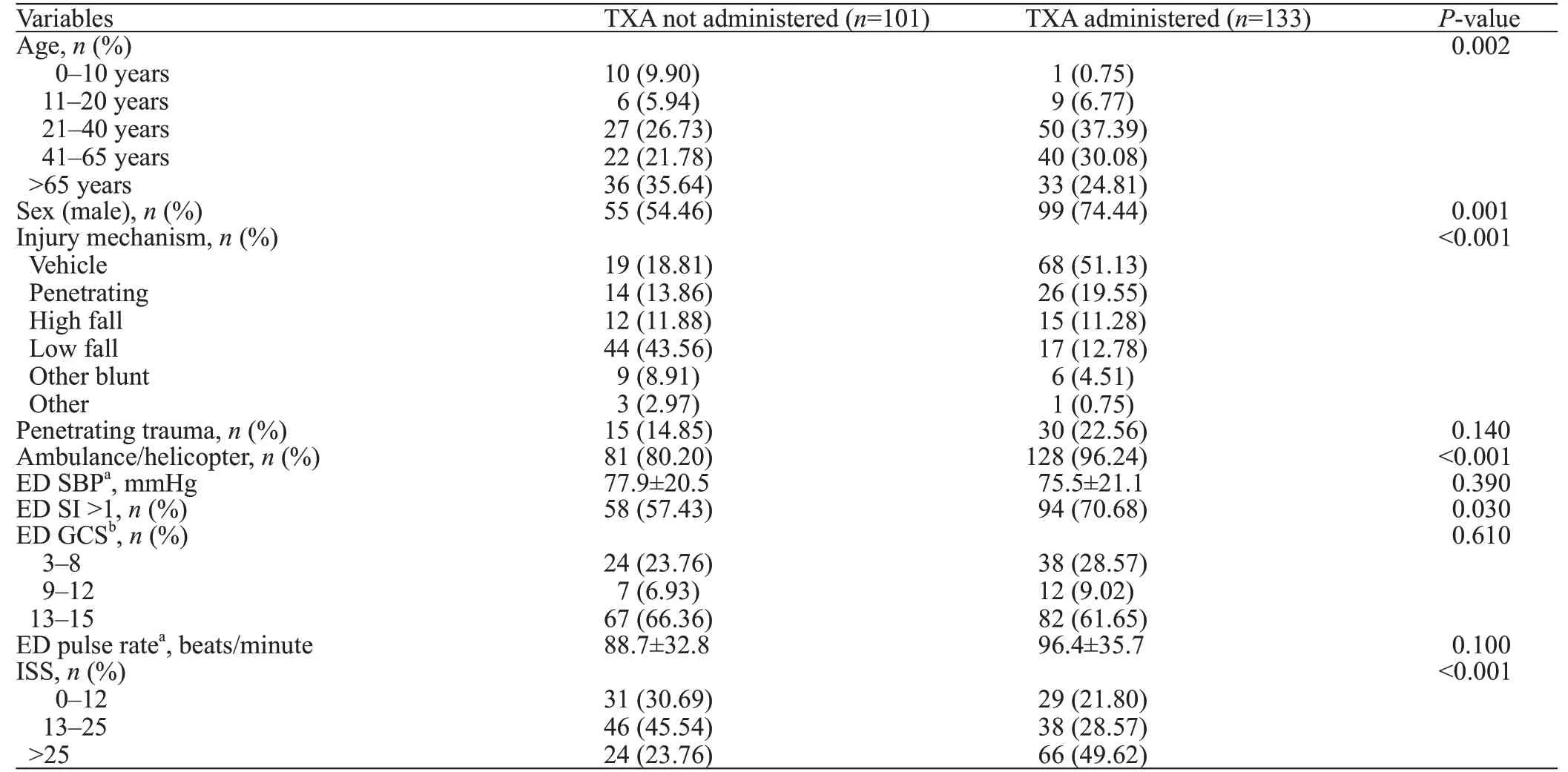
Table 1. Demographics and baseline variables
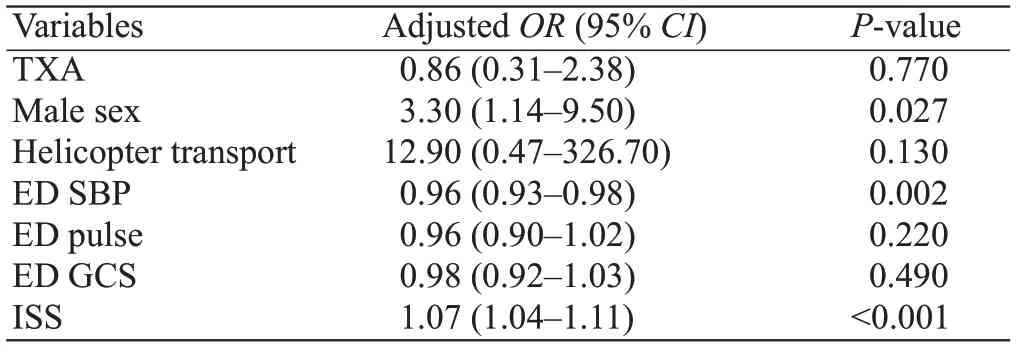
Table 2. Association between variables and mortality
The model demonstrated good discrimination(Figure 2) with AUROC 0.83 (95%0.75-0.91). The-value for Hosmer-Lemeshow GOF was 0.21. The mean variance inflation factor was 1.24. There was no signif icant interaction between ED SBP and ISS (-value for interaction term=0.60).
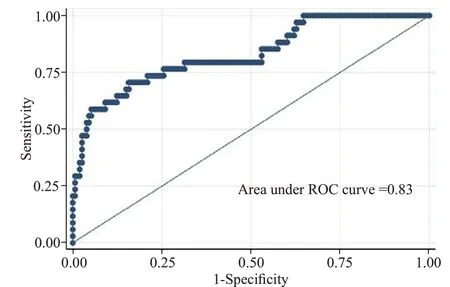
Figure 2. ROC curve for logistic regression model used.
After publication of CRASH-2 trial results,TXA was administered to 57% of injured patients in hemorrhagic shock arriving at EDs in Ireland. TXA was administered to patients with high SI and higher ISS more commonly, with significant baseline differences among cohorts. When adjusted for known confounders,TXA administration was not associated with improved survival to hospital discharge.
In this selected cohort of injured patients who were hypotensive and received blood transfusion, the proportion of patients that received TXA was lower than expected. Coats et alfound that since the publication of CRASH-2, 68.7% of injured patients in the UK who received blood products also received TXA. This f inding was in keeping with current literature that shows uptake of TXA post-publication of CRASH-2 has not been universal.
One reason that may contribute to the lower implementation of TXA in Ireland compared to the UK, is the lack of a coordinated national trauma system in Ireland. Without a coordinated national system,no national statutory body exists to standardize and provide governance for trauma patients in Ireland. Many individual hospitals and national bodies, e.g., Irish Association for Emergency Medicine (IAEM), have proposed and implemented guidelines on TXA use.However, this approach is neither universally mandated nor adopted throughout all 26 trauma-receiving hospitals in Ireland. Therefore, it is difficult to estimate how many of the 26 separate units have a major hemorrhage protocol (MHP) in place and have TXA administration included as an integral constituent. Ireland is currently in the process of restructuring to implement a coordinated national trauma system.This will see two strategically placed Major Trauma Centers (MTC) in Ireland as hubs to a number of smaller spoke trauma units throughout the country. As part of this major reorganization of services, a National Trauma Office will be implemented,which will provide key statutory oversight for national guidelines and major policies including TXA use for major trauma patients as a part of MHP.
While our hypothesis of improved outcomes associated with TXA administration was not proven,the differential administration of TXA to more severely injured patients may have been a contributing factor.While the attempt to account for confounders resulted in a model of good fit and discrimination, it is possible that unknown confounders were present. Retrospective cohort studies examining the use of TXA have previously reported a wide heterogeneity of outcomes, and results have not always been consistent with the f indings of the CRASH-2 trial. For example, while Valle et alfailed to show significant benefit from TXA, other studies such as the Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) Trialand a study reported by Shiraishi et aldemonstrated the possible benef its of TXA administration.
This study was limited in being a retrospective analysis of registry data and the possibility of unknown confounders. However, it provides a framework for describing current management within the Irish trauma system and assessing quality improvement strategies.Specification by strict inclusion criteria to select a population with bleeding and shock was designed to maximize any potential effect, however it resulted in a small sample size. Furthermore, information on delivery of TXA and timing of injury were not available. The timing of TXA administration has been associated with potential harm when administered >3 hours of injury.In addition, data on secondary outcomes such as thromboembolic events were not available. Whether TXA results in increased thromboembolic events in the trauma setting is still unknown. The CRASH-2 trial did not f ind an increase in thromboembolic events, however, retrospective cohort studies have previously shown an increase in propensitymatched cohorts.In our dataset, the incidence of venous thromboembolism (VTE) was poorly recorded with only 17 (7.2%) data entries and improved data collection will assist secondary analyses in future.
External validation of CRASH-2 trial findings may enable better translation. Several RCTs are currently underway examining the use of TXA for injured patients in countries with mature trauma systems in an attempt to externally validate the findings of the CRASH-2 trial. The Pre-hospital Anti-fibrinolytics for Traumatic Coagulopathy and Hemorrhage Study (PATCH) is an international, multicenter, double-blinded, placebocontrolled trial examining TXA in advanced trauma systems based out of Australia and New Zealand. PATCH will enroll 1,200 severely injured patients deemed to be at risk of acute traumatic coagulopathy (ATC) using the coagulopathy of severe trauma (COAST) scoring system. The primary outcome of the PATCH trial will be the proportion of patients with a favourable outcome at 6 months, as def ined by Glasgow Outcome Scale-Extended(GOS-E) of 5-8, with recruitment expected to complete in the second half of 2020.
Study of Tranexamic Acid during Air Medical Prehospital transport (STAAMP) was a US-based multicentre, double-blinded, placebo-controlled trial examining TXA in trauma patients. The primary outcome of all-cause mortality difference was not demonstrated.However, the study generated the hypothesis that TXA may be of benef it in subgroups of patient who receive the drug early or have initial hypotension.
Tranexamic Acid Mechanisms and Pharmacokinetics in Traumatic Injury (TAMPITI) was a US-based RCT. By analyzing pharmacokinetic, pharmacodynamic, and immune parameter samples of patients randomized to three arms(placebo, 2 g TXA intravenous, 4 g TXA intravenous), the TAMPITI Trial concluded minimal immunomodulatory effects with respect to leukocyte phenotypes and circulating cytokine levels after TXA administration.
Among injured patients in Ireland presenting with hypotension and managed with blood transfusion, TXA was administered to 56.8% of patients who were severely injured. However, a mortality benefit could not be demonstrated, which may be due to the low proportion of patients receiving TXA. We recommend ongoing efforts to standardize the care of injured patients across Ireland with development of a national coordinated trauma system using robust guidelines combined with ongoing surveillance of TXA administration.
We would like to thank Maydul Islam for extraction of data. We would like to acknowledge all of the audit coordinators and clinical leads in the Irish hospitals who submit data to the Major Trauma Audit governed by the National Office of Clinical Audit.
None.
Approval by the Mater Misericordiae University Hospital (MMUH) ethics board with reference to the Health Service Executive National Consent Protocol was awarded.
Biswadev Mitra is a chief investigator in the PATCH-Trauma trial indicating a belief in equipoise between TXA and placebo for injured patients. No other authors have conflicts to declare.
KW wrote the f irst draft. All authors contributed to the study and to further drafts.
