Study on the Application of Quality Risk Management on Drug Collinear Production
Xu Wenxiu,Ma Hui,Yang Dianzheng,Wei Jing,
(1.School of Business Administration,Shenyang Pharmaceutical University,Shenyang 110016,China;2.Liaoning Certification and Evaluation Institute,Shenyang 110036,China)
Abstract Objective To study how to ensure the quality of listed drugs by using quality risk management to control the risks in the drug collinear production because some pharmaceutical companies produce drugs with multiple dosage forms and specifications on the same line to save costs in China.Methods The application status of quality risk management in the production of drugs was analyzed by consulting literature,field investigation and research related policies.Results and Conclusion When introducing co-line products,we should focus on combining product reality,establish a cross-professional team in comprehensive quality,R&D,equipment,and production.Then we can coordinate quality risk management to integrate with each other to avoid“shortcomings”in different links,which will affect the effectiveness of risk management.When a company conducts co-line production,it should rely on quality risk management theories to establish a scientific quality management system,reasonably use quality risk assessment tools to evaluate the types of co-line production,and control key risk factors effectively.These methods can ensure the risk of co-production within an acceptable range.
Keywords:collinear production;cross contamination;quality risk management
In the process of drug production,the problem of collinear production is inevitable when the product is introduced into the appropriate production line to make good use of resources and save costs.The major problem of collinear production is the pollution or cross contamination in the production process.Cleaning is the main way to prevent the pollution or cross contamination in the process.Cleaning method and verification standard are very important in collinear production.China’s Good Manufacturing Practices (GMP) 2010 requires that special protective measures should be taken in collinear production and necessary verification should be carried out to assess the risk of collinear production according to the principle of risk management.In China,since quality risk management cognition of pharmaceutical production enterprises is different,the level of quality risk management is various.Only a few enterprises can apply quality risk management to the management system,and few can apply it freely[1].This article focuses on how to apply quality risk management scientifically to pharmaceutical collinear production to reduce the risk of drug contamination or crosscontamination.
1 Literature review of relevant research
This paper studies the quality risk management theory applied in drug collinear production management,so“quality risk management”“drug collinear production”“cross contamination”“cleaning validation”are taken as keywords to search in CNKI and related full-text literature database during 2010-2020.A total of 120 articles is obtained.Based on the analysis and summary of the existing literature,these articles are classified into the following four categories.
1.1 Study on the risk management of drug production
Lu Haitao (2012) analyzed the problems in the lack of relevant risk awareness and experience of Chinese drug collinear manufacturers in“Talking about the Problems and Corresponding Measures in the Implementation of Drug Production Risk Management”and put forward some feasible suggestions based on the actual situation[2].In“The Problems and Strategies of Drug Production Risk Management”,Wang Lixue (2016) concluded that risk management was an effective method to manage and control risks,which had a significant effect on the risks for pharmaceutical production.He also expounded the concept of risk management for pharmaceutical production,emphasizing the importance of the risk management.Besides,he analyzed the existing problems in the production process[3].
1.2 Risk assessment of drug collinear production
Liu Zhiyong,et al.(2011) clarified the current situation and existing problems of the collinear production of hormone and anti-tumor chemicals in China through investigation in“Management for the Risk of Drug Production with Hormonal and Anti-Tumor Drugs in One Production Line”.Then,he made a systematic analysis with the help of quality risk management tools.Examples of quality risk management tools were provided for hormone enterprises,and how to carry out risk assessment for collinear production of drugs was expounded[4].Zhang Lingfei,et al.(2016) expounded the key contents of risk assessment and related risk control measures in the process of collinear production of traditional Chinese medicine in“Risk Assessment of Traditional Chinese Medicine Products”.Due to the particularity of traditional Chinese medicine varieties,her paper was of great significance to guide the collinear production management of traditional Chinese medicine enterprises[5].
1.3 The effect and specific application of quality risk management on drug collinear production
In“Quality Risk Management in the Collinear Production Assessment of Several Traditional Chinese Medicinal Injections”,Sun Qiuying,et al.(2012)elaborated the application of quality risk management tools in the collinear production of TCM.Then,she pointed out the application of failure mode,effect and analysis (FMEA) in quality risk management and risk control in the production process of TCM injections[6].Tian Yingna (2013) expounded the risks in the production process of oral solid preparations in“Research on the Quality Risk Management in Production of Oral Solid Dosage Form of Drug”and put forward several suggestions for the risk control of collinear production of oral solid preparations[7].
1.4 Cleaning verification of drug collinear production
Liu Yuansheng,et al.(2014) analyzed the regulations on cleaning validation of EMA,WHO,and FDA in“Current Status and Regulatory Countermeasures of Clean Verification in Pharmaceutical Manufacturing Enterprises”.They all made clear requirements of regulatory organizations and institutions for cleaning validation and the standard of residue limits.Through the combination of investigation and research,questionnaire and field research,the status quo and existing problems of cleaning verification of pharmaceutical enterprises in China were clarified,and corresponding suggestions were put forward[8].In“Research on the Key Points of Cleaning Verification of Collinear Production Based on Life Cycle Theory”,Xiong Lang,et al.(2016) analyzed instances of collinear production in pharmaceutical enterprises,and discussed the key points of the common cleaning validation work,which covered all stages of cleaning validation life cycle[9].
To sum up,there are plenty of studies on drug collinear production management in China,but few of them are on how to apply quality risk management in drug collinear production in a scientific way.The research direction is mainly on quality risk management of specific varieties.In this paper,through literature review,field investigation,case study and other methods,the problems in China’s drug collinear production management are clarified,and the application status of quality risk management in drug collinear production and how to apply it scientifically and rationally are analyzed in details.Then,some suggestions are put forward for ensuring product quality in collinear production.
2 Regulatory requirements and management status of collinear production in China’s pharmaceutical enterprises
2.1 Regulatory requirements for drug collinear production
In China,article 46 of the fourth chapter in GMP 2010 stipulated the following items.To reduce the risk of contamination and cross-contamination,factory buildings,production facilities and equipment shall be designed,arranged,and used reasonably according to the characteristics of the drug production,technological process and the corresponding cleanliness level.In principle,collinear production is allowed for products that are not clearly stipulated by law,but the following assessments and regulators authorization need to be provided,mainly including the collinear production,management,risk assessment and the conclusion of the effectiveness of the process,equipment,facilities management,cleaning validation,management level,and control ability[10].
From the perspective of regulatory requirements,China only demands special and (or) independent production facilities and equipment for certain drugs with special properties,such as penicillin,β-lactam structural drugs,and sex hormone contraceptive drugs,which cannot be co-produced with other products.Regulations and laws do not explicitly prohibit collinear production for other types of drugs,but it is required to assess the risks of in-line production and a series of protective measures should be taken.In China,pharmaceutical manufacturers can use facilities for products that are required to be produced separately by regulations.However,for most other co-line production,there are currently no relevant guidelines to guide companies to conduct scientific risk assessments for the collinear production of varieties and effectively control the risk affecting product quality.In summary,the legal requirements for the co-production of drugs in China are too broad,and the details of the relevant clauses should be improved,and the content of risk assessment should be supplemented.
2.2 Current situation of drug collinear production in China’s pharmaceutical enterprises
2.2.1 Analysis of defects in drug inspection
Based on the national GMP compliance inspection data of Center for Food and Drug Inspection of National Medical Products Administration in 2019,the defects existing in the enterprises during the inspection were summarized and analyzed.It was found that there were 41 major defects,409 general defects and 122 unclassified defects.Among the major defects,there were 10 cleaning verification defects(24%),and 6 contamination and cross-contamination defects (15%),as shown in Fig.1.The general defects included risk assessment,personnel training,plant facilities,equipment,cleaning verification,pollution,and cross-contamination,etc.The top 10 defects are shown in Fig.2.
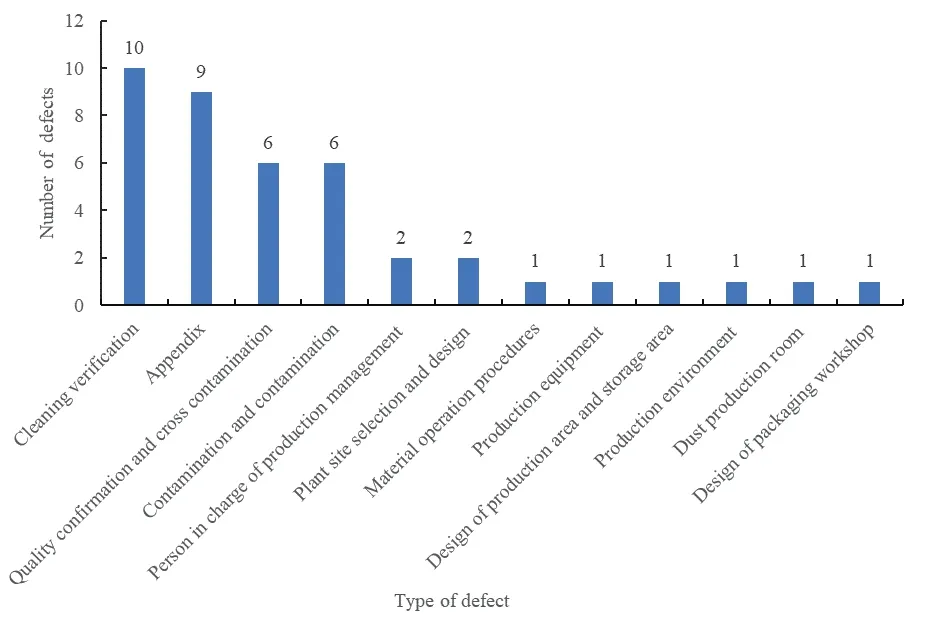
Fig.1 The main defects of national GMP inspection in 2019

Fig.2 The top 10 national GMP inspections by the number of general defects in 2019
It can be seen that conducting risk assessment,preventing pollution and cross-contamination,and cleaning verification in the pharmaceutical production process have always been challenges for enterprises.These challenges are even more severe in China’s drug symbiotic enterprises.Therefore,the prevention of pollution and cross-contamination and establishing standards for quality evaluation and cleaning verification in the production of drugs in the same line is crucial.
2.2.2 Field research of collinear production
The purpose of the field investigation is to better grasp the current situation of the collinear production and the problems in China’s pharmaceutical manufacturers.From September to October 2020,the research team went to Zhejiang Province (1 company),Jiangsu Province (3 companies),Sichuan Province (2 companies),Guangdong Province (1 company),and Hainan Province (1 company).The field survey of production lines and varieties were carried out in a total of 8 companies in five provinces,aiming to understand the companies’ general situation,the production,risk assessment and feasibility assessment methods,the cleaning verification method and compliance with standards,and the problems of collinear production.
From the results of the field survey,the risk control measures adopted by different companies are different,and the standards for cleaning verification are also different.Among them,two collinear production companies applied a large number of advanced disposable technologies (disposable bioreactors,disposable liquid dispensing bags,disposable filling appliances,hoses,etc.),and the robot filling production lines.Through the application of advanced technology,enterprises greatly reduced the risk of collinear production.Due to the excessive cost of one-off technology,most of the co-line production companies have not introduced this technology.However,these risks can be controlled through quality risk management,risk assessment and feasibility assessment,and most enterprises take FMEA as their risk assessment tools.The standards adopted by different companies in the cleaning verification reference standards are different,mainly as follows.(1) 10 ppm principle:based on the general principle of safety requirements,that is,10 × 10-6is the basic requirement,and the residual of the previous batch of products is not allowed to exceeds 10 × 10-6in the next batch of products.(2) 1/1 000 principle:the last batch of products is not allowed to enter the next batch of products with the residual exceeding 1/1 000 of the minimum daily dose.(3) The visual residual limit is less than 4μg/cm2(PDA TR 29).
2.3 The problems existing in China’s co-line production enterprises
Through GMP compliance inspection data and the on-site investigation,it is found that at present,the verification management of drug co-production companies in China is not perfect.A certain number of companies only deal with the inspection of the regulatory authorities.The main problems are as follows.(1) Incomplete understanding of the regulations.For example,most companies know that the high-active,high-toxic,and cytotoxic products specified in Article 46 of the GMP can be coproduced,but they do not know what products belong to high-active and high-toxic products.(2) Lack of the definition of the above products.Some“hormones”in GMP are not clearly clarified.Therefore,enterprises do not know whether they can co-produce other hormone products except for“sex hormones”with special requirements.(3) GMP requires risk assessment of coline production to go through“necessary verification”,but the content of the verification is not clearly stated.Therefore,although some protective measures are taken by such pharmaceutical manufacturers,they still cannot guarantee the quality of their products.
3 Application of quality risk management in drug production
3.1 Overview of risk management theory
Quality risk management is a scientific method,which is often used in the process of drug production and quality management.In order to guide the identification of quality risks,International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use(ICH) issued ICH-Q9 (Guideline on Quality Risks) in 2005.According to the definition of ICH,quality risk management refers to the systematic process through forward-looking or retrospective methods to control the life cycle process of product,from quality risk assessment to control,to notification and review[11].The strength,form and document requirements of the implementation of quality risk management program shall be scientific and reasonable,and match the degree of risk.The process of quality risk management includes risk assessment (risk identification,risk analysis,risk evaluation),risk control (risk reduction,risk acceptance),risk audit and review.The risk management process requires risk communication.The mapping of these steps can be expressed in Fig.3.
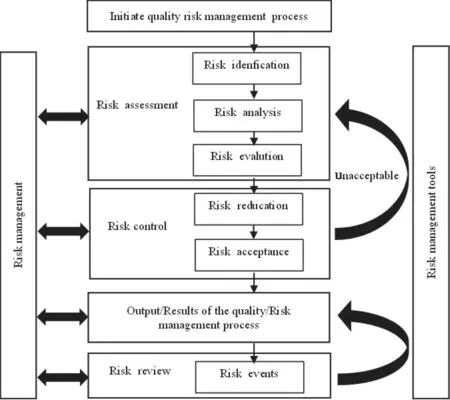
Fig.3 Quality risk management process
3.2 Risk assessment in the production of drugs
3.2.1 Analysis of the factors of multi collinear product evaluation
The main risk assessment factors for collinear products are as follows.(1) The characteristics of the collinear production varieties,which mainly include the category of the varieties (raw materials,chemical drugs,traditional Chinese medicines,and biological products,etc.),toxicity,activity,sensitization,solubility,and traits.(2) The process of the collinear production variety mainly includes the process flow,process description,and terminal sterilization or non-terminal sterilization of the collinear variety.(3) The intended use of the production variety mainly includes drug users,clinical indications,and route of administration.If the characteristics,technology and intended use of the collinear production varieties are similar,the next step of collinear production evaluation can be carried out.If the collinear production varieties have special characteristics,collinear production is not allowed.
3.2.2 Multi-product co-production evaluation
Multi-product co-line production evaluation is a shared risk analysis of facilities,systems and equipment,which include the applicability of plant,air purification system,public engineering system,and equipment cleanability.
The applicability analysis of the plant mainly includes the area of planned co-line production workshop,design of the flow of people,design of logistics,and the design of waste flow.The applicability analysis of the air purification system covers the control area of each air conditioning system,the return and exhaust mode,the number of air changes,the air level design,the temperature and relative humidity,and the room pressure difference.The applicability analysis of the public engineering system includes water system,pure steam system,and compressed air system.It means whether the purified water,water for injection,pure steam,nitrogen,and compressed air can guarantee the use of coline production and affect the operation of process equipment.The equipment suitability analysis mainly includes weighing equipment,preparation equipment,filling equipment,freeze-drying equipment,and packaging equipment.The purpose of equipment cleaning and verification analysis is to confirm the effect of the cleaning procedure on the product.Cleaning verification is a tool to verify the success of cross-contamination and pollution control measures,and it is decisive for the compliance of multi-product co-production.From the perspective of regulatory or industry,cleaning verification is considered to be an important task that can control product crosscontamination and ensure patient safety and product quality.If the multi-product co-line production analysis can meet the requirements of co-line production,the next step is to conduct risk analysis.If the requirements are not met,co-line production cannot be performed.
3.2.3 Analysis of multi-product co-line risk
Risk analysis is to study all related hazard factors that have been confirmed,each potential hazard source and the worst impact after failure.The risk analysis mainly starts from the five links of human,machine,material,law and environment.In the process of risk analysis,a key point for manufacturers is to define a scientific and acceptable residue standard.Then,they can take effective measures to ensure that the residue does not exceed the acceptable standard.The main hazards in the co-line production of drugs are shown in Table 1.

Table 1 Main hazards in collinear production of drugs
3.2.4 Risk assessment and control of multi-product co-production
Multi-product co-production risk assessment mainly relies on evaluation tools.Meanwhile,the quality risk management includes some formal risk management tools,such as hazard analysis critical control point (HACCP),FMEA,risk ranking and filtering (RRF)[1].In China’s pharmaceutical industry,most companies use the FMEA method.This article focuses on application of FMEA for risk assessment in the production.
FMEA is a forward-looking reliability analysis and safety assessment method.It is an engineering technology used to determine,identify,prevent or eliminate the known or potential failure,problems and errors in system design and production.It has been widely used in aerospace,machinery,automobiles,ships,medical engineering,logistics management,safety management and many other fields at present[12].When using FMEA method for risk assessment,we must confirm the risks first.These risks may affect product quality,output,process operations or data integrity.Secondly,we should make risk judgments,including confirming the risk consequences before the assessment,based on the severity,possibility,and detectability (Table 2).
After the risk level is divided,the risk level matrix and the risk priority level matrix can be established (Fig.4).
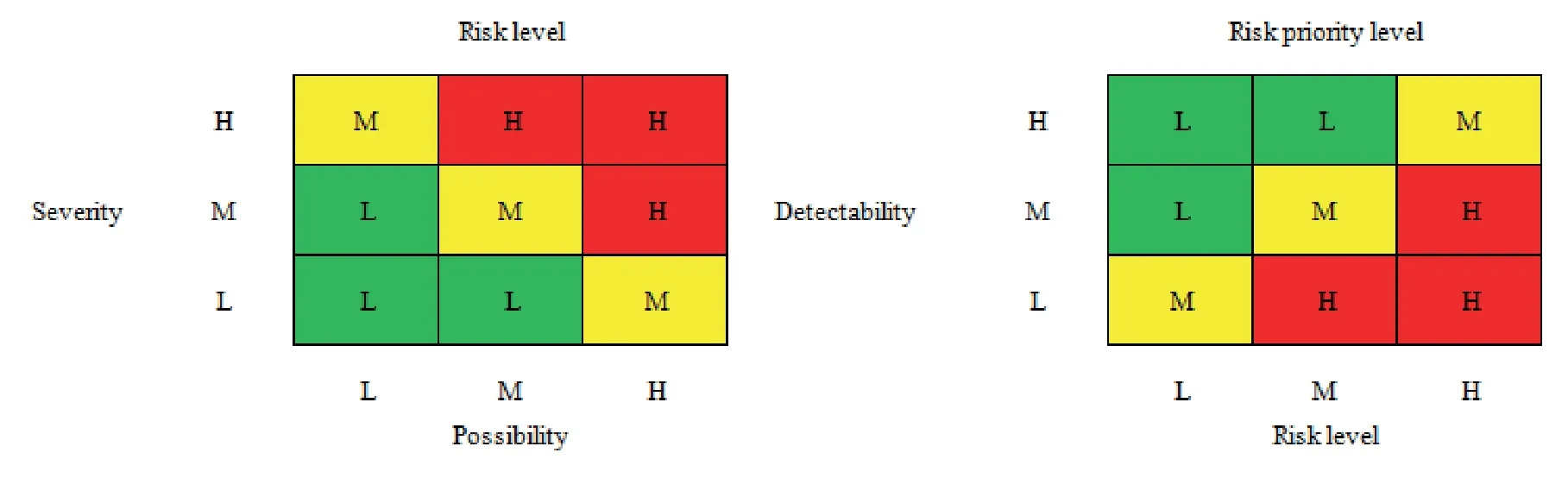
Fig.4 Risk matrix diagram
The risk matrix is mainly to conduct a preliminary qualitative screening of risks.The next step is to perform detailed FMEA scoring.According to“ICH Quality Risk Management Guidelines(ICH Q9)”,the severity of risks is assessed using the FMEA matrix method.Then,the risk priority number (RPN) is calculated,RPN=S*P*D,ifRPN≥ 16 orS=4,the collinear risk level is high,if 15 ≤RPN≤ 8,the collinear risk is medium.WhenRPN≤ 7,the collinear risk is low.After completing the FMEA score of the product itself and the production process,the low-risk points will no longer be analyzed,which means the risk is acceptable.For the medium-risk and high-risk points,corresponding measures need to be taken to reduce the risk to the acceptable low-risk level.
3.2.5 Multi-collinear product risk review and communication
From the above evaluation results,we can obtain the risk factors that affect product quality.Enterprises need to understand whether the control measures are effective.Some of the control measures for risk factors need to set a risk review cycle,which can review risks regularly to ensure that the risks are within an acceptable range.When the implementation of risk control measures is reviewed,we can find whether there are any violations.If there are factors that affect the implementation of the original control measures,such as equipment changes and control system changes,we can make the adjustment in time.Meanwhile,we should find out whether the implementation of the control measures will introduce new risks.If relevant deviations occur in production activities,the incident should be corrected immediately.
4 Examples of the application of quality risk management in the workshop
This study uses examples to analyze the practices of China’s pharmaceutical companies to control co-line production risks,including the way to conduct risk assessment and cleaning verification.These companies produce small-volume injections and therapeutic biological products (recombinant humanized anti-PD-1 monoclonal antibody injection,recombinant humanized anti-VEGF monoclonal antibody injection,recombinant humanized antihuman interleukin 6 receptor monoclonal antibody injection,recombinant Fc glycosylation modified anti-CD20 humanized monoclonal antibody injection,and adalimumab injection).
4.1 Overview of the enterprise’s production workshop
This paper analyzes the enterprise’s 3000 mL stock solution co-line workshop,including adalimumab,bevacizumab,tocilizumab recombinant humanized anti-PD-1 monoclonal antibody,and recombinant humanized anti-HER2 monoclonal antibody.The type of collinear is shared area,air purification system and some production equipment.The main shared equipment is 500 mL seed tank.The category of collinear is biological product.
4.2 Collinear production risk assessment of 3000 L stock liquid line
The company evaluates the feasibility of the collinear production workshop.(1) Characteristics analysis includes collinear product characteristics,active ingredients,mechanism of action,indications,toxicological and pharmacological effects,sensitization,compatibility and contraindications,metabolism,and water solubility information.(2) Feasibility analysis includes whether it contains highly allergenic ingredients,whether the biological product contains live microorganisms,whether it contains beta lactam,sex hormones or contraceptive ingredients,whether it contains certain hormones,cytotoxicity,high active ingredients,whether it is highly toxic,whether highly toxic materials are used in the production process or high toxic intermediate products are produced.(3) Applicability analysis includes the workshop,shared system,public system,situation of production equipment,and facilities.According to the characteristics of batch/equipment applicability/container,combined with the activity/toxicology and process risks of the drug itself,the shared special workshop/area/equipment are listed.(4) Risk assessment is conducted and the specific conditions are shown in Table 3.

Table 3 Table of risk assessment
For the above-mentioned risk sources of medium and high-risk levels,the company has adopted corresponding control measures and carried out risk reassessment.The risk assessment status is shown in Table 4.
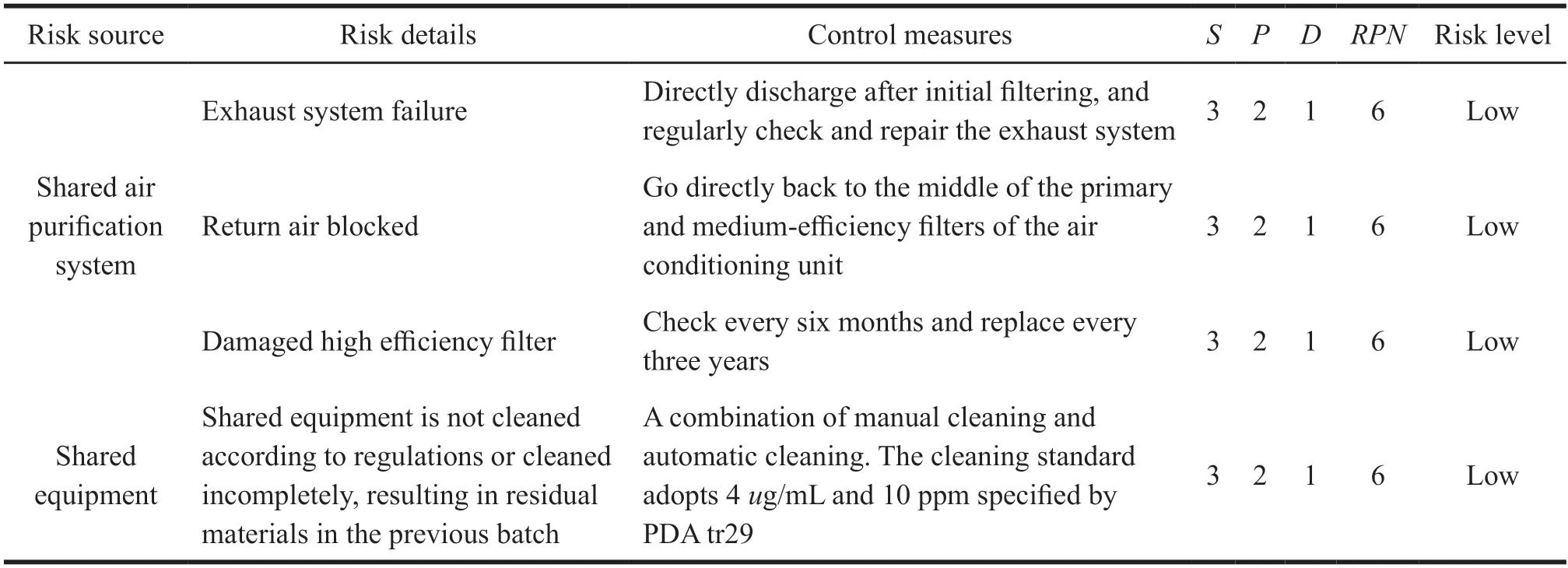
Table 4 Table of risk reassessment
4.3 Conclusions of risk assessment of enterprise’s coproduction
Through the risk assessment of the multivariety shared workshops,facilities,and equipment,the enterprise take corresponding control measures for high and medium risk sources and re-evaluated them.Then,the original high and medium risk levels are reduced to the low risk.Therefore,the company concludes that the risk of co-line production in the 3 000 mL stock solution workshop is acceptable,and co-line production can be carried out.
4.4 Summary and analysis of risk assessment of enterprise’s collinear production
Judging from the content and process of risk assessment conducted by the above-mentionedcompanies,they have made a comprehensive risk assessment for the collinear production workshop and formulated relevant risk control measures.They have also applied the quality risk management theory to the management of the collinear production.However,these companies also have some shortcomings,such as not establishing health-based exposure limits and related toxicology databases during the risk assessment process,failing to calculate the residual limits of related products,and disconnecting from international advanced concepts of cleaning and related management concepts.Enterprises should solve the problems and deficiencies in the abovementioned risk assessment,which is more conducive to the control of the risks of drug collinear production to ensure drug quality.
5 Conclusions and recommendations
In China,for products that are not mandated by laws and regulations,pharmaceutical enterprises will believe that they can have collinear production.At the current stage,the collinear production of enterprises in China has problems such as large differences in management levels,lack of rationality in risk assessment,low standards for testing indicators,and various factors in the evaluation of different varieties.In response to the above problems,this paper puts forward the following three suggestions.
5.1 Regulators should expedite the development of relevant guidelines for collinear production
Regulatory agencies should speed up the development of relevant guidelines based on the concept of quality risk management,guiding companies to further conduct risk analysis and assessment of collinear production.Besides,they should unify the inspection standards of drug for the collinear production,which will effectively control product confusion and product residues.The problems of confusion and cross-contamination in implementation such as transfer operations,air particle transmission,and cleaning verification will further ensure the safety of drugs and improve their quality.It is of far-reaching significance to the realization of the intended use of drugs.
5.2 Enterprises should build a scientific and reasonable quality risk management system
Enterprises should rely on quality risk management theory to establish a scientific and reasonable quality control system,using quality risk assessment tools to comprehensively evaluate the types of collinear production,effectively controlling the key factors that generate risks,and ensuring that the risks of collinear production can be reduced.Carrying out drug quality risk management and continuous improvement of drug quality are important responsibility for drug manufacturers.Drug manufacturers are the first object responsible for implementing drug quality risk management and ensuring drug quality.They should conduct quality risk assessment based on scientific theory,establishing a cross-professional team in comprehensive quality,R&D,equipment,and production.Besides,they must coordinate the development of quality risk management as a whole to avoid“shortcomings”in individual links,which will affect its effectiveness.Quality risk management should be dynamic and continuous,thus improving the enterprise’s quality management level,and ultimately realizing the safe use of medicines for the public.
5.3 Increasing the frequency of personnel training
With the development of quality risk management,the success of quality management depends on key personnel with professional experience and clinical trial experience.Those on the production line must have considerable professional knowledge.Without the direct participation of frontline production personnel,the effectiveness of quality risk management will be greatly affected.Therefore,enterprises should increase the frequency of personnel training to ensure that daily work is carried out in compliance with regulations.
- 亚洲社会药学杂志的其它文章
- Research and Suggestions on the Development of Smart Hospital -Taking Hospital A in Liaoning for Example
- The Development Opportunities and Dilemmas of Telemedicine-Base on the Perspective of Medical Resource Distribution
- Development Status and Enlightenment of Precision Medicine in China
- A Review of the Development and Effect of Contraceptive Counseling After Abortion
- Design of Pharmaceutical Care Process for Retail Pharmacies Based on Pareto Analysis
- Analysis and Enlightenment of Pediatric Drug Registration Data in China

