Risk profiles and outcomes of patients receiving antibacterial cardiovascular implantable electronic device envelopes:A retrospective analysis
David A Woodard,Grace Kim,Kent R Nilsson
David A Woodard,Kent R Nilsson,Department of Cardiology,Piedmont Heart Institute,Athens,GA 30606,United States
Grace Kim,Kent R Nilsson,Department of Medicine,Augusta University-University of Georgia Medical Partnership,Athens,GA 30606,United States
Abstract BACKGROUND Cardiovascular implantable electronic devices (CIEDs) are implanted in an increasing number of patients each year,which has led to an increase in the risk of CIED infection.Antibacterial CIED envelopes locally deliver antibiotics to the implant site over a short-term period and have been shown to reduce the risk of implant site infection.These envelopes are derived from either biologic or nonbiologic materials.There is a paucity of data examining patient risk profiles and outcomes from using these envelope materials in the clinical setting and comparing these results to patients receiving no envelope with their CIED implantation.AIM To evaluate risk profiles and outcomes of patients who underwent CIED procedures with an antibacterial envelope or no envelope.METHODS After obtaining Internal Review Board approval,the records of consecutive patients who underwent a CIED implantation procedure by a single physician between March 2017 and December 2019 were retrospectively collected from our hospital.A total of 248 patients within this period were identified and reviewed through 12 mo of follow up.The CIED procedures used either no envelope (n=57),a biologic envelope (CanGaroo®,Aziyo Biologics) that was pre-hydrated by the physician with vancomycin and gentamicin (n=89),or a non-biologic envelope (Tyrx™,Medtronic) that was coated with a resorbable polymer containing the drug substances rifampin and minocycline by the manufacturer (n =102).Patient selection for receiving either no envelope or an envelope (and which envelope to use) was determined by the treating physician.Statistical analyses were performed between the 3 groups (CanGaroo,Tyrx,and no envelope),and also between the No Envelope and Any Envelope groups by an independent,experienced biostatistician.RESULTS On average,patients who received any envelope (biologic or non-biologic) were younger (70.7 ± 14.0 vs 74.9 ± 10.6,P =0.017),had a greater number of infection risk factors (81.2% vs 49.1%,P < 0.001),received more high-powered devices (37.2% vs 5.8%,P =0.004),and were undergoing more reoperative procedures (47.1% vs 0.0%,P < 0.001) than patients who received no envelope.Between the two envelopes,biologic envelopes tended to be used more often in higher risk patients (84.3% vs 78.4%) and reoperative procedures (62.9% vs 33.3%) than non-biologic envelopes.The rate of CIED implant site pocket infection was low (any envelope 0.5% vs no envelope 0.0%) and was statistically equivalent between the two envelope groups.Other reported adverse events (lead dislodgement,lead or pocket revision,device migration or erosion,twiddler’s syndrome,and erythema/fever) were low and statistically equivalent between groups (biologic 2.2%,non-biologic 3.9%,no envelope 1.8%).CONCLUSION CIED infection rates for biologic and non-biologic antibacterial envelopes are similar.Antibacterial envelopes may benefit patients who are higher risk for infection,however additional studies are warranted to confirm this.
Key Words:Cardiovascular implantable electronic device envelope;Defibrillator;Extracellular matrix;Implantable cardioverter-defibrillator;Infection;Pacemaker
INTRODUCTION
Expanding indications for cardiovascular implantable electronic devices (CIEDs) have increased the number of these devices that are implanted[1],but considering the common comorbidities seen in this patient population,complications such as infection are also increasing[2-4].Reported infection rates of de novo CIED implantation range between 0.7%-4.6%,and can be as high as 7% for re-operations[5].Thus,a better understanding of patient risk factors and available prophylactic techniques could potentially lower the risk of infection in this population[5-8].CIED envelopes are intended to securely hold pacemakers or defibrillators when implanted in the body,and antibacterial CIED envelopes additionally provide short-term local antibiotic delivery which can reduce the risk of infection at the device implant site[9].Available antibacterial CIED envelopes are either fabricated from biologic material (extracellular matrix hydrated with antibiotics by physician choice) or from non-biologic material (synthetic mesh coated with antibiotics by the manufacturer).The biologic envelope (CanGaroo®,Aziyo Biologics,Inc.,Roswell,GA,United States) is made of decellularized extracellular matrix derived from porcine intestinal submucosa (SIS-ECM) which is rehydrated in solution for 1-2 min prior to use,whereas the non-biologic envelope (TYRX™,Medtronic PLC,Mounds View,MN,United States) is made from an absorbable synthetic substrate mesh coated with a bioresorbable polymer containing the drug substances rifampin and minocycline.Both envelopes have been reported to release antibiotics over a period of seven days in separate studies[10-13].
Although both envelopes have similar indications and antibiotic elution abilities,the material each envelope is created from may affect the biologic response upon implantation into the patient.Synthetic (non-biologic) absorbable and non-absorbable materials have been reported to initiate a strong foreign body reaction,resulting in chronic inflammation leading to hypovascular fibrotic tissue surrounding the implanted material[14-18],which a previously-marketed non-absorbable synthetic envelope leveraged to stabilize the electronic device upon implantation[19].Conversely,ECM (the material that the biologic envelope is made from) has been shown to promote constructive remodeling and healthy tissue restoration[20-23].Both biologic and non-biologic envelopes have been reported to support clinical infection prevention strategies[12,24-26].
This is an analysis of a retrospective,real-world study which assessed the risk profiles and clinical outcomes of patients who underwent a CIED procedure and received an antibacterial envelope (biologic or non-biologic),or no envelope (CARE Plus,NCT04351269).To our best knowledge,this study contains the first reporting of biologic and non-biologic antibacterial envelopes reported together in the clinical setting.
MATERIALS AND METHODS
Records of consecutive patients undergoing CIED procedures from a single center performed by a single physician between March 2017 and December 2019 were retrospectively reviewed for up to 12 mo of follow-up.The study protocol was reviewed and approved by an independent internal review board (IRB) [WIRB-Copernicus Group®(WCG)] prior to the chart review.A waiver of informed consent and HIPAA was obtained due to the retrospective nature of the study.
The study aimed to determine risk profiles and clinical outcomes of patients who were undergoing a CIED procedure and received either no envelope,a biologic envelope (CanGaroo®) hydrated by the implanting physician for 1 - 2 minutes with a vancomycin and gentamicin solution before implantation,or a non-biologic envelope (TYRX™) coated by the manufacturer with a bioresorbable polymer containing the drug substances rifampin and minocycline.The implanting physician made all decisions regarding device type,which envelope and envelope size was used,and biologic envelope hydration solution (if one was used).Aside from the pre-hydration of the biologic envelope,the implanting technique of both the biologic and non-biologic envelope was similar.The no envelope group’s CIED implantation procedure was identical to the envelope CIED implantation procedure,just without the use of an envelope.The pre- and post-operative protocol was the same for all 3 groups.
Information was extracted in detail from medical records,including medical history,infection risk factors,surgical details,and adverse events from the initial procedural visit out to 12 mo post-op.Infection risk factors were defined by previous literature[4,27,28] which identified elements that were significantly associated with increased risk for CIED infection,including renal insufficiency,diabetes,obesity,peripheral vascular disease,chronic obstructive pulmonary disease,congestive heart failure,malignancy,coronary artery disease,hypertension,chronic steroid use,oral systemic anticoagulants,malnutrition,smoking,the presence of two or more leads,pocket re-entry within 2 wk of the initial implant,prior device infection,and reoperative procedure.The number of risk factors was counted for each patient to examine the relative levels of infection risk between patient groups.Infection risk was categorized for each patient as lower risk (0-1 infection risk factors) or higher risk (2 or more risk factors),based on the quantity of established clinical risk factors present in each patient from above.An independent,biomedical statistician performed analyses between the 3 groups (CanGaroo,Tyrx,and no envelope),and also between the no envelope and any envelope groups by using means with standard deviations for continuous variables and counts with percentages for categorical variables.Continuous variables were checked for normality.Fisher’s exact tests were used when ≥ 1 expected cell counts were < 5,and Pearson chi-square tests were used for categorical variable comparisons when cell counts were ≥ 5.Statistical significance was set to aP< 0.05.SPSS version 26 (IBM,Armonk,NY,United States) was used for statistical analyses.
RESULTS
Among 248 enrolled patients who underwent CIED procedures,191 (77%) received an envelope.These included 89 (46.6%) biologic and 102 (53.4%) non-biologic envelopes (Table 1).
Surgical procedure details
Patients who received high-powered devices,including implantable cardioverter defibrillators (ICDs) and cardiac resynchronization therapy (CRT) devices,were more likely to receive an envelope (P=0.001) (Table 1).Patients undergoing reoperative procedures (generator changes,upgrades,other reoperative procedures such as lead or pocket revisions) received an envelope significantly more often than no envelope (100.0%vs0.0%,P< 0.001) and tended to be more likely to receive a biologic than a non-biologic envelope (n=56,62.9%vs n=34,33.3%).Those with de novo implants tended to be more likely to receive a non-biologic envelope (n=68,66.6%) than a biologic envelope (n=33,37.1%).
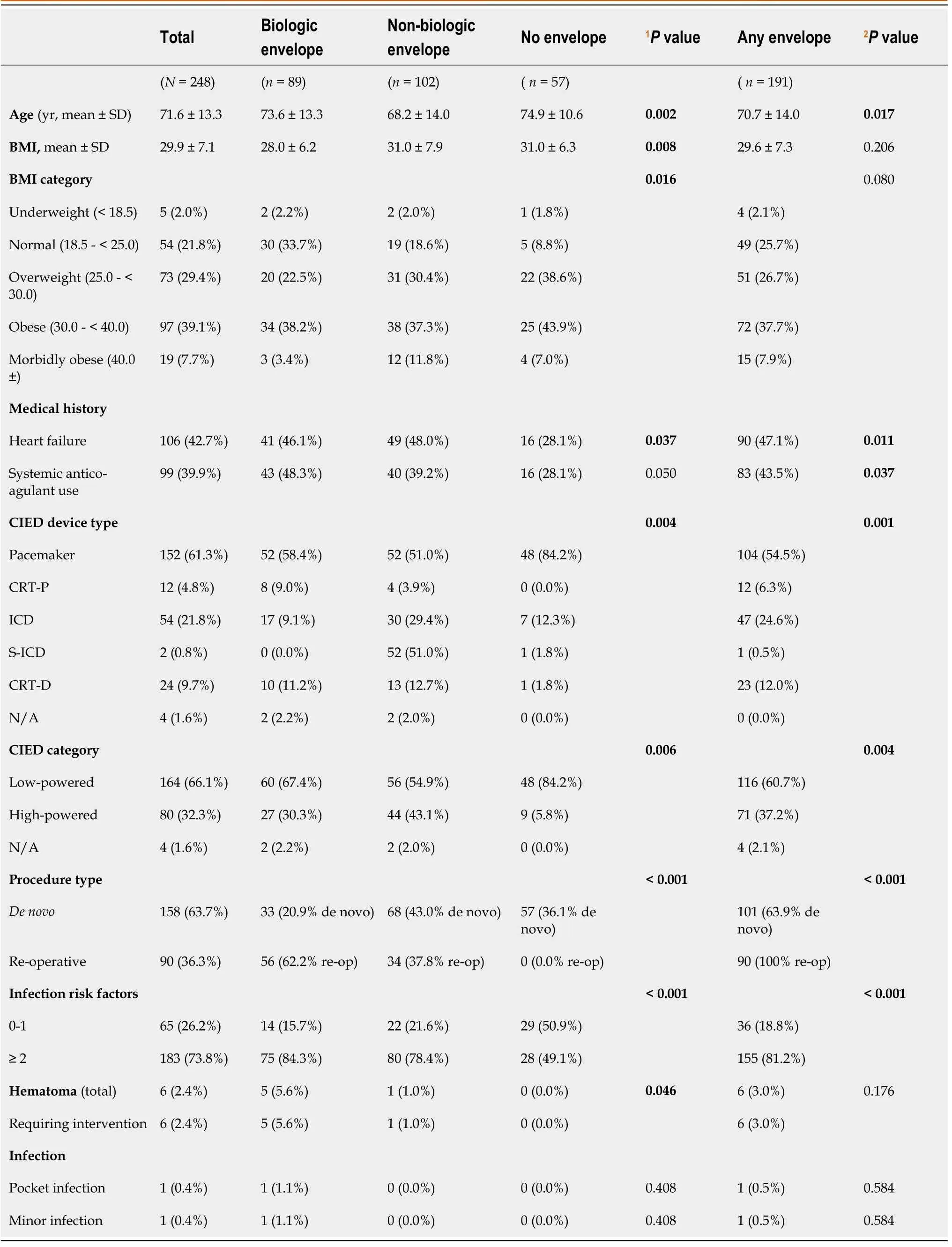
Table 1 Comparison across cohorts
Clinical characteristics and infection risk factors
Patients who received any envelope were younger on average (70.7 ± 14.0vs74.9 ± 10.6 years,P=0.017) and had higher rates of comorbid risk factors such as heart failure (47.1%vs28.1%,P=0.011) and systemic anticoagulation (43.5%vs28.1%,P=0.037) than those who did not receive an envelope (Table 1).Patients with biologic envelopes tended to be somewhat older (mean 73.6 ± 13.3vs68.2 ± 14.0 years) and less overweight (22.5%vs30.4%) than those with non-biologic envelopes.Differences in systemic anticoagulation among the 3 groups were statistically significant (biologic 48.3%,non-biologic 39.2%,no envelope 28.1%,P=0.050).Patients who received any envelope had a significantly higher number of infection risk factors (≥ 2) than those with no envelope (81.2%vs49.1%,P< 0.001),and biologic envelopes tended to be used more frequently for these higher risk patients (84.3%vs78.4%).
Infection outcomes
Pocket infection rates were low (envelope 0.5%,no envelope 0.0%),with no significant difference between biologic and non-biologic envelopes (Table 1).Among the patients who received an envelope,one (0.5%) developed a major CIED infection (pocket infection),and one (0.5%) developed a minor CIED infection (superficial surgical site infection).However,the incidence of major or minor infection did not significantly differ between the 3 cohorts.
Other adverse events
Pocket hematoma (requiring surgical intervention) developed in 6 patients (2.4%):5 patients (5.6%) with biologic envelopes,1 patient (1.0%) with a non-biologic envelope,and 0 patients without an envelope (0.0%) (P=0.046) (Table 1).However,there was no significant difference in hematoma between any envelope (3.0%) and no envelope (0.0%).There were no reported hematoma that led to infections in this study.Other adverse events included 3 Lead dislodgements (1 in the biologic group,2 in the nonbiologic group),1 Lead revision (non-biologic group),1 hemothorax (non-biologic group),and 1 site drainage (biologic group) in the envelope cohorts and erythema/fever in 1 patient in the no envelope cohort.Rates of adverse events other than pocket hematoma did not significantly differ among the 3 cohorts.
DISCUSSION
This retrospective study examined clinical profiles and outcomes of patients receiving CIEDs implanted with antibacterial biological envelopes hydrated with gentamicin and vancomycin (biologic envelopes),CIEDs implanted with synthetic (non-biologic) antibacterial envelopes,and CIEDs with no envelope.Non-biologic antibacterial envelopes have been previously shown in a large,randomized study to reduce infection risk in patients who are at increased risk for CIED infection[12].To the best of our knowledge,this is the first reporting of clinical outcomes from using either biologic or non-biologic antibacterial envelopes,or no envelope within the same dataset.
Patient selection for envelope use
Patient selection by the implanting physician is reflected in the study findings.Envelopes were selected significantly more often for younger patients,patients undergoing device replacement procedures,highpowered device implantations,those on systemic anticoagulation,patients with heart failure,and patients with 2 or more risk factors for CIED infection.Treatment preferences can be observed by envelope usage for at-risk patients who may benefit most from the local delivery of antibiotics to their CIED implant site.Interestingly,there was no statistical difference in observed infection rates between the envelope and no envelope groups,even though the envelope group contained significantly more patients with ≥ 2 infection risk factors.Our results and those of other studies[9,12,24,26],support that the utilization of antibacterial envelopes (biologic or non-biologic) may reduce the potential risk burden of patients with multiple concurrent infection risk factors who are undergoing CIED procedures.However,further studies are needed to determine if there are specific patient types that could benefit the most from receiving an antibacterial envelope.
Complications
There were no significant differences in individual adverse event rates between groups,except that more patients with biologic envelopes were reported to have hematoma requiring intervention compared to the other two groups.However,this observation may have been due to the greater use of systemic anticoagulation and reoperative procedures in the biologic envelope group,which have both been shown to be risk factors for hematoma formation in previous studies[29,30].In fact,a recent analysis of hematoma from the 6800 patients included in the WRAP-IT trial reported a hematoma occurrence of 2.2%,which was significantly associated with an increased risk of infection for the no envelope (control) group and a significantly lower risk of major infection in the non-biologic envelope group (2.5%vs13.1%,P=0.03)[31].No hematoma in our dataset led to subsequent infection,which further supports a potential benefit from using antibacterial envelopes (biologic or non-biologic) to reduce the risk of hematoma manifesting to CIED implant site infection.
Infections at the CIED implantation site have serious morbidity,mortality,and economic consequences[1,32].The use of antibacterial envelopes may reduce the risk of infection and could potentially reduce these serious complications and healthcare costs[33].In our dataset,antibacterial envelopes were used significantly more often to treat patients with multiple comorbid risk factors,and biologic envelopes tended to be used more often in higher risk patients than non-biologic envelopes.We observed a 0.4% overall rate of pocket infection,which is lower than previously-reported studies of 0.7% to 4.6% for de novo implantations and up to 7% for reoperative procedures[5-8].No significant difference was found in major CIED (pocket) infection rates between the 3 groups.A previous study reported that infection rate can differ depending upon various patient- and procedure-related circumstances (such as device type,procedure type,antibacterial envelope use,or perioperative antibiotics)[7],thus along with the major infection rates reported for high risk patients in the WRAP-IT (0.7%)[12] and PADIT (0.7%)[34] studies,the low pocket infection rate observed in our preliminary results (0.4%) supports that high infection risk factors can be countered with infection prophylaxis techniques such as the use of antibacterial envelopes.
Antibacterial CIED envelope types
There are currently two commercially available CIED envelopes in the United States.The biologic envelope (CanGaroo®) is manufactured from two sheets of 4-ply SIS-ECM material which can be hydrated by the implanting physician with an antibiotic solution prior to implantation,and the nonbiologic envelope (TYRX™) is fabricated from an absorbable synthetic substrate mesh coated with a bioresorbable polyarylate polymer containing the drug substances rifampin and minocycline.In separate studies,the release of antibiotics occurs similarly from both envelopes over a period of seven days[10-13].Both envelopes are intended to stabilize the CIED post-implantation,yet the host response to these different materials may vary.All biomaterials (biologic and non-biologic) interact with the body upon implantation,and certain characteristics of these materials can influence the host response to the implant[35,36].
Extensive studies have shown that implanted biologic materials (such as non-crosslinked decellularized SIS-ECM) stimulate the production of site appropriate,functional tissue (termed “constructive remodeling”[37])[20-23,36].The ability to elicit a remodeling response post-implantation is due to the natural degradation of the implanted ECM by proteases which release intrinsic bioactive peptides and growth factors such as FGF-2 and VEGFin situ[22,38-40].When implanted,for example into a CIED pocket,these bioavailable signaling molecules can influence the healing milieu surrounding the implant site by directing cellular activities such as differentiation,chemotaxis,adhesion,and angiogenesis[22,41-43].Non-biologic materials do not contain these bioactive components.
Limitations
Limitations to this study include non-randomization of patients to the treatment groups,a limited period of follow up,and all implantations performed by a single physician at one institution.The choice of patients receiving an envelope (and which envelope was used) creates selection bias observed in the differing patient factors between groups.However,the intent of this report was to evaluate and define physician practice patterns instead of assessing superiority between the three therapies.Longer-term (> 1 year) follow up may have captured late adverse events,which cannot be ruled out in this study.
CONCLUSION
In this real-world study,patients at higher risk for CIED infection received antibacterial envelopes and lower infection risk patients did not receive envelopes,yet the CIED pocket infection rate did not differ between groups.There was also no significant difference in observed pocket infection rates for patients receiving biologicvsnon-biologic antibacterial envelopes.These findings support that use of an antibacterial envelope may benefit patients who are at higher risk for infection,however further work will continue to refine patient selection and clinical decision-making for optimal utilization of antibacterial envelopes during CIED implantation.
ARTICLE HIGHLIGHTS
Research background
An increase in cardiac implantable electronic device (CIED) implantation has led to an increase in observed complication rates,including infection.Antibacterial CIED envelopes have been shown to reduce the risk of infection complications,even in high-risk patient groups.There are currently two different CIED envelopes in clinical use which differ in the material from which they are made.
Research motivation
There is a paucity of data describing real-world physician practice patterns when using antibacterial CIED envelopes.Understanding clinical rationale and outcomes from the use of this prophylactic therapy could improve future patient outcomes.
Research objectives
Patient risk profiles and outcomes were compared from patients undergoing CIED procedures receiving either no envelope,or one of two antibacterial envelopes.
Research methods
In this retrospective analysis,the records of consecutive CIED procedure patients were reviewed at one center through a follow-up time of 12 mo.
Research results
Patients who were selected to receive an antibacterial CIED envelope were at significantly higher risk for infection than patients who did not receive an envelope (81.2% vs 49.1%,P < 0.001).Among the infection risks,envelope patients were undergoing more reoperative procedures (47.1% vs 0.0%,P <0.001) and received more high-powered devices (37.2% vs 5.8%,P=0.004) than patients who received no envelope.There was a propensity for the physician choosing a biologic envelope in patients who were higher risk than non-biologic patients (84.3% vs 78.4%),and those that were undergoing reoperative procedures (62.9% vs 33.3%).The rate of pocket infection was low (any envelope 0.5% vs no envelope 0.0%),with no significant difference between the two envelope groups.
Research conclusions
There is an apparent benefit for using antibacterial envelopes in patients who are at higher risk of implant site infection.When using antibacterial envelopes,there was no significant difference in infection rate for biologic and non-biologic envelopes.
Research perspectives
Future studies should further explore patient and procedural factors that play a role in antibacterial envelope usage for specific patient types to further improve patient outcomes.
ACKNOWLEDGEMENTS
The authors acknowledge Sherrie Webb for writing and editing assistance and Kristina Chapple,PhD for her biostatistical analysis expertise.
FOOTNOTES
Author contributions:Woodard D led the conception,design,data collection and analysis,and drafting of the manuscript;Nilsson K and Kim G contributed their expertise to the analysis/interpretation of data and editing of the manuscript;and all authors accept accountability for the accuracy of this work,and drafted,revised,and approved the final version of the manuscript to be published within this journal.
Supported byAziyo Biologics,Inc.
Institutional review board statement:This study was reviewed and approved by the Ethics Committee of the WIRBCopernicus Group®.
Informed consent statement:A waiver of informed consent and HIPAA due to the retrospective nature of this study was obtained.This study was conducted in accordance with the ethical principles in the Declaration of Helsinki and conducted according to United States and international standards of Good Clinical Practice in accordance with applicable Federal regulations,International Council for Harmonization guidelines,and institutional research policies and procedures.
Conflict-of-interest statement:Woodard D is a consultant for Aziyo Biologics,Inc.The other authors have no relevant financial relationships to disclose.
Data sharing statement:The dataset supporting the conclusions of this article is available upon reasonable request to the corresponding author.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See:https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:United States
ORCID number:David A Woodard 0000-0001-7046-911X;Grace Kim 0000-0002-8769-8864;Kent R Nilsson 0000-0001-8000-9686.
Corresponding Author's Membership in Professional Societies:American Heart Association.
S-Editor:Ma YJ
L-Editor:A
P-Editor:Ma YJ
 World Journal of Cardiology2022年3期
World Journal of Cardiology2022年3期
- World Journal of Cardiology的其它文章
- Fundamentals of percutaneous coronary bifurcation interventions
- Arrhythmic risk stratification in ischemic,non-ischemic and hypertrophic cardiomyopathy:A two-step multifactorial,electrophysiology study inclusive approach
- Climatic influences on cardiovascular diseases
- Predictors of persistence of functional mitral regurgitation after cardiac resynchronization therapy:Review of literature
