Giant ooids of microbial origin from the Zhangxia Formation (Cambrian Miaolingian Series) in North China
Ming-Yue Di , Hu-Shn Zhng , Wei Zheng ,*,Yong-An Qi ,**, Zhi-Feng Xing , Zhen Zhng
a School of Resources and Environment, Henan Polytechnic University, Jiaozuo 454003, Henan Province,China
b Key Laboratory of Biogenic Traces and Sedimentary Minerals of Henan Province, Henan Polytechnic University, Jiaozuo 454003, Henan Province, China
Abstract The role of microorganisms in the formation of giant ooids is one of the areas of long-term controversy in ooidal research, but it has not been confirmed conclusively. Abundant giant ooids developed in the Zhangxia Formation of the Cambrian Miaolingian Series in North China. Giant ooids in the study area were examined by using Polarized Light Microscopy and Field Emission Environmental Scanning Electron Microscopy. The nuclei of the ooids consist of micritic pellets or radial ooids with diameters less than 2 mm and are formed in a weak-agitating seawater environment.Their cortices are concentric,and are characterized by the alternations of the dark laminae of micritic calcite or Girvanella filaments and light laminae of microsparry calcite. In the environments of inter-bank sea with the alternating development of medium and low energy and chiefly weak-agitating conditions, giant ooids were formed under the joint action of Girvanella filamentous growth, biologically-induced calcification and/or biologically-influenced calcification and inorganic calcium carbonate precipitation. The microfossils of Girvanella are distributed in inner and outer cortices of giant ooids,especially dense in the latter. This distinctly indicates that microbes play a significant role in the formation of giant ooids, and also provides a vital example for discussing the microbial origin of giant ooids.
Keywords Girvanella filament, Microbial origin, Giant ooid, Cambrian Miaolingian Series, Zhangxia Formation, North China
1.Introduction
Ooids are carbonate or non-carbonate particles composed of nucleus and cortex. The microfabrics,mineral components, abundance and size of ooids can reflect the physical and chemical conditions of sedimentary environments in marine and non-marine settings.Ooids,therefore,are of great significance for the prediction of palaeoenvironment such as water energy,temperature,salinity and water depths(Flügel,2004).Generally,ooids are smaller than 2 mm in diameter and mostly range between 0.5 mm and 1 mm (Simone,1980; Richter, 1983). For particles exceeding 2 mm diameter and similar to ooidal particles in structures are known as pisoids,especially referring to the ooidal grains of non-marine origin (Middleton et al., 2003;Flügel, 2004; Mei, 2008; Mei and Gao, 2012). In contrast, those ooids with a diameter of more than 2 mm, formed in marine environments, are defined as giant ooids (Sumner and Grotzinger, 1993; Mei, 2008,2012a, 2012b; Li et al., 2010, 2013; Trower and Grotzinger, 2010; Paul et al., 2011; Dai et al., 2014;Tang et al.,2015;Thorie et al.,2018;Trower,2020).
As to the origin of giant ooids, some studies have closely related the formation of ooids with their internal structure and environmental conditions, and have given a good explanation (Sorby, 1879; Zhou, 1982;Zhao et al., 1984; Duguid et al., 2010). It is generally believed that most of the ooids are formed in shallow water environment, especially in turbulent water environment (Tucker and Wright, 1990; Sumner and Grotzinger, 1993; Flügel, 2004; Duguid et al., 2010).The main factors that control the growth of concentric laminae of ooids are the chemical properties of water and turbulent water.The inorganic origin of giant ooids is deduced through the study of the Neoproterozoic giant ooids by Sumner and Grotzinger(1993).Although the theory of inorganic origin of ooidal particles has been dominant for a long time, there is an obviously strong biological control during the formation of ooids(Flügel, 2004). Both Bathurst (1975) and Wang et al.(1983) found organic residues in ooids interior, and the research results of other scholars further confirmed that the significant role of organisms in the ooidal formation (Davies et al., 1978; Folk, 1993; Folk and Leo Lynch, 2001; Brehm et al., 2004, 2006; Pl′ee et al.,2008,2010;Woods,2013). Hence,the relationship between ooids and microbes has become a hot spot in the research of oolites. Mitterer (1968, 1972) discovered the close relationship between ooids and microorganisms in the study of ooids from modern oceans. However, in modern ooids in the Bahamas, Duguid et al.(2010) found that in the early diagenetic stage,microorganisms changed the structures and chemical properties of ooids through microboring activities,rather than playing a constructive role in the formation of ooids, which impacts the hypothesis of microbial genesis of oolites. The microbial mechanism in the formation of giant ooids is supported by the example in the Lower Triassic South China (Mei and Gao, 2012; Li et al., 2013).The major controlling factors of the ooidal growth are microbial activities(Gerdes et al.,1994;Reitner et al., 1997; Davaud and Girardclos, 2001).Cyanobacteria and other bacteria are conducive to the formation of ooids,especially in low-energy conditions(Hillgartner et al., 2001). Subsequently, Xin et al.(2020)found a large number of calcified microbial residues (e.g., Epiphyton, Girvanella, Renalcis) in microbial carbonate rocks, further confirming that the formation of microbial carbonates is closely related to microbial metabolic activities dominated by cyanobacteria.Meanwhile,these important findings indicate that microorganisms play a significant role in organomineralization, and also confirm to a certain extent that microbial activities participated in almost all sedimentary processes throughout the evolutionary history of life on Earth(Chen et al.,2017;2019a).
Although there is growing evidence that microbes are involved in the formation of ooids, some researchers believe that the available evidence is insufficient to confirm the biogenic origin of ooids,especially for ancient ooids (Duguid et al., 2010; Tang et al.,2015). Abundant giant ooids occur in carbonates of the Zhangxia Formation of the Cambrian Miaolingian Series in North China(Dai et al.,2014),in which a large number of well-preserved Girvanella filaments were found in nuclei and cortices of giant ooids. This indicates that microorganisms play an important role in the formation of giant ooids,and provides an important example for the study of the microbial origin of giant ooids.
2.Geological setting and stratigraphic characteristics

Fig. 1 A) Comparison chart for the chronostratigraphy, biostratigraphy (fossil zone), and lithostratigraphy of the Cambrian Ren Village section in Western Henan subregion, North China (modified from Pei et al., 2008; the red box line represents the study horizon); B)Generalized geological map showing the locality of Ren Village section (the red star) on the North China Platform; C) The giant ooidal limestone developed in carbonate rocks in the lower part of the Zhangxia Formation (Cambrian Miaolingian Series), intercalated with mudstripes; D) Fresh surface of the giant ooidal limestone unit; E) Weathered surface of the giant ooidal limestone unit.
Henan Province is tectonically located in the junction of the southern part of the North China Platform and the Qinling Fold Belt.Based on the analysis of the Cambrian palaeogeography and palaeotectonic framework, the North China stratigraphic region of the Cambrian can be divided into Taihang, Western Henan and Eastern Henan plain subregion (Pei et al., 2008).The study section is located in Ren Village of the Western Henan stratigraphic subregion in North China stratigraphic region(Fig.1B).During the Cambrian,the North China Platform was mainly a sedimentary basin of epeiric sea, which was overall dominated by carbonate rock precipitation(Meng et al.,1997;Shi et al.,1997). The Cambrian strata developed continuously in this area.The Xinji Formation of the Cambrian Series 2 is characterized by conglomerate and sandstone deposits in the first transgression period of the Phanerozoic.The Zhushadong Formation of the Cambrian Series 2 is marked by coastal-shallow marine dolomites and the first member of the Mantou Formation (Cambrian Series 2) is clastic rocks intercalated with carbonate deposits in tidal-flat facies. The second and third members of the Mantou Formation(Cambrian Miaolingian Series)are mainly composed of clastic deposits in tidal-flat facies and neritic facies, followed by carbonate rocks. The Zhangxia Formation (Cambrian Miaolingian Series) is characterized by oolitic limestones, oolitic dolomites, bioclastic limestones and microbialites in coastal-shallow marine facies. The Sanshanzi Formation of the Cambrian Furongian Series is dominated by dolomites in tidal-flat facies.
The giant ooid samples were collected from the Zhangxia Formation of the Cambrian Miaolingian Series from the Ren Village section of Western Henan, North China (Fig. 1A and B). The medium-thick giant ooidal limestone is developed in carbonate rocks in the lower part of the Zhangxia Formation,which contains a small amount of bioclasts intercalated with mud-stripes(Fig. 1C). The thickness of oolitic limestone in the study stratum is about 90 cm, and the diameter of ooids progressively increases from 0.5-1.5 mm in the bottom to 5 mm in the top (Fig. 1D and E), in which includes trilobite fossils Bailiella lantenoisi (Mansuy),Proasaphiscus honanensis Chang.It belongs to the topmost fossil zone of Wuliuan Stage.
The giant ooidal limestone unit with a thickness of 3.87 m is located in the lower part of the Zhangxia Formation of the Cambrian Miaolingian Series,and can be divided into 8 beds from bottom to top (S1-S8)(Fig. 2). Bed S1 is an oolitic limestone subdivision containing ooids with particle sizes of less than 2 mm.The ooidal nuclei are irregular micritic calcite masses,while some nuclei are not developed. The inner cortices of ooids are composed of calcite crystals with radial fabric. The outer cortices of ooids are characterized by microsparry and micritic calcite in concentric fabric, and surrounding rocks are cemented by microsparry calcite (Fig. 2A). Bed S2 is oncolitic limestone containing ooids, in which the particle size of oncolites is about 3-10 mm,most oncolites are elliptic or irregular and most of them are single nucleus. The concentric cortices containing the Girvanella filaments are not obvious and irregular, and the sorting of the concentric cortices is poor.Bed S3,similar to Bed S1,is composed of ooids mostly smaller than 2 mm in diameter.A large number of aggregates of suspected organic masses can be found among grains (Fig. 2B). The average size of these aggregates is about 200 μm and their maximum size is more than 500 μm, which is distinctly distinguished from peloids (Fahraeus et al.,1974; Bathurst, 1975; Flügel, 2004). Beds S4-S6 are giant ooidal limestone beds,in which the particle size of ooids is 2-5 mm.The size of the giant ooids in bed S4(Fig. 2C) is relatively small, generally between 2 mm and 3 mm. The diameter of the nucleus is small,generally 0.5-0.6 mm.The diameter of some common ooids is less than 2 mm.These common ooids are nearly circular, and their nuclei are composed of inconspicuous or irregular dark micritic calcite masses. Their cortices are concentric, and the thickness of the cortices is large,accounting for 4/5 of the diameter of giant ooids. They are characterized by a regular alternation of the light and dark laminae and generally have 5-7 couplets formed in the alternate laminae,in which the light laminae are obviously thicker than the dark laminae. Some of the larger ooids develop dark concentric micritic calcite laminae in the outer cortices, and some irregular micritic masses are scattered among ooids. The matrices between ooids are microsparry calcite formed by recrystallization of micrite.In Bed S5 and Bed S6(Fig.2D),the diameter of giant ooids is relatively large; generally, between 3.5 mm and 4.7 mm, and parts of them can be larger than 5 mm. The nucleus diameter accounts for 1/4 to 1/3 of the size of the giant ooids, which is unstructured. The thickness of the concentric laminae accounts for 2/3 to 3/4 of the diameter of the giant ooids.The light and dark laminae are developed irregularly,and the thickness of the dark laminae is obviously thicker than the light laminae.The boundary between them is blurred and often difficult to distinguish, and there are only 1 to 3 couplets of laminae in cortex.There is basically no contact between giant ooids,and the matrix of giant ooids is composed of microsparry calcite formed by recrystallization of micrite.Bed S7 is marked by thrombolite limestone and the morphology of thrombolites is irregular, which is accompanied by oolites and bioclastic rocks as well as earthy yellow mudstone.The uppermost Bed S8,similar to the characteristics of S1 ooids,is composed of oolitic limestone with ooid diameter of less than 2 mm(Fig.2F).
3.Material and methods
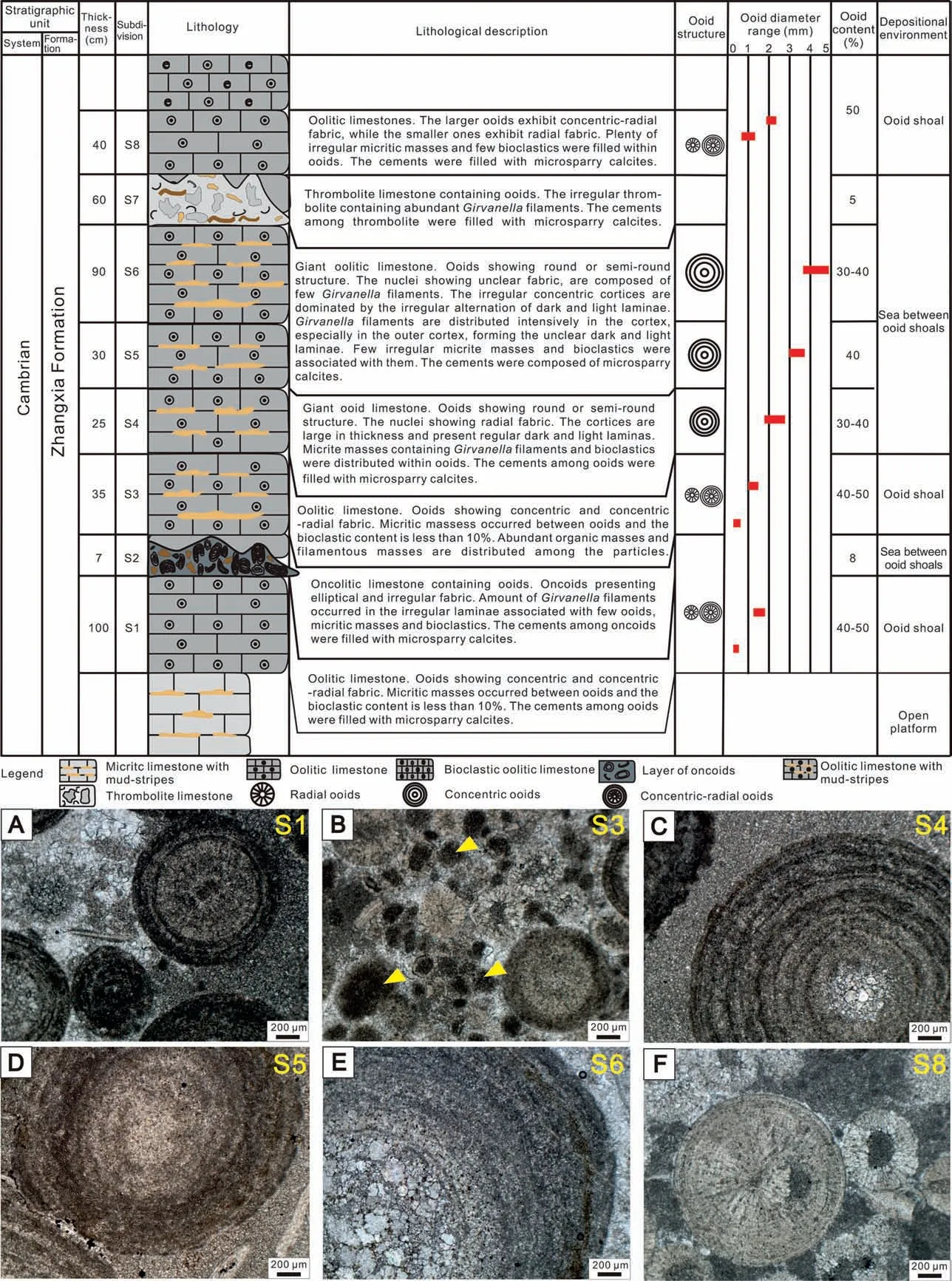
Fig. 2 Comprehensive columnar section of giant ooidal limestone and micrographs of ooids. A) Characteristics of ooids in Bed S1; B)Characteristics of ooids in Bed S3(the arrows pointing to suspected organic masses);C)Characteristics of ooids in Bed S4;D)Characteristics of ooids in Bed S5; E) Characteristics of ooids in Bed S6; F) Characteristics of ooids in Bed S8. The scale is 200 μm.
The macromorphological characteristics of ooids were observed in outcrops and hand specimens. The microstructures of oolites were mainly studied by observing thin sections with optical microscope. The microtopography, microfabric and mineral studies were carried out by observing freshly fractured rock samples using FEI Quanta 250 FEG Field Emission Scanning Electron Microscopy (FESEM). The elemental compositions were determined by Energy Dispersive Xray Spectroscopy (EDS) connected to FESEM.
FESEM was operated at 10 kV voltage with a working distance of 10.4-14.8 mm. All laboratory analyses were performed at the Key Laboratory of Biogenic Traces and Sedimentary Minerals of Henan Province, Henan Polytechnic University.
4.The characteristics of giant ooids
The giant ooids developed in the oolitic limestone are spherical or subspheroidal. The particle size is mostly 2-4 mm (Fig. 2C and D), and the maximum is 5 mm (Fig. 2E), which is obviously different from ordinary ooids(Fig.2A,B,F).On the weathered surface,the giant ooid nucleus is dark in color (Fig.2B and C),the cortices are concentric, and the light and dark laminae are clearly discernible. There are several irregular earthy yellow mudstone layers or mud-stripes in giant ooidal limestone.
Under polarized light microscope, most nuclei in giant ooids display unclear microfabric and are mainly filled with microsparry calcite formed by micritic recrystallization, while a few are filled with dolomite with a better idiomorphic degree. A few nuclei have radial microfabric and the boundaries between nuclei and cortices are not very clear (Fig. 2C, E). The concentric cortices of giant ooids with abundant Girvanella filaments are composed of light and dark laminae. The light laminae are relatively thin, about 100-150 μm, and are composed of microsparry calcite. The dark laminae vary greatly in thickness.The thinner dark laminae are about 50-80 μm thick(Fig.2C),which are organic matter laminae composed of micritic calcite. The thicker dark laminae are about 200-400 μm thick (Fig. 2E), in which there are a large number of Girvanella filaments and the intervals between filaments are filled with micritic calcite.These micritic calcite particles with different sizes and uneven distribution are accompanied with microcrystalline-microsparry cements, so it is difficult to identify the stages of cementation and their generational structures are rare. The outer boundaries of giant ooids are often destroyed by the cements of the surrounding rock, and the secondary metasomatic edges are occasionally found (Fig. 3A).Certain incompletely enveloped filamentous cortices or filamentous masses are found in the surrounding rocks(Fig.3B and C).Fig.3B exhibits that the nucleus is enveloped by mutually tangled filaments,but is not formed in a uniform cortex structure, because the contact between the nucleus with surrounding rocks presents a state of fracture, pulling and separation.Before the formation of the cortex, the edges of filamentous laminae have been eroded and/or pulled to change the original thickness and morphology. In the end, after experiencing the processes of capture,bonding, rolling, jumping, pulling, erosion, compaction, etc., the cortex then is formed with a relatively uniform concentric laminae. The filamentous masses may be exfoliated during the formation process of the concentric laminae or free molecules in the environment, which may also become the giant ooidal nucleating particles.
5.Girvanella filaments in giant ooids
The high magnification of microscopy observations shows that the Girvanella filaments in the concentric cortices of giant ooids appear as slightly curved, unbranched, unsegmented and elongated tubular texture, with diameters of 10-12 μm and extension lengths of 100-150 μm. The Girvanella filaments present a densely intertwining arrangement, parallelly-, obliquely- and/or vertically-distributed to the direction of the tangent line of cortices(Fig.3E and F).The filamentous tube wall is dark grey, about 2 μm thick and composed of micritic calcite. The tube core is light grey with thickness of 5-7 μm and is filled with microsparry calcite.
A large amount of Girvanella filaments developed in giant ooids, suggesting a relatively well-preserved microbial feature in ooids larger than 2 mm. The microbial traces appear in the forms of single organicrich cortices and masses, but the filamentous fossils are abundant in the nuclei and laminae of giant ooids and surrounding rocks. Herein, the distribution of Girvanella filaments in giant ooidal cortices can be classified into three types:1)the filaments distributed closer to the nuclei are slightly sparse. They grow outwards through one end,attaching to the nuclei and interconnect with other filaments to form a grid, and form the irregular cortices with large thickness(Fig.3E,G);2)the filaments arrange in the direction of the tangent to the laminae in the inner part of the cortices and intertwine and overlap densely in the outer part of the cortices(Fig.3E).Some dark laminae composed of filaments do not form completely enveloping cortices and gradually taper out at both ends,resulting in a large and irregular thickness of the dark laminae, which then are difficult to be distinguished from the light laminae (Fig. 3H); 3) the filaments distributed in the edge of giant ooids are darker,denser and more clutter than those in the cortices(Fig. 3F and G).
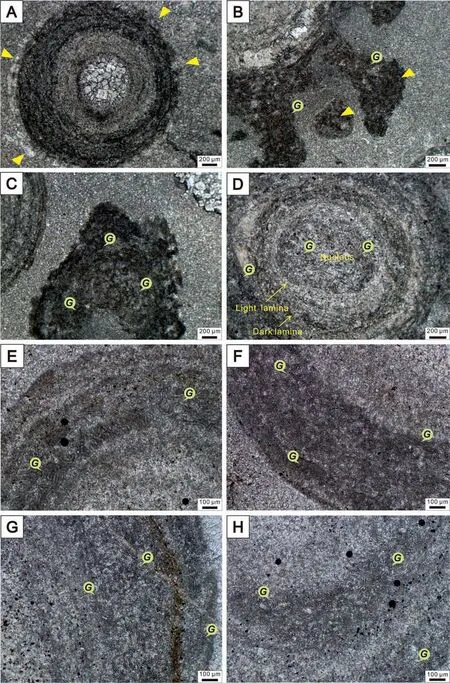
Fig.3 Polarized light microscope photos of giant ooids.A)The boundary between ooids and surrounding rocks(the arrows point to the ooid edge); B) Incomplete filamentous cortices, showing that the nucleus of giant ooid is enveloped by mutually tangled filaments, but is not formed in a uniform cortex structure,because the contact between the nucleus and the surrounding rocks presents a state of fracture,pulling and separation. The arrows point to organic masses that consist of Girvanella filaments; C) Girvanella filamentous masses in surrounding rocks;D)Girvanella filaments in the nucleus and cortices of a giant ooid;E-H)Girvanella filaments in dark cortices of giant ooids.The scale in A-D is 200 μm; the scale in E-H is 100 μm. The symbol G points to Girvanella filaments.
FESEM observations indicate that slightly curved tubular filaments and round transverse section of tubular filaments are densely distributed in the giant ooidal cortices, and the distinct boundary between ooids and surrounding rocks can be observed(Fig.4A).There are obvious secondary metasomatic surrounding rocks at the edge of giant ooids and embedded with calcite cements. The surrounding particles of giant ooids are distinctly larger than those in the giant ooidal cortices. They vary in size and distribute unequally,and have no obvious generation structure.The micritic calcite inclusions are found at the edge of giant ooids.The calcites in giant ooids display granular mosaic texture with poor idiomorphism and may be formed by micritic recrystallization.The secondary metasomatic edge and the well-preserved Girvanella filamentous fossil can be seen locally. The diameter range of the filaments is 9-11 μm, the maximum length is 143.4 μm; the diameter of the tube core is approximately 5-6.5 μm and the tube wall is about 1.5 μm thick (Fig. 4B-F). Further magnification shows that there are extremely tiny calcites spreading intensively around the Girvanella. Minerals in the tube wall and tube core of Givenalla are distinctly different. The tube wall is composed of elongated tiny idiomorphic calcites which have the diameters of 0.7-1.5 μm(Fig. 4C and D). However, calcites in tube cores and surrounding rocks have different crystal sizes with poorly idiomorphic degree. The tiny calcites forming the tube core coat outside the core and are arranged in an orderly and regular manner on the tube wall, and arranged radially perpendicular to the tube core of filaments in the direction of the long axis of calcite particles.The alveolate uniform pits presenting on the exposed tube wall are the mosaic texture left by the tiny calcites (Fig. 4F). The tiny size and idiomorphic degree of the orderly arranged calcite particles in the Girvanella tube core and tube wall indicate that the calcification of the tube wall is biogenic and the tube wall should be the colloid sheath formed by the calcification of extracellular polymeric substances(EPS) secreted by Girvanella. This process of calcification is extremely likely to happen during the growth period of microorganism,thus preserving the compact and well-euhedral original calcites.The calcites in the tube core and surrounding rocks may be formed by recrystallization in diagenetic stage. In surrounding rocks,we capture the micritic calcite masses(Fig.4G),which are composed of microcrystalline calcite with uniform particle size. After magnification, it is observed that the major long axis of the particle size of these micritic calcite masses is mostly about 4-6 μm(Fig.4H),which is consistent with the diameter of the Girvanella tube core. This indicates that the microsparry calcite may be derived from the decomposition of organic matter left by Girvanella filaments.
6.The formation process of giant ooids
In the study area, the giant ooids form in two stages: the formation of nucleus and the formation of cortex. The nucleus of giant ooids are of two types.The first type of nucleus is made of common (normalsized) radial ooids (Fig. 5A), which are formed by the radial growth of calcium carbonate around micritic masses in the low energy,weak agitating,and calcium carbonate supersaturated water environment (Tucker and Wright, 1990; Sumner and Grotzinger, 1993;Flügel,2004;Duguid et al.,2010).No obvious trace of microbes occurs in this kind of nucleus, and those normal-sized radial ooids were taken here from nearby ooidal bank by current, providing nucleating particles for the development of giant ooids (Fig. 5B). The second type of nucleus is an unstructured micritic pellet, in which Girvanella filaments can be seen(Fig. 5A), and the boundary between this kind of nucleus and its concentric cortex is blurry.Under the lowenergy environmental condition, Girvanella has sufficient time to grow and preserve (Dai et al., 2014,2016; Qi et al., 2017), and is able to secrete the extracellular polymeric substances (EPS), which may possess abundant functional groups that can bind Ca2+or Mg2+to promote the carbonate precipitation(Dupraz et al., 2009). The nucleus is composed of irregular micritic masses, which are formed by microbial mud crystallization, or formed by carbonate particles bound and captured by the Girvanella-secreted EPS in the environment(Fig.5C).With the increase of water energy, these irregular micritic particles are stirred up to present a suspending or rolling state,and are abraded by the water flow to form the micritic pellets of the nuclei of giant ooids (Fig. 5B).

Fig. 4 Field emission environmental scanning electron microscope images of filaments. A) The boundary between giant ooids and surrounding rocks; B) Girvanella filaments (symbol G) in the cortices; C)The longitudinal section of the filamentous tube, and microcrystalline calcites (the arrows) perpendicular to the tube wall; D) The transverse section of the filamentous tube, and microcrystalline calcites (the arrows) arranged radially within the tube wall; E) The tube cores of filaments; F) The alveolate uniform pits (the arrows) on tube cores; G)Micritic calcite masses in the surrounding rock; H) The enlarged version of Fig. 4G.

Fig.5 Models of the formation of giant ooids.A)The Girvanella masses and radial ooids in the environment are the nucleation particles of giant ooids; B) The formation of the nuclei of giant ooids. When the water shows low energy with weak agitating, these irregular microorganism masses and radial ooids are stirred up to present a suspending or rolling state,and are abraded by the water flow to form the nuclei of giant ooids.At the same time,the microorganism Girvanella begins to attach to the surface of nuclei;C)The formation of the dark laminae of giant ooids.Girvanella can not only promote carbonate precipitation through metabolic activities such as photosynthesis,but also can secrete EPS to bind and capture carbonate particles in the environment,thus promoting the formation of asymmetric dark laminae;D)The formation of the light laminae of giant ooids.When the water energy increased,the development of Girvanella is inhibited.The supersaturated calcium carbonate in the water began to precipitate around the irregular dark laminae of giant ooids,forming the light laminae of microsparry calcite;E)The formation of giant ooids.As the growth of cortices of giant ooids reached a certain state when the surface of giant ooids and seawater were in an equilibrium state, the deposition stopped and then the giant ooids were formed; F) The precipitation of giant ooids.
The concentric cortices of giant ooids stand for their precipitation in an agitated beach environment(Tom′as et al., 2013; Antoshkina, 2015; Thorie et al., 2018).In this paper,the cortices of giant ooids also formed in the water environment with an alternation of medium to low energy and mainly with weak agitation. When the water environment is relatively weak and shows weak agitation, the microorganism Girvanella begins to attach to the surface of ooids and grows (Fig. 5B; Liu and Zhang, 2012; Dai et al., 2014, 2016; Qi et al.,2017). These microorganisms can not only promote the carbonate precipitation by increasing the microenvironmental alkalinity through metabolic activities such as photosynthesis(Pl′ee et al.,2008,2010;Dupraz et al., 2009; Pacton et al., 2012; Diaz et al., 2014,2017;Tan et al.,2017),but also can secrete EPS to bind and capture carbonate particles in the surrounding environment and provide a template for the nucleation of giant ooids(Fig.5C;Dupraz et al.,2009;Diaz et al.,2013, 2014, 2017; O'Reilly et al., 2016; Tan et al.,2017). The deposition of calcium carbonate caused by this continuous biologically-induced mechanism and biologically-influenced mechanism leads to the formation of asymmetric dark laminae (Fig. 5C; Liu and Zhang, 2012). When the water energy increased, the deposition rate of carbonate particles is accelerated,meanwhile the development of Girvanella is inhibited(Dai et al., 2014, 2016; Qi et al., 2017). The supersaturated calcium carbonate in the water begins to precipitate around the irregular dark laminae of giant ooids, forming the light laminae that primarily consist of microsparry calcite(Fig.5D).At the same time,the current washes the giant ooids back and forth promotes the abrasion of the light and dark laminae along with the precipitation and accretion of calcium carbonate,and thus makes the giant ooids remain spherical(Brehm et al., 2006; Mei and Gao, 2012). As the growth of cortices of giant ooids reaches a certain state when the surface of giant ooids and the seawater are in an equilibrium state, the deposition stops and then the giant ooids are formed(Fig.5E).
The thinner dark laminae in the cortices are mainly formed by the biologically-induced and/or biologically-influenced calcification and the inorganic calcium carbonate precipitation. Under short-term weakly-agitated environmental conditions, microorganisms promote calcium carbonate precipitation and calcification through photosynthesis and other metabolic activities. The dark laminae are the results of the precipitation and calcification of calcium carbonate and other microcrystals on the surface of microbial biofilm promoted by EPS absorbing Ca2+(Reitner et al.,2005;Dupraz et al.,2009).Girvanella filaments are rarely developed in the dark lamina,and are indeterminate in the boundary between the dark and the light laminae, but the dark laminae near the outer cortex contain a large number of Girvanella filaments and are thicker. This indicates that the longer duration of the weak-agitating conditions allows sufficient time for the microorganisms to grow freely, developing abundant calcified Girvanella filaments (Dai et al., 2014, 2016; Qi et al., 2017).Therefore, the formation of giant ooids occurs in an alternation of medium to low energy with a mainly weak-agitating water environment. The biologicallyinduced and/or biologically-influenced calcification and the inorganic calcium carbonate precipitation together contribute to the development of the cortices of giant ooids, and then control the formation of giant ooids (Fig. 5).
7.Discussion
7.1. Difference between microbiogenic giant ooids and oncoids
In the study area, giant ooids are confused with oncoids because of the direct evidence of microbial participation in the formation of giant ooids.Although giant ooids and oncoids both have nucleus and cortex structure, they are distinctly different in many aspects.In terms of the size of an individual particle,the diameter of a giant ooid is generally 2-5 mm, rarely exceeds 10 mm, and the particle size of giant ooids is uniform. However, the diameter of oncoids is relatively larger with a range of 5-20 mm and individually can exceed 20 mm.In terms of morphology,giant ooids are mostly spherical or subspherical, and their morphology is relatively regular,while the oncoids are commonly irregular with minor hemispherical or spherical types (Qi et al., 2013, 2016). The cortex texture of giant ooids is regular with the continuous and uniform thickness of dark and light laminae, and the number of ooid laminae can exceed 90 (Newell,1960; Bathurst, 1967). However, the cortex texture of oncoids often exhibits non-concentric with mostly discontinuous and uneven dark and light laminae(Tucker and Wright, 1990; Flügel, 2004; Mei et al.,2019; Mei, 2021), and the number of oncoid laminae is less than that of ooids.The giant ooids develop in the sea between ooid shoals with an alternation of medium to low energy, but the oncoids are formed in the subtidal environment with low energy(Dai et al.,2016;Qi et al., 2017).
7.2. Connection between Givanella and giant oolitic genesis
According to the typical viewpoint on ooid formation,ooids are the inorganic carbonate particles formed by the direct chemical precipitation of seawater.Although this viewpoint has been widely accepted,recent studies emphasize the important role of microorganisms in the formation of ooids.Brehm et al.(2006)found the spherical microbial communities (i.e., a symbiosis of cyanobacteria,diatoms and heterotrophic bacteria) would cause calcification during the nucleation and formation of ooids. Based on the study of ooidal sands of Great Bahama Bank, Diaz et al. (2014)found that the carbonate precipitation in marine ooidal biofilms involves many biogeochemical processes,such as photosynthesizers, denitrifiers, sulfate reducers, etc. Based on the study of modern ooids from the Bahamas,Duguid et al. (2010)found that microorganisms do not play a role in the formation of ooids,but change the structure and chemical properties of ooids through microbial microboring activities during the early stage of diagenesis. However, in subsequent studies, organic residues were found in the cortices of ooids(Jones and Peng,2012;Woods,2013;Tang et al.,2015;Mei,2021).EPS secreted by microorganisms were well documented in both ancient and modern ooids(Diaz et al., 2013, 2017; Tang et al., 2015; O'Reilly et al., 2016; Li et al., 2017), which further indicate that microorganisms play a vital role in the formation of giant ooids. Based on the viewpoint that EPS play an important role in organo-mineralization (Dupraz and Visscher, 2005; Jones and Peng, 2012; Tang et al.,2015; O'Reilly et al., 2016; Diaz et al., 2017; Li et al.,2017), Diaz and Eberli (2019) speculated that microorganisms control the organo-mineralization process of ooids by two ways,namely biologically-induced mineralization and biologically-influenced mineralization.The biologically-induced mineralization mechanism is the result of an interaction between biological activities and environments (Dupraz et al., 2009; Diaz and Eberli, 2019), and the biologically-influenced mineralization mechanism is that the EPS provide a nucleation template for carbonate precipitation (Dupraz et al.,2009;Fang et al.,2016;Diaz and Eberli,2019).
Although previous studies have shown that microorganisms participated in the formation of ooids, the proofs are not direct and sufficient, especially for ancient ooids. Compared with Li et al. (2017) who demonstrated that calcified microbial biofilms occur in the dark laminae of the Lower Triassic ooids, the microbial traces in this paper not only existed in the form of organic-rich cortices and masses(Fig.4G and H),but also existed as hosts of Girvanella filaments in nuclei(Fig. 3D) and cortices (Fig. 3E-H) of giant ooids and surrounding rocks (Fig. 3C), which provides a vital evidence that microorganisms control the formation of ooids through organo-mineralization (Liu and Zhang,2012; Tang et al., 2015; Li et al., 2017; Tan et al.,2017; Diaz and Eberli, 2019). In terms of the morphology, Girvanella filaments in the nuclei and cortices of giant ooids are very similar to those in the Cambrian microbialites (i.e., stromatolites, oncoids,thrombolites, etc.; e.g., Qi et al., 2013, 2016, 2017;Dai et al.,2014,2016;Zhang et al.,2014;Chen et al.,2019b; Xin et al., 2019, 2020; Mei et al., 2019; Mei,2021), which indicates that the formation of giant ooids in this study area is affected by microorganisms.Herein, through SEM observation, we found that the calcification of the tube wall (Fig. 4C and D) has biological characteristics, which might be the gel sheath formed by the calcification of EPS secreted by the microorganisms.
The concentric laminae of ooids indicate their precipitation in an agitating beach environment above wave base with ample microbial activities (Tom′as et al., 2013; Antoshkina, 2015; Thorie et al., 2018).From the preservation of microfossils in giant ooids in the study area,a large number of Girvanella filaments were found in giant ooids(Fig.3).In the early stage of formation of giant ooids,Girvanella filaments took the existing radial ooids in the environment as nucleating particles,fixed the particles by intertwining with each other and grew around them to form a giant ooid nucleus(Figs.3D and 5A,B).In the absence of particles,the giant ooid nuclei are composed of the irregular micritic masses formed by microbial micritization or the particles bonded and captured by EPS secreted by microbes. In the inner cortices near the cores, Girvanella filaments grow outwards through one end attaching to the core and interconnect with other filaments to form a grid,forming thick and irregular dark laminae (Figs. 3E, G and 5C). This reflects that Girvanella filaments begin to attach to the surface of giant ooid cores at the water environment with low energy and weak agitation (Fig. 5B; Liu and Zhang, 2012; Dai et al., 2014, 2016; Qi et al., 2017), and that CaCO3precipitation caused by continuous biologicallyinduced and/or biologically-influenced calcification leads to the formation of asymmetric dark laminae(Fig. 5C; Liu and Zhang, 2012; Diaz et al., 2017; Tan et al., 2017). Girvanella filaments close to the inner part of cortices are roughly arranged into several layers along the cortices, while the filaments close to the outer part of cortices are densely intertwined and overlapped (Fig. 3E). The reason why it is difficult to distinguish between dark and light laminae is that some laminae characterized by filaments are not completely enveloped by filaments (Fig. 3H). This indicates that the development of Girvanella filaments is inhibited or even stops with the increase of the water energy (Dai et al.,2014,2016;Qi et al.,2017),and then the Girvanella filaments show overlapping forms along cortices(Fig.5D).At the same time,when the water energy increases, the ooid particles are stirred up to be at a suspension state (Fig. 5D). The supersaturated calcium carbonate in the water began to precipitate around the irregular dark laminae of giant ooids, forming the light laminae of microsparry calcite(Fig.5D).The filaments distributed on the edge of giant ooids are more compact and disordered than those in the cortices,and they are separated from the surrounding rocks with microsparry calcite and organic masses (Fig. 5D). At the late stage of the giant ooid formation, plenty of particles brought by the strong current destroyed environment that were needed for the growth of Girvanella filaments (Dai et al., 2014,2016),indicating the end of the growth of giant ooids.Therefore, the filaments distributed in giant ooids indicate that they take part in the formation of the nuclei and cortices of giant ooids, and also serve as vital evidence that the microbes participate in the formation of giant ooids.
7.3. Depositional environment of giant ooidal limestone
According to the regional researches of lithofacies palaeogeography and sedimentology in Western Henan,during the depositional period of the Zhangxia Formation of Cambrian Miaolingian Series, the transgression continued and the seawater depth increased, and an extensive platform shoal was developed on the uplift belt.The ooid shoals in the Western Henan stratigraphic subregion developed from Zhengzhou City to Sanmenxia City (Feng et al., 1990), and the concentric or concentric radial ooidal limestone of up to 30 m thick were formed in the Zhangxia Formation of Dengfeng area,Zhengzhou City.However,the thickness of ooidal limestone of the Zhangxia Formation in Mianchi area,Sanmenxia City was generally 1-3 m,but varied greatly with the maximum of 8 m.The facies transformation of the Zhangxia Formation in Mianchi area was fast, and the ooidal limestone often developed alternately with microcrystalline limestone. The inter-bank sea between shoals(Fig.6)was weakly agitated(Feng et al.,1990).The ooidal limestone of the Zhangxia Formation in Mianchi area was not developed at the core part of the ooid shoals, but formed at the edge of the ooid shoals with poor lateral continuity and irregular distribution, and formed within the inter-bank sea (Fig. 6).Nucleus of giant ooids is composed of micritic pellets or radial ooids,and the laminae are characterized by the alternate development of the dark laminae composed of micrites or Girvanella filaments and the light laminae composed of microsparry calcite. Other associated particles are a small amount of ooids with particle sizes less than 2 mm and bioclastics,and their total content is less than 10%.The intervals or the cores of giant ooids are filled with microsparry calcite, and the brightness of the crystal is relatively low.The remnants of micrite can be seen locally, which are caused by the micritic recrystallization, rather than the cement of sparry calcite (Feng and Zhang, 2013). In addition, there are several layers of mudstone or argillaceous bands with different thickness in the giant ooidal limestone. The underlying strata are composed of interbeds containing radial or concentric radial ooidal limestone, bioclastic limestone, microcrystalline limestone and mudstone,representing ooid shoals and inter-bank sea.The overlying strata are ooidal limestone intercalated with microcrystalline limestone, and gradually evolve into the concentric or concentric thick oolitic limestone. It reflects that the hydrodynamic conditions fluctuated between weak to strong energy and the sedimentary environment gradually changed from the inter-bank sea to ooid shoals. Based on all the above analyses, the giant ooidal limestone beds of the Zhangxia Formation in the Mianchi area, Western Henan, were formed in the weak-agitating inter-bank seawater environment with an alternating medium to low energy during the Cambrian Miaolingian Epoch.

Fig.6 Schematic diagram showing depositional environment of giant ooidal limestone.The giant ooidal limestone of the Zhangxia Formation(Cambrian Miaolingian Epoch)in Mianchi area formed mainly in the weak-agitating environment such as the inter-bank sea with an alternation of medium to low energy (arrows point to the environment where they formed).
8.Conclusions
A large number of giant ooids are developed in the Zhangxia Formation of Cambrian Miaolingian Series in Western Henan, North China. Their nuclei consist of radial ooids with diameters less than 2 mm or micritic masses with filaments. Their concentric laminae are characterized by the alternations of dark laminae composed of micrites or Girvanella filaments and light laminae composed of microsparry calcite.
The distribution of Girvanella filaments in the cortices of giant ooids are divided into three types:1)the filaments distributed close to the nuclei are sparse and grow outwards by one end attaching to the nuclei,presenting a meshwork type by winding each other,and forming a circle with large and irregular thickness;2) the filaments arranged in the direction of the tangent to the laminae in the inner part of the cortices and intertwine and overlap densely in the outer part of the cortices;3)the filaments distributed at the edge of giant ooids are darker, denser and more clutter than those in the interior of the cortices.
Although many previous studies have shown that microorganisms are involved in the formation of ooids,the intuitive evidence of microorganisms participating in the formation of ancient ooids is insufficient. The giant ooids developed in the study area are formed in the environment of inter-bank sea with alternating medium-low energy dominated by weakly agitated hydrodynamic conditions. The well-preserved Girvanella filaments in the nuclei and cortices of giant ooids directly confirm that microorganisms control the formation of nuclei and cortices of giant ooids through biologically-induced and/or biologically-influenced calcification.
Funding
This work was financially supported by the National Natural Science Foundation of China (Giants No:41872111 and 41902115).
Authors' contributions
All the authors have actively participated in the preparation of this manuscript. MYD, WZ and YAQ conceived the idea of the study, and HSZ wrote the manuscript. All authors did the fieldwork sampling,and observed thin sections. MYD and HSZ finished the final version of the manuscript that was then read and approved by ZFX and ZZ.All authors read and approved the final manuscript.
Availability of data and materials
All data and materials generated or analyzed during this study are included in this published paper.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.The manuscript is approved by all authors for publication.
Acknowledgements
The authors sincerely thank the Editor-in-Chief Prof. Zeng-Zhao Feng, and the anonymous reviewers for their detailed comments and constructive suggestions, which greatly improved this manuscript.
 Journal of Palaeogeography2022年1期
Journal of Palaeogeography2022年1期
- Journal of Palaeogeography的其它文章
- Bioturbation enhanced petrophysical properties in the Ordovician carbonate reservoir of the Tahe oilfield, Tarim Basin, NW China
- Book Review on River Planet:Rivers from Deep Time to the Modern Crisis by Martin Gibling
- Palaeoenvironmental reconstruction for the Permian (lower Gondwana) succession of the Godavari Valley Coalfield in southern India based on a combined palynofacies, carbon isotope, and biomarker study
- Lagoonal carbonate deposition preceding rifting-related uplift: evidence from the Bartonian-Priabonian (Eocene) of the northwestern Gulf of Suez (Egypt)
- Middle Jurassic climate oscillations from paleosol records of the Sichuan Basin,SW China
- Silurian ostracods from the Nyalam region,southern Tibet,China and their implications on palaeoenvironment and palaeobiogeography
