Offspring Sex Is Not Determined by Gestation Temperature in a Viviparous Lizard (Eremias multiocellata) from the Desert Steppe of Inner Mongolia
Qiyu LI ,Tingting ZOU ,Wenqi TANG ,Yang WANG ,Weiguo DU and Xifeng WANG*
1 Key Laboratory of Animal Ecology and Conservation Biology,Institute of Zoology,Chinese Academy of Sciences,Beijing 100101,China
2 University of Chinese Academy of Sciences,Beijing 100049,China
3 Key Laboratory of Animal Physiology,Biochemistry and Molecular Biology of Hebei Province,College of Life Sciences,Hebei Normal University,Shijiazhuang 050024,Hebei,China
Abstract Sex-determining systems show a striking diversity not only among species,but also among populations.In reptiles,sex-determination is a continuum,from temperature-dependent sex determination (TSD) to genetic sex determination (GSD).The multi-ocellated racerunner (Eremias multiocellata) is reported to be a cryptic ZZ/ZW chromosomal TSD species,with male-biased sex ratios at high temperatures in two Gansu populations.However,the generality of the sex-determining pattern in different populations of this species remains unclear.To investigate the mode of sex determination in a population of E.multiocellata from the desert steppe of Inner Mongolia,we first identified sex chromosomes via comparative genomic hybridization (CGH).We then conducted a thermal manipulation experiment to determine the effect of gestation temperature on offspring sex ratios.From the CGH studies we found that lizards from the Inner Mongolia population possessed ZZ/ZW sex chromosomes.However,our thermal manipulation experiment showed that gestation temperature did not affect the sex ratio of neonates in this population.In combination,these results rule out TSD in the Inner Mongolia population of E.multiocellata,and suggest that there is widespread geographic variation in the sexdetermining system of this species.
Keywords geographic variation,maternal thermal environment,reptile,sex chromosome,sex determination,sex ratio,sex reversal
1.Introduction
Vertebrates exhibit two broad categories of sex-determining systems:genotypic sex determination (GSD) where specific genetic elements direct the development of the gonads,and environmental sex determination (ESD) where external cues,such as temperature (temperature-dependent sex determination,TSD),determine the sex of an individual.All mammals and birds exhibit GSD.A dominant sex gene on the Y chromosome region (Sry) initiates male development in mammals (Kashimada and Koopman,2010;Koopmanet al.,1990).In birds,the dosage effects of double sex and mab-3 related transcription factor 1 (Dmrt1) on the Z chromosome control sex determination (Ellegren,2009;Smithet al.,2007;Smithet al.,2009).In contrast,sex determination systems are highly variable among ectotherms.Both ZZ/ZW and XX/XY sex-chromosome systems occur in all these taxa including fish,amphibians and reptiles,as well as TSD in certain reptiles and fish (Bachtroget al.,2014;Capel,2017;Lambertet al.,2019;Nemesháziet al.,2020;Ospina-Alvarez and Piferrer,2008;Janzen and Krenz,2004).For example,reptiles have multiple sex-determining mechanisms.In all snakes,and some lizards and turtles (Ezazet al.,2006a;Ezazet al.,2006b;Janzen and Krenz,2004;Schwanzet al.,2013) sex determination involves GSD (with ZZ/ZW or XX/XY sex chromosomes),whereas in all crocodiles,the tuatara,many turtles and several lizard species (Rhen and Schroeder,2010;Shineet al.,2007) nest temperature (in oviparious species) or gestation temperature (in viviparous species) is responsible for determining gonadal sex of offspring.
Environmentally influenced sex determination has been found in some ectothermic vertebrates with sex chromosomes,in which genes and environment interact to determine the phenotype of the sex (Holleleyet al.,2015).Temperature can override the GSD effect and result in sex reversal in some fishes (Baroiller and D’Cotta,2016;Ospina-Alvarez and Piferrer,2008),amphibians (Alhoet al.,2010;Bachtroget al.,2014;Flament,2016;Lambertet al.,2019;Nemesháziet al.,2020) and reptiles (Holleleyet al.,2015;Quinnet al.,2007;Radderet al.,2008;Sarreet al.,2004;Shineet al.,2002).These species have sex chromosomes but exhibit thermal sensitivity in the development of gonadogenesis,representing mixed sex-determination systems.For example,high incubation temperatures reverse genotypic males (ZZ) to phenotypic females in the Australian central bearded dragon lizard (Pogona vitticeps),which has GSD with female heterogamety (ZW) (Holleleyet al.,2015;Quinnet al.,2007).Analogously,low temperatures override chromosomal sex to generate XX male offspring in a montane scincid lizard (Bassiana duperreyi) from south-eastern Australia,which has male heterogamety (XY) (Radderet al.,2008;Shineet al.,2002).Further work on the interactive effects of sex chromosomes and early developmental (embryonic or larval) temperatures on offspring sex ratios is needed to elucidate the sex-determining systems across a greater diversity of lineages in ectothermic vertebrates.
Interestingly,recent studies have demonstrated that sexdetermining mechanisms may also differ among populations within a species.For example,the association between phenotypic sex and the underlying genetic factor (linkage group 2) was perfect in a northern Swedish population,but weak and variable among families in a southern one in the common frog (Rana temporaria) (Rodrigueset al.,2016).Three Australian lizards,the snow skink (Niveoscincus ocellatus) (XX/XY system),the bearded dragon (ZZ/ZW system) and another skink (Bassiana duperreyi) (XX/XY system),are GSD species possessing a mixed sex determination system with temperatureinduced sex reversal in some populations (Castelliet al.,2020a;Cunninghamet al.,2017;Dissanayakeet al.,2021;Hillet al.,2018;Penet al.,2010).In the snow skink,variation in the magnitude of between-year temperature fluctuations might lead to natural selection on sex determination across altitudes (Cunninghamet al.,2017;Penet al.,2010).Similarly,climatic effects on sex determination also occur in the Atlantic silverside,Menidia menidia,which exhibits an exceptionally high level of clinal variation in sex determination across its geographic range,with TSD in southern populations,GSD in northern populations,and mixed systems in between (Duffyet al.,2015).However,whether such intra-specific divergence in sex-determining systems exists in other lineages remains largely unknown.
The multi-ocellated racerunner (Eremias multiocellata) is a small viviparous lizard (about 65 mm snout-vent length SVL) with an extensive distribution ranging from Mongolia,Kazakhstan,Kyrgyzstan to Xinjiang,Gansu,and Inner Mongolia of China,with the highest known population found at an altitude of 2900 m above sea level (asl) (Zhao,1999).The chromosomal system of this species was identified by traditional Giemsa staining as 2n=38,with no obvious sex chromosomes.However,a recent study detected female-specific amplified signals in two Gansu populations by using comparative genomic hybridization (CGH),suggesting this species possesses a female-heterogametic sex chromosome system (ZZ/ZW) (Wanget al.,2015).Interestingly,gestation temperature has been shown to affect offspring sex ratio in two Gansu populations at elevations of 1400 m and 2900 m asl,with male-biased sex ratios at 35°C and female-biased sex ratios at 25°C (Tanget al.,2012;Zhanget al.,2010).In addition,compared to the lowelevation population from a relatively warmer climate,the high-elevation population ofE.multiocellataexhibited a less skewed offspring sex ratio (Tanget al.,2012).In the Inner Mongolia population ofE.multiocellataat a lower elevation of 1036 m asl,the edge of the species’ altitudinal distribution range,extreme environments (i.e.,low precipitation and extreme high temperatures) during reproductive season affect offspring sex (Wanget al.,2016).The effect of temperature on offspring sex varied between years with different precipitation levels (Wanget al.,2016),so a controlled experiment is needed to test if high temperature itself (without accompanying aridity) affects sex determination in the Inner Mongolia population ofE.multiocellata.Therefore,here we used CGH to identify the sex chromosome system,and conducted temperature manipulation experiments to investigate the effects of materal thermal (gestation) environment on offspring sex.
2.Materials and Methods
2.1.Study site and speciesTheE.multiocellataused in this study were collected from Shierliancheng countryside (40°12'17"N,111°07'43"E;elevation 1036 m),Jungar Banner,in the desert steppe region of Inner Mongolia,China.Shierliancheng is characteristic of a cold semi-arid climate with an average annual temperature of 6-7°C and annual precipitation of 300-380 mm (http://www.nmic.gov.cn).This lacertid lizard is a viviparous species (adult SVL 54.0-67.3 mm,BM 4.7-8.2 g) that generally occupies arid or semi-arid regions.FemaleE.multiocellatahave a gestation period of two months and produce a single litter of 2-5 offspring between July and August (Zhao,1999).The selected body temperature and critical thermal maximum ofE.multiocellatain Inner Mongolia population are (35.2±0.2)°C and (45.1±0.1)°C,respectively (Liet al.,2017).
2.2.Identification of sex chromosomesThree male and three female adultE.multiocellatawere used for sex chromosome identification,using the established protocol of CGH (Martinezet al.,2008).we prepared both female and male metaphase spreads of mitotic chromosomes from peripheral blood leukocytes following the published method of Ezazet al(2005).Approximately 100 μL blood was used to set up 2 mL culture in RPMI1640 (Gibco) supplemented with 10% fetal bovine serum,100 units/mL penicillin,0.1 mg/mL streptomycin,40 μg/mL phytohaemagglutinin M (PHA M).After incubated at 30°C in 5% CO2incubators for 72 h,we replaced new medium.Eight and six hours before harvesting,35 mg/mL 5-bromo-2-deoxyuridine (BrdU;Sigma) and 75 ng/mL colcemid (Roche) were added to each culture.Metaphases were fixed in 3:1 methanol-acetic acid.Cell suspension was dropped onto wet cold slides prechilled at 4°C,and slides were dried on a hotplate at 60°C and saved at -80°C.
Total genomic DNA (gDNA) was extracted from whole blood following the phenol-chloroform method,and the DNA probe was then labeled with SpectrumGreen-dUTP and SpectrumRed-dUTP (Enzo Life Science,Farmingdale,NY).Metaphase chromosome slides dried for 2 h at 60°C and denatured for 2 min at 60°C in 70% formamide and 2×SSC,then dehydrated through a 70%,80% and 100% ethanol series for 1 min each,and air-dried at room temperature until hybridization.
The probe mixture containing 20 mg glycogen,500 ng female probe,and 500 ng male probewere co-precipitated with 5 mg of boiledE.macquariifemale DNA.Following an overnight co-precipitation at -20°C,the probe mix was centrifuged at 15 000 g at 4°C for 15 min.The supernatant was discarded and the probe DNA pellet was resuspended in 40 μL of 37°C pre-warmed hybridization buffer and resuspended for at least 30 min at 37°C.The hybridization mixture was denatured at 70°C for 8 min,immediately placed on ice for 2 min,and 16 μL of the probe mixture was placed as a single drop per slide.For the purposes of this experiment we assumed thatE.multiocellatahave a female ZZ/ZW chromosome system,and therefore,non-labeled male gDNA was used to compete with DNA probes;in the reciprocal experiment,female gDNA was the competitor.After a humid hybridization chamber at 37°C for 3 days,slides were washed at 55°C in 0.4×SSC,0.3% Tween-20 for 2 min,followed by a second wash in 2×SSC,0.1% Tween-20 for 1 min at room temperature.Slides were immersed into a DAPI solution with a concentration of 1 μg/mL for 30 s.This procedure resulted in the respective hybridization of the competing DNA probes,with male and female metaphase spreads.Finally,we conducted fluorescene microscopy (Zeiss) to detect if there was any sex-specific signal in the spreads.
2.3.Thermal treatmentsA total of 60 gravid female and 18 adult maleE.multiocellatawere captured and brought back to the laboratory in Beijing in early May 2017.This timing of capture allowed us to start treating females prior to the temperaturesensitive period (TSP) of offspring sex determination,because the TSP is in the middle third of embryonic development in reptiles (Wibbelset al.,1991).After recording snout-vent length (SVL,±0.01 mm) and body mass (BM,±0.001 g),lizards were evenly assigned into three temperature treatments:low (29°C),moderate (35°C),and high (38°C).Within each treatment,lizards were randomly distributed into three terraria (55.6 cm×42.5 cm×34.6 cm,length×width×height) that were placed in temperature-controlled incubators,with each terrarium containing 6-7 females and 2 males.Incubators were set at 23°C for the low (29°C) and moderate (35°C) temperature treatments,and at 29°C for the high temperature treatment (38°C).We then used electronic heating pads with temperature control devices (YWK-F,Shanghai Medical Instruments,China) attached to each terrarium to increase the ambient temperature of terraria to 29°C,35°C,and 38°C for the low,moderate,and high temperature treatments respectively,from 08:00 to 18:00 h each day.Data loggers (iButton,DS1921;Maxim Integrated Products Ltd.,USA) were used to monitor the thermal environment within each terrarium for the duration of the study.Additionally,a 12:12 h (light:dark) photoperiod was provided in each terrarium from 07:00 to 19:00 h,using full UV-spectrum lamps.Each terrarium contained a sand substrate (5 cm deep) and several shelter items.Food (mealworms and crickets dusted with extra vitamins and minerals) and water were providedad libitum.We recorded body temperatures of active female lizards four times on May 25th,June 1st,June 8thand June 15thby inserting a probe of UT325 electronic thermal meter into cloacae.
2.4.Female reproduction and neonate traitsFemales in the experimental terraria were palpated for gravidity every three days.Individual females containing soft and oval eggs (i.e.,late developmental-stage embryos) were then transferred into small boxes with the same temperature as their original thermal treatments to give birth.Then,we checked these boxes every day for neonates.Once found,neonates were measured (SVL;±0.01 mm) and weighed (BM;±0.001 g).We calculated gestation length as the days between female capture and the parturition.
We obtained a total of 104 neonates,of which 22,40 and 42 neonates produced by females from the low,moderate and high temperature treatment,respectively.In addition,10 out of 22 neonates produced by gravid females from the low temperature treatment were stillborn.We identified the sex of these 104 neonates by the presence/absence of hemipenes.A subset of those neonates (n=34) did not survive after birth and were used to validate our morphological sex identification technique,by inspecting the gonads.Intact gonads were dissected out,snap-frozen in liquid nitrogen and embedded in an optimal cutting temperature compound (OCT) at -20°C to -30°C.Frozen gonads were cut into 6 μm slices with the microtome portion of the cryostat.Each slice was then placed on a glass slide and stained with hematoxylin and eosin (H&E stain).Sex was identified under an optical microscope based on standard testis and ovarian morphology (Figure 1).The sex of all of neonates identified by gonadal inspection was consistent with our morphological sex-identification technique (everting hemipenes;Figure 1);subsequently,neonate sex was identified using presence/absence of hemipenes.
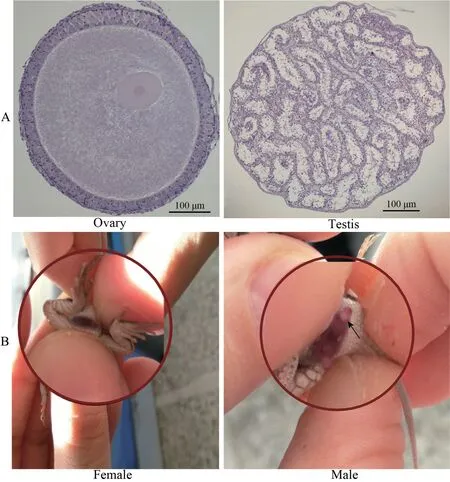
Figure 1 Methods of sex identification for Eremias multicellata used in this study.(A) Microscopy of frozen sections of gonads from E.multicellata.(B) Manual eversion of hemipenes.Sex of E.multicellata was determined by presence/absence of hemipenes.Arrow points to the hemipenes.
2.5.Statistical analysisWe used linear models (LM;“stats”package) (R Core Team.,2019) to detect the differences of maternal SVL and BM,and the ambient temperatures inside terraria between thermal treatments.We used the generalized linear models (GLM;“stats”package) (R Core Team.,2019) to detect the effects of thermal treatments on female reproductive success using a binomial distribution with a logit link.Generalized least squares models (GLS;“nlme”package) (Pinheiroet al.,2017) were used to detect the effects of thermal treatments on gestation period and litter mass with maternal SVL as a covariate,because within-group variance differed between treatment groups,we allowed for treatment-dependent variances in the GLS model by the varIdent function.The Kruskal-Wallis test was used to detect the effects of thermal treatments on litter size (the total number of offspring born by a female including stillborn).We used linear mixed-effects models (LME“nlme”package) (Pinheiroet al.,2017) to test the effects of thermal treatments on neonate SVL and BM with maternal ID as a random factor.The same model LME was used to detect the effects of thermal treatments on body temperature of theE.multiocellata.In order to detect the effects of thermal treatments on neonate survival,total neonate (including both surviving and dead individuals) sex ratio,sex ratio of neonates that were dead after birth,and sex ratio of neonates that survived after birth,we used generalized linear mixed effect models (GLMMs;“lme4”package) (Bateset al.,2015) with a binomial distribution and a logit link,and maternal ID as a random factor.We compared each model including thermal treatments with a model that only contained the random factor using the“anova”function to get an overall chi-squared andP-value for the thermal treatments effect.Posthoc tests for the analysis of litter mass and gestation period were performed by emmeans function with“tukey”method (“emmeans”package) (Lenth R.V.,2018) and we used pairwise Mann-Whitney tests to carry out post-hoc tests for significant Kruskal-Wallis test and corrected theP-values by the“fdr”method (“stats”package) (R Core Team.,2019).We checked the GLMMs with binomial distribution for the dispersion parameter by“dispersion_glmer”function (“blmeco”package) (Korner-Nievergeltet al.,2015),and the dispersion parameters of all binomial models are (close to) 1.We conducted all statistical analyses in R version 3.5.1 (R Core Team,2019).
3.Results
3.1.Sex chromosomes of E.multiocellataWe detected a significantly enhanced and specific hybridization signal in one of the microchromosomes in cells from all females,but not in males (Figure 2).This female-specific chromosome is therefore,by definition,a W chromosome,which means thatE.multiocellatafrom the Inner Mongolia population possess a ZZ/ZW sex chromosome system.
3.2.Female body temperature and reproductionThe ambient temperatures inside terraria were significantly different among the three temperature treatments (F2,616=126.59,P< 0.001;Figure 3A).Correspondingly,daily average body temperatures of gravid females from 08:00 to 18:00 were also significantly different among thermal treatments (F2,36=247.4,P< 0.001).Mean (±SE) body temperatures of gravid females were as follows:high temperature treatment (37.9±0.1°)C,moderate temperature treatment (34.6±0.1)°C,low temperature treatment (28.9±0.1)°C (Figure 3B).
Gestation period of gravid females was significantly affected by thermal treatment,decreasing as female gestation temperature increased (Table 1 and Figure 4).Female reproductive success (the proportion of females producing live offspring) was 65.0% (13/20) for females in the high temperature group,80.0% (16/20) in the moderate temperature group,and 50.0% (10/20) in the low temperature treatment (Figure 4).This difference in reproductive success among temperature treatments was not statistically significant (Table 1).Thermal treatment significantly affected litter mass and litter size.Females from the high temperature treatment had greater litter mass and larger litter size than those from the low temperature treatment (Table 1 and Figure 4).
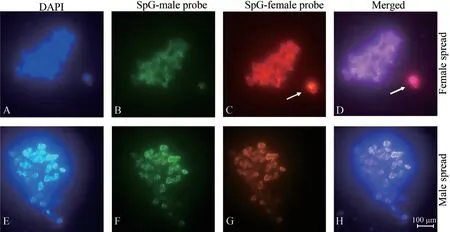
Figure 2 Results of comparative genomic hybridization (CGH) in female (upper) and male (lower) metaphases from Eremias multiocellata in our study.(A,E) DAPI stained metaphase chromosome spread;(B,F) Metaphase hybridized with SpectrumGreen-dUTP labeled male whole genomic DNA;(C,G) Metaphase hybridized with SpectrumRed-dUTP labeled female whole genomic DNA;(D,H) Merged images of metaphase hybridized with male and female whole genomic DNA.Scale bar indicates 100 μm.Arrow indicates W chromosome.
3.3.Neonate phenotypes and sex ratioNeither neonate SVL nor BM differed among thermal treatments (Table 1 and Figure 5).The proportion of neonates that did not survive after birth was higher in the high (78.6%) and low (75.0%) temperature treatment than the moderate (45.0%) temperature treatments (Figure 5).
Most notably,female gestation temperature did not affect neonate sex ratio (Table 1).The male ratio of neonates (54.5%,52.5% and 52.4% for the low,moderate and high temperature treatments,respectively,which was very close to 50:50 in all three groups,did not differ between the three temperature treatments (Figure 5 and Table 1).
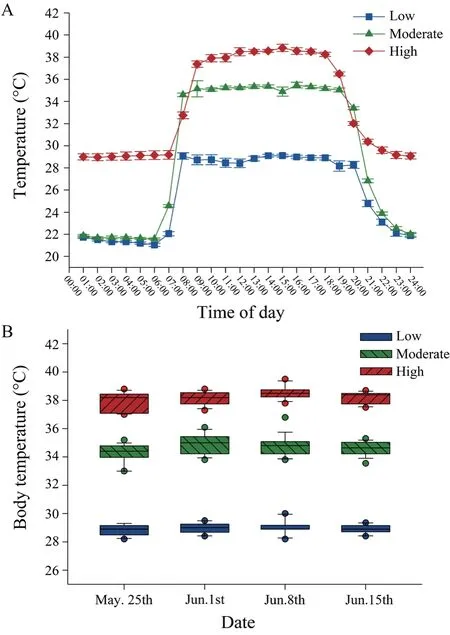
Figure 3 Experimental thermal treatments.(A) Ambient temperatures recorded in the terraria from the three temperature treatments,and (B) active body temperatures recorded during gestation in gravid female Eremias multicellata.Values are expressed as mean±SE.
4.Discussion
Our study revealed thatE.multiocellatafrom the Inner Mongolia population have a female ZZ/ZW chromosome system,and that female gestation temperature does not affect offspring sex ratio;this is inconsistent with the TSD pattern found in the two Gansu populations ofE.multiocellata(Tanget al.,2012;Zhanget al.,2010).This between-population discrepancy in sex-determining system adds another example of intraspecific variation in TSD and GSD patterns in reptiles,in addition to the phenomenon first reported in a viviparous Australian montane lizard,the snow skink,Niveoscincus ocellatus(Cunninghamet al.,2017;Penet al.,2010).Sex-determining systems are quite variable in reptiles,involving environmental and genetic sex determination,and only some reptiles have been identified to possess sex chromosome differentiation (Bachtroget al.,2014;Georgeset al.,2010;Martínez-Juárez and Moreno-Mendoza,2019).Traditional Giemsa staining methods did not elucidate sex chromosomes inE.multiocellatabecause the traditional way identifies chromosome pairs based on morphological differences (Wanget al.,2015).Instead,we used CGH,which distinguishes sex chromosomes through the non-homologous region,to reveal thatE.multiocellatafrom the Inner Mongolia population had ZZ/ZW sex chromosomes (Figure 2).The sex chromosomes of some reptiles are extremely cryptic,and can only be detected via such high-resolution cytogenetic techniques (Badenhorstet al.,2013;Ezazet al.,2005;Ezazet al.,2006b;Kawaiet al.,2007;Martinezet al.,2008).The Gansu populations ofE.multiocellataalso have ZZ/ZW sex chromosomes,but the sex was affected by temperature (Wanget al.,2015).These results suggest that theE.multiocellatapopulations we have studied all have sex chromosomes,but that the genetic effects on offspring sex can be overridden by extreme temperatures in the Gansu populations (Tanget al.,2012;Zhanget al.,2010).
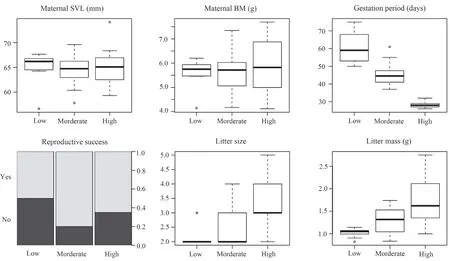
Figure 4 Differences between gestation temperature treatments in maternal snout-vent length (SVL),body mass (BM),gestation period length,reproductive success,litter size and litter mass in the multi-ocellated racerunner Eremias multicellata from Inner Mongolia.
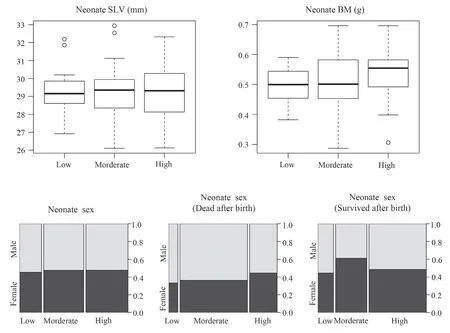
Figure 5 Neonate snout-vent length (SVL),body mass (BM),and sex ratio in different thermal treatments during maternal gestation of the multi-ocellated racerunner Eremias multicellata from Inner Mongolia.
The gestation temperatures we used in our experiment,which mimicked female body temperatures measured in the field during the reproductive season (Liet al.,2017),did not affect offspring sex ratio in the Inner Mongolia population ofE.multiocellata.However,a field warming experiment on this population showed that females produced maleskewed offspring under extremely high temperatures and dry environment,but not in response to extremely high temperatures alone (Wanget al.,2016).The sex-ratio bias found under warm and dry conditions could have been due to water deprivation or maternal manipulation of offspring sex ratios in response to nutrient deficiency caused by the extreme conditions.For example,water deprivation during pregnancy could induce a male-biased secondary sex ratio inAspic viperandZootoca vivipara(Dupouéet al.,2019).In addition,in an agamid lizard (Amphibolurus muricatus) from Australia,females fed a poor-quality diet produced highly malebiased sex ratios (Warner and Shine,2007).These instances indicate that female-to-male sex reversal may be triggered by stressors,perhaps due to the effects of glucocorticoid hormones and reactive oxygen species on sex determination (Castelliet al.,2020b).In addition,the ecological driving forcesof different sex determining systems among populations ofE.multiocellataremain unrevealed,but could be related to amongpopulation differences in climate conditions.Our study site at Inner Mongolian has lower ambient temperatures in terms of average,the lowest and highest temperatures and higher precipitation than two sites of Minqin and Tianzhu in Gansu Province (Table 1).The dry and warm condition in Minqin and Tianzhu might be a reason for the evolution of a mixed sexdetermining system with temperature-induced sex reversal in these populations.
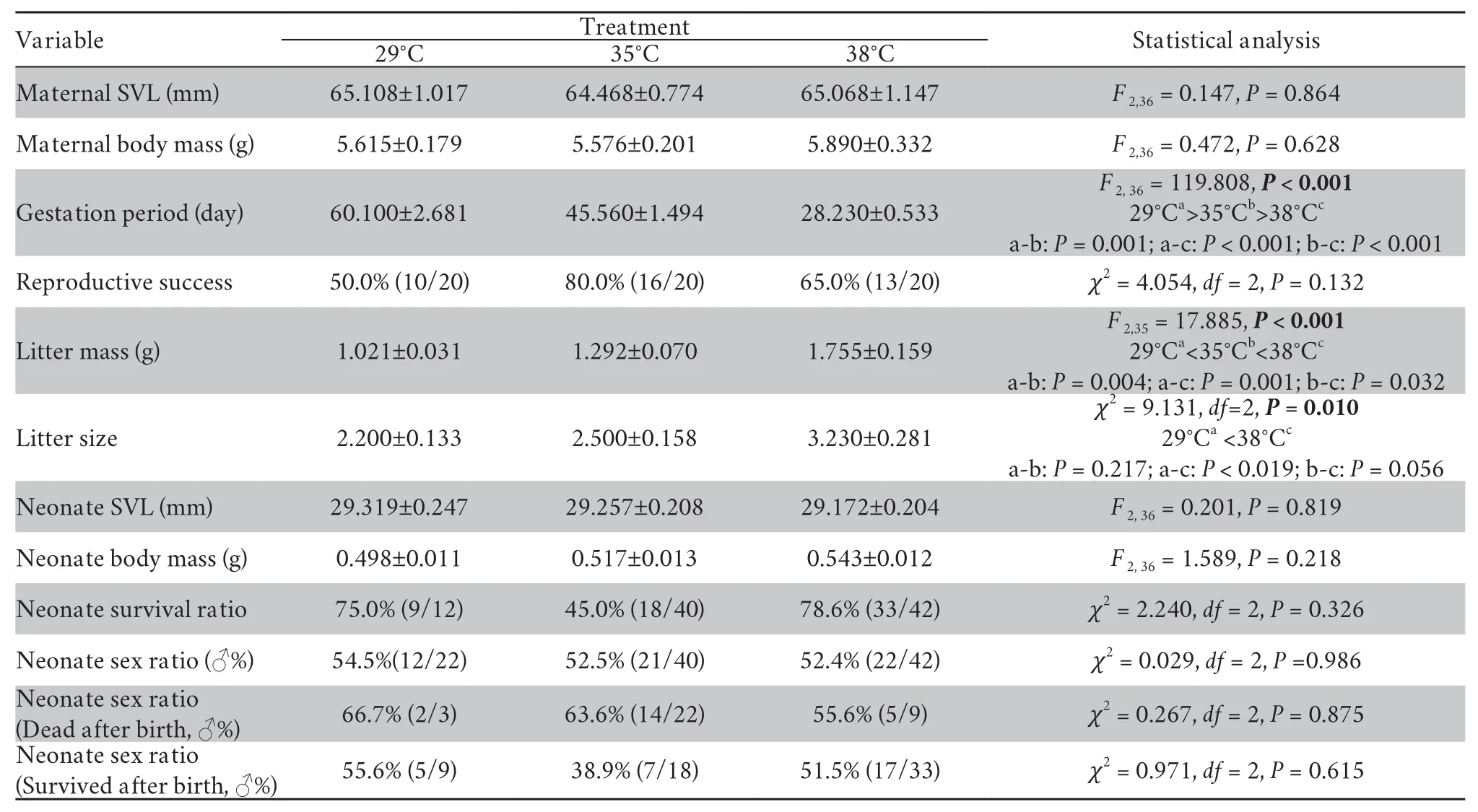
Table 1 Differences between gestation temperature treatments in maternal and neonate traits in the multi-ocellated racerunner Eremias multicellata from Inner Mongolia.
Currently there is a knowledge gap on intraspecific variation in sex-determining systems,concerning the evolutionary driver of different sex-determination patterns among populations.For example,climate or elevation did not explain within-species variation in sex reversal in the Australian dragon lizard (Cornejo-Páramoet al.,2020a) or sexchromosome differentiation in common frogs (Phillipset al.,2020).The population divergence of sex-determining systems found inN.ocellatuswas attributable to the increased rate of female maturation in the lowland population,and the higher magnitude of between-year temperature fluctuations in the highland population (Penet al.,2010).Therefore,climatic
effects on lizard life history may induce divergent natural selection forces on sex determination across altitudinal clines e.g.,a relatively long activity season favours an evolutionary shift from GSD to TSD in lowland populations ofN.ocellatus(Cunninghamet al.,2017;Penet al.,2010).However,this explanation cannot be applied to theE.multiocellatasystem,because TSD occurs in high-altitude populations rather than low-altitude populations in this species.In addition,a metaanalysis on sex-determining systems of non-avian reptiles falsified the assumption that populations living in either highly variable or cold climatic conditions should evolve GSD to buffer the populations from extreme sex ratios (Cornejo-Páramoet al.,2020b).The selective forces acting on the evolution of TSD-GSD systems remain a mystery and warrants further study in future.
AcknowledgementsThanks to Xin HAO and Xingzhi HAN for their assistance and help in field work and lab work.We also give the gratitude to Baojun SUN,Zhongwen JIANG and Pengfei WU for their assistance in data analysis.This work is supported by grants from the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB31000000),
the Second Tibetan Plateau Scientific Expedition and Research Program (STEP) (2019QZKK0501),and Joint Grant from Chinese Academy of Sciences-People’s Government of Qinghai Province on Sanjiangyuan National Park (LHZX-2020-01).Ethics approval and protocol (IOZ14001) for the collection,handling and husbandry of the study animals was given by Animal Ethics Committees at the Institute of Zoology,Chinese Academy of Sciences.
Appendix
File 1Excel 1 presented the data of female gestation traits including maternal SVL,BM,Gestation period,litter size,litter mass and reproductive success in the multi-ocellated racerunnerEremias multicellatafrom Inner Mongolia with different temperature treatment.Excel 2 presented the data of neonate traits (SVL,BM,sex and survival) with different temperature treatment of their mother.The file can be downloaded from the website https://pan.baidu.com/s/1M8MuOyWLkyzBH_rp_S9sqw (access code:k9og).
File 2Meteorological data of Tianzhu,Minqin and Eerduosi (Inner Mongolia) from http://data.cma.cn in recent 20 years.LowestT means the lowest temperature,highestT represents the highest temperature,and averageT means the average temperature.RH represents relative humidity.The file can be downloaded from the website https://pan.baidu.com/s/1AZ3OW91K-lij3LHjXsZ1BQ (access code:v9n5).
 Asian Herpetological Research2022年1期
Asian Herpetological Research2022年1期
- Asian Herpetological Research的其它文章
- Comparative Osteology of Two Far Eastern Species of Ratsnakes (Serpentes:Colubridae),Elaphe dione (Pallas,1773) and E.schrenckii (Strauch,1873),for the Purpose of Palaeontological Studies
- Sex But Not Altitude,Modulates Phenotypic Covariations Between Growth and Physiological Traits in Adult Asiatic Toads
- Circadian Rhythm and Intersexual Differences in Auditory Frequency Sensitivity in Emei Music Frogs
- High-elevation Adaptation of Motion Visual Display Modifications in the Toad-Headed Agamid Lizards (Phrynocephalus)
- An Annotated List of Lizards (Sauria:Squamata) Recorded from the People’s Republic of China
- Appendix 1
