Sintilimab-induced autoimmune diabetes:A case report and review of the literature
INTRODUCTION
Immune checkpoint inhibitors (ICIs) have been used widely in the treatment of various advanced malignances.Programmed cell death (PD)-1 (also known as CD279) is one of the best-known ICs,and is expressed on T cells,B cells,activated monocytes,dendritic cells (DCs) and natural killer cells[1].Its ligand PD-L1 (B7-H1,CD274) is expressed on antigen-presenting cells,macrophagocytes,nonhematopoietic cells and parenchymatous organs such as heart,lungs,placenta and liver.When PD-1 binds to PD-L1 (B7-H1,CD274)/PD-L2 (B7-DC,CD273),a signal that inhibits the proinflammatory ability of T cells and attenuates the function of cytotoxic T cells is delivered.T cell tolerance protects human tissues from immune-mediated tissue damage[2].However,PD-1 and PD-L1 pathways are also seized by tumors,which impairs tumor immunity and facilitates tumor survival.PD-1/PD-L1 inhibitors remove the inhibitory signals of T cells,enhance cytotoxicity and increase cytokine production.Thus,ICIs can enhance the antitumor effect,but they also increase the chance of inflammatory injury (Figure 1).
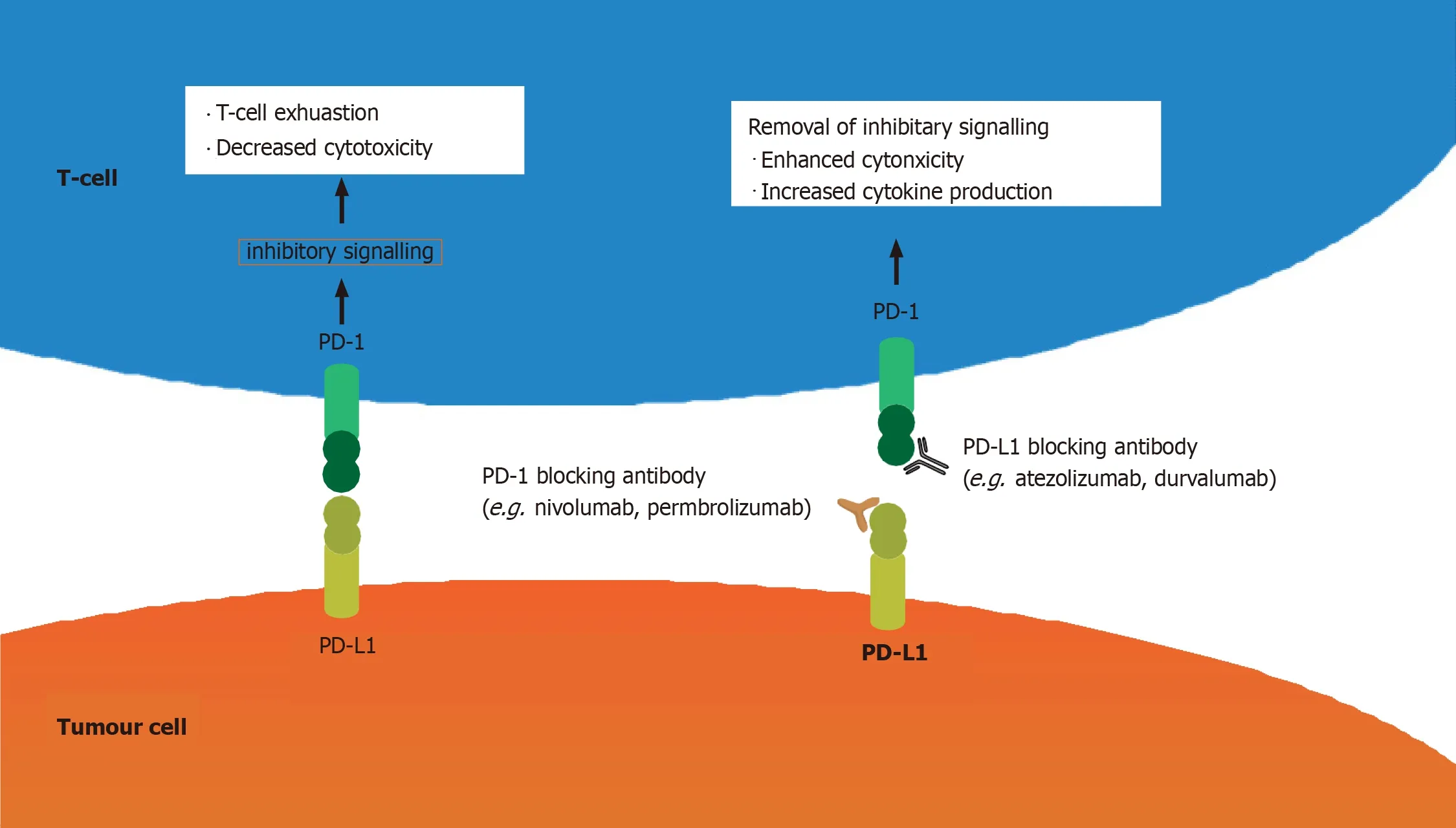
According to recent research,ICIs induce immune-related adverse events (irAEs) that involve the whole body,including skin (46%–62%),gastrointestinal tract (22%–48%),autoimmune hepatitis (7%–33%),endocrine system (12%–34%),respiratory system (3%–8%) and urinary system (1%–7%)[3].PD-1/PD-L1-inhibitor-associated autoimmune diabetes mellitus (DM) is rare,with an incidence rate of 0.1% in clinical trials[4].ICI-induced DM (ICI-DM) is an irreversible event that can be life-threatening if not promptly recognized.Its incidence has increased with the widespread use of immunotherapy.Therefore,it is important for clinicians to fully understand the pathogenic mechanisms of these treatments and their potential irAEs.
Sintilimab is a PD-1 inhibitor that was newly approved in China for treatment of relapsed or refractory Hodgkin’s lymphoma in February 2019[5],and it is now also a feasible treatment for a variety of solid tumors,including non-small cell lung cancer and esophageal cancer.Small cell lung cancer (SCLC) is a malignant tumor with rapid metastasis and poor prognosis.Treatment of SCLC with sintilimab alone or combined with other chemotherapeutic drugs is rare and there are no reports published to describe its clinical effects.Here,we present the first case of new-onset autoimmune DM in a patient with SCLC during treatment with sintilimab,along with marked antitumor efficacy.We also provide a review of case reports of ICI-DM.
A short walk from my house in Hampshire, on a hill overlooking the heathland, is a plaque1 marking the spot where Richard Pryce Jones deliberately2 crashed his Halifax bomber3 during the war. He could have parachuted to safety, but that would have meant crashing into the village. The epitaph(,) reads: He died that others might live.
Two weeks went by with hardly a word from my father. Then, one afternoon, I came home from school to find my mom sitting at the dining room table waiting to talk to me. I could see in her eyes that she had been crying. She told me that Dad had been there and that they had talked for a long time. They decided that there were things that the both of them could and would change and that their marriage was worth saving. Mom then turned her focus to my eyes.
The patient was diagnosed with SCLC on October 29,2019 in the First Affiliated Hospital of Zhejiang University.She underwent endobronchial ultrasound-guided transbronchial needle aspiration,and the results were suggestive of poorly differentiated cell carcinoma,considered to be SCLC.Immunohistochemical staining demonstrated CKpan(+),P40(),P63(+),Ki67 (50%+),TTF-1(+),CgA(+),Syn(+),CD56(+) and CD45().The patient immediately underwent concurrent chemotherapy and immunotherapy for SCLC (extensive).She received her first treatment,etoposide 82 mg,days 1–3;cisplatin 20 mg,days 1–3;and sintilimab 200 mg,day 1;EP plan) on October 30,2019.The patient came to our hospital to continue treatment.After we assessed her condition,she continued the EP treatment plan,but we reformulated the doses as follows:etoposide 240 mg,days 1–3;cisplatin 250 mg,days 1–3;and sintilimab 200 mg,day 1.This therapy did control her disease well,with decreased tumor markers and no metastases found on imaging.In the following days,she came to our hospital monthly for evaluation.Her blood glucose level was normal after treatment.After five cycles with the EP plan,we changed to sintilimab 200 mg and anlotinib 8 mg q.d.because of severe gastrointestinal adverse reactions.After three cycles of the new treatment,the patient developed lower urinary tract infectionsuch as urinary frequency,difficulty urinating,pain with urination,and hematuria.Therefore,we had to stop anlotinib and used levofloxacin to treat the infection.Hence,we used sintilimab monotherapy,and imaging showed good antitumor effects (Figure 2).During the treatment,the patient only had mild gastrointestinal symptoms such as nausea and poor appetite.
CASE PRESENTATION
Chief complaints
58. They lived happily ever afterward: The Grimms changed the story considerably138 to try to justify139 the father s redemption and ability to live happily ever after. However, many critics, such as Hans Dieckmann, find the ending disturbing and even unethical. The father, who was too weak to resist the evil suggestion of his wife and with her abandoned the children in the forest, is not only not punished for his highly immoral140 way of acting141 but even gets to enjoy the treasures the children bring back (Dieckmann 1986). Also note that part of their happiness centers on their acquisition of material wealth.Return to place in story.
History of present illness
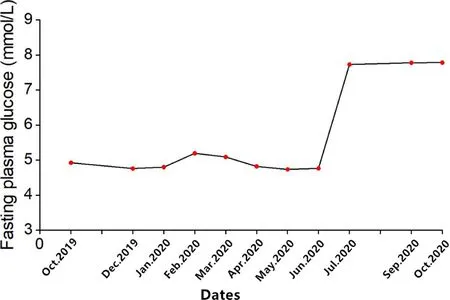
The patient initially developed polyuria and polydipsia and her blood glucose level showed a mild increase after 12 cycles of sintilimab,but the treatment was continued.Two months later,the patient presented with hyperglycemia up to 23.4 mmol/L (random blood glucose level) with strong positive uric sugar (++++) and hemoglobin A1c of 8.2%.
History of past illness
This study was conducted according to the advice of the Ethics Center of Zhejiang Provincial People’s Hospital.The patient’s written informed consent was obtained for publication of this case and any images or information that may identify the patient.
So the jackal came up to the panther, and asked politely, Panther, would you like me to look after your children while you are out hunting? I should be very much obliged, said the panther; but be sure you take care of them
Personal and family history
cells.Subsequently,she was switched to once-daily basal insulin detemir (long-acting insulin,10 U) plus thrice-daily premeal insulin aspart (fast acting insulin,11 U,respectively 3U,4U,3U three meals) subcutaneous injection for long-term treatment.
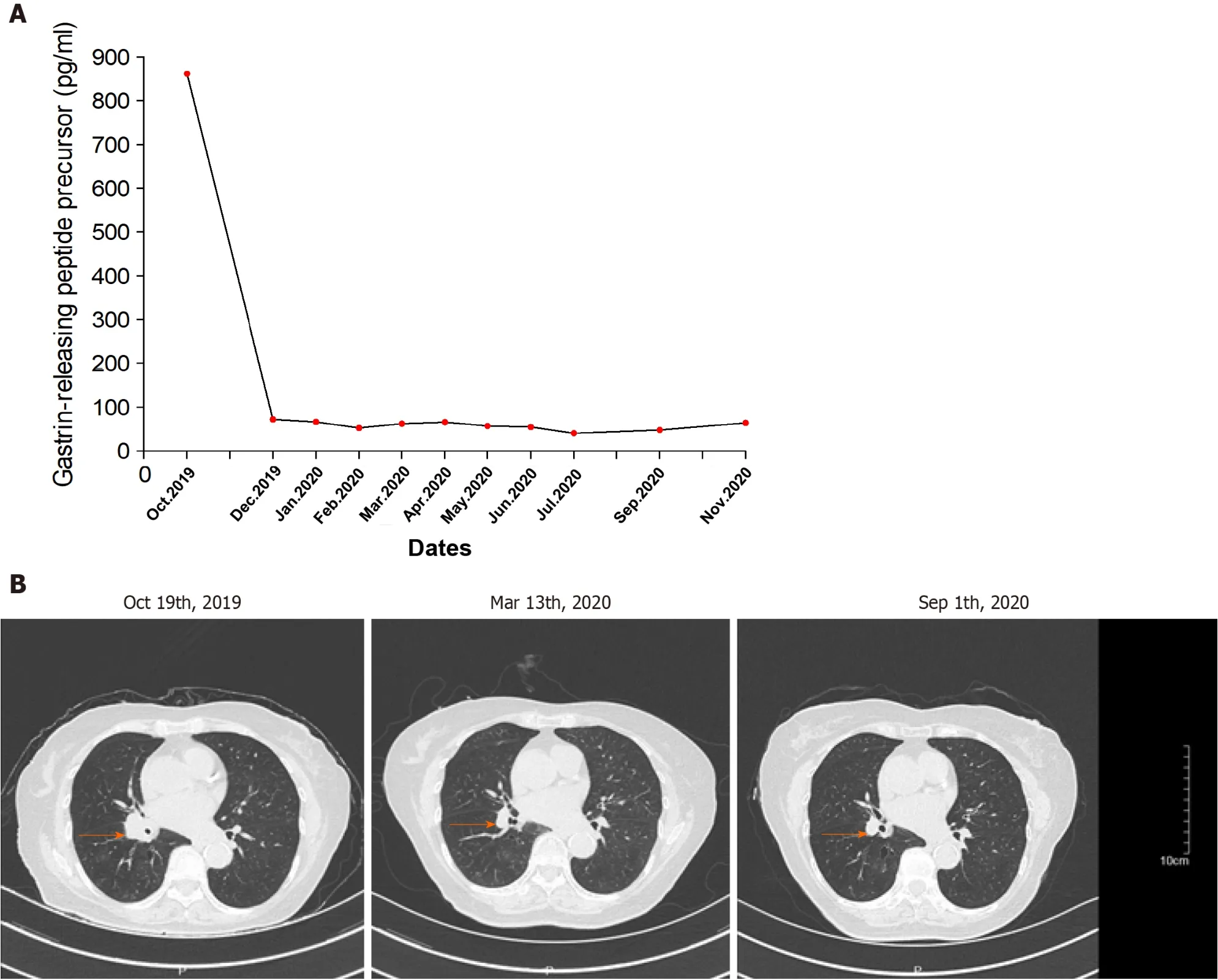
A 78-year-old Chinese woman was diagnosed with SCLC 1 year ago with no history of DM who presented with hyperglycemia up to 23.4 mmol/L (random blood glucose level) after 14 courses of sintilimab.The plasma glucose line shown in Figure 2.
Physical examination
According to the computed tomography scanning of the patient’s chest (Figure 3B),the tumor was shrinking,which indicated that anti-PD-1 therapy was effective.
First she chased away her cat, lest it should run away when the door was opened, then he heard her talking to herself and made out that her lamp wanted trimming, that she might see better who it was that knocked, and then that it lacked fresh oil, and she must refill it
Laboratory examinations
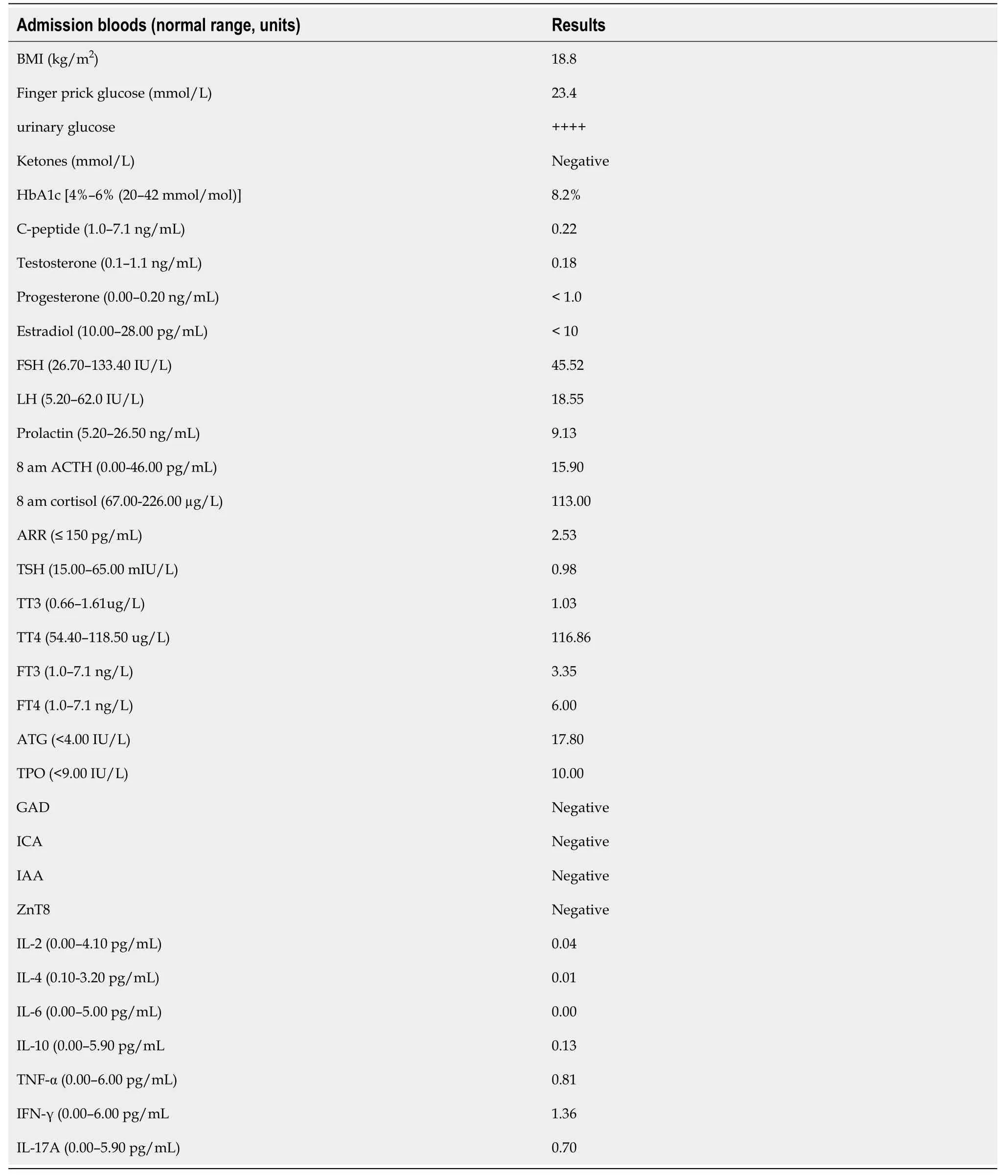
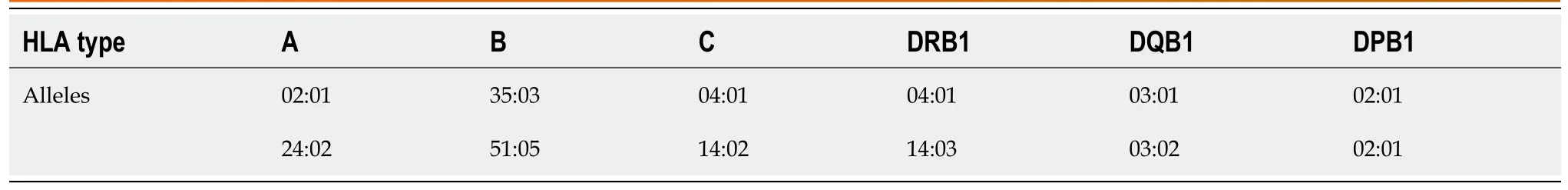
Other laboratory evaluation (Table 1) showed that urinary ketones were negative and blood pH was normal and the hypothalamic–pituitary–gonadal axis and hypothalamic–pituitary–adrenocortical axis were negative;but antithyroid autoantibodies were 17.80 IU/mL (normal<4.0 IU/mL) and antithyroid peroxidase autoantibodies were 10.0 IU/mL (normal<9.0 IU/mL).The islets antibodies tests were all negative,including anti-glutamic acid decarboxylase 65 (GADA) antibody,anti-islet cell antibody,and anti-insulin antibody tests were all negative.Moreover,zinc transporter 8 antibody levels were unavailable in our hospital.Human leukocyte antigen (HLA) class I and II,which was shown in Table 2,including HLA-A,B,C,DRB1,DQB1 and DPB1,were tested by polymerase chain reaction-sequence based typing (PCR-SBT).
Imaging examinations
Height:151 cm;weight:40.4 kg;body mass index:17.71 kg/m.Physical examination was no positive signs.
FINAL DIAGNOSIS
Intravenous fluid infusion,continuous subcutaneous insulin infusion (insulin infusion pump therapy) and other supportive treatments were administered.After 10 d of insulin infusion pump therapy,the patient’s plasma glucose returned to normal levels.We performed a simple oral glucose tolerance test that revealed that the fasting and 0–2-h insulin levels were 0.29 and 2.50 IU/mL;fasting and 0–2-h C-peptide levels were 0.22 and 0.52 ng/mL,which indicated an insufficient function of pancreatic islet β
If a sailor unties4 one knot, he has a fair wind; when he unties the second, it blows hard; but if the third and fourth are loosened, then comes a storm, which will root up whole forests
TREATMENT
The patient had no history of DM or autoimmune disease before treatment,and there was no medication,infection,thromboembolic event,or other factor that could cause hyperglycemia;according to the laboratory evaluation,thus,sintilimab-induced,newonset autoimmune DM was diagnosed.
The patient denied any other specific personal history.But she has family history of cancer,her grandmother died of lung cancer,whereas her father died of colorectal cancer.
OUTCOME AND FOLLOW-UP
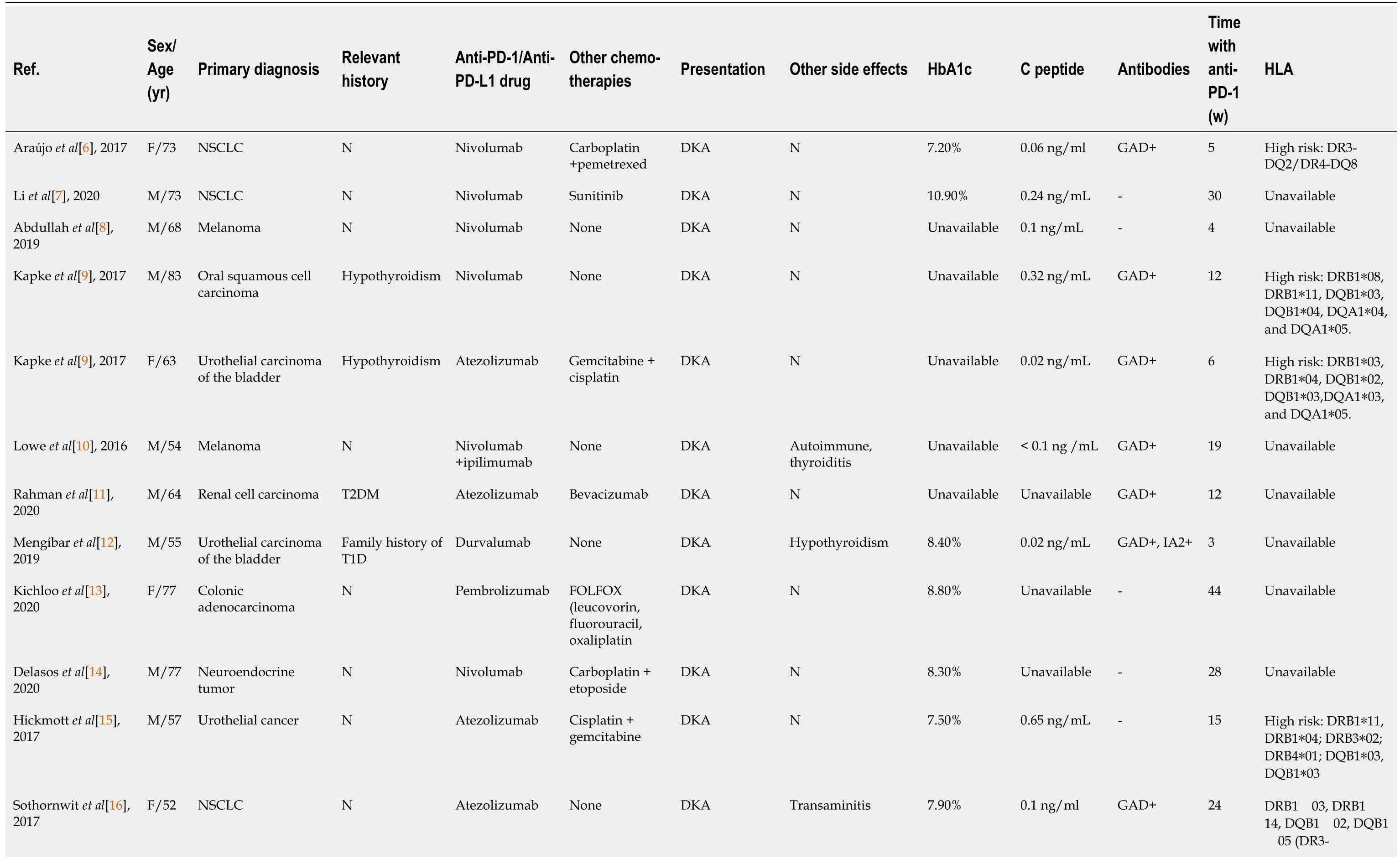
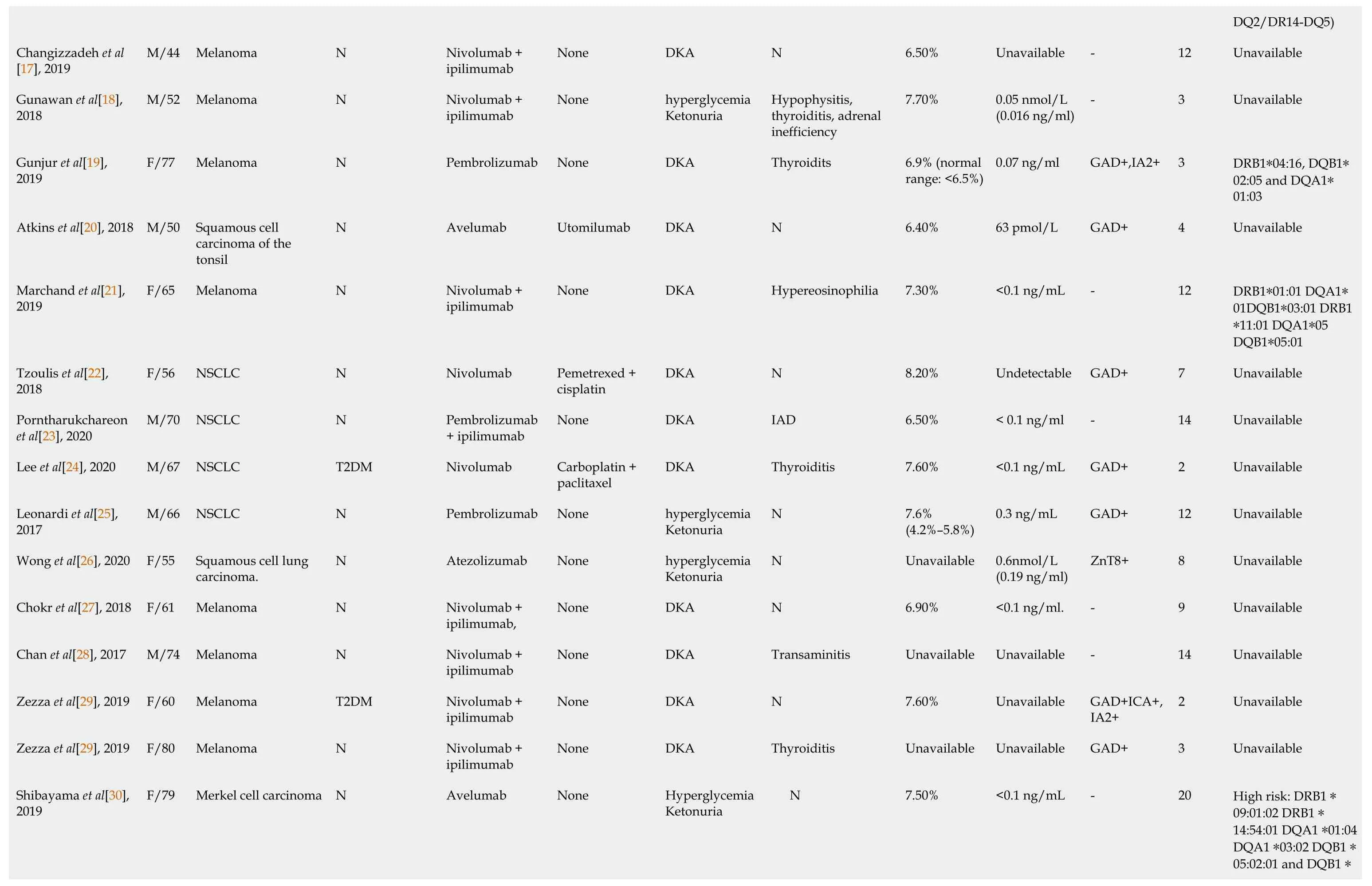
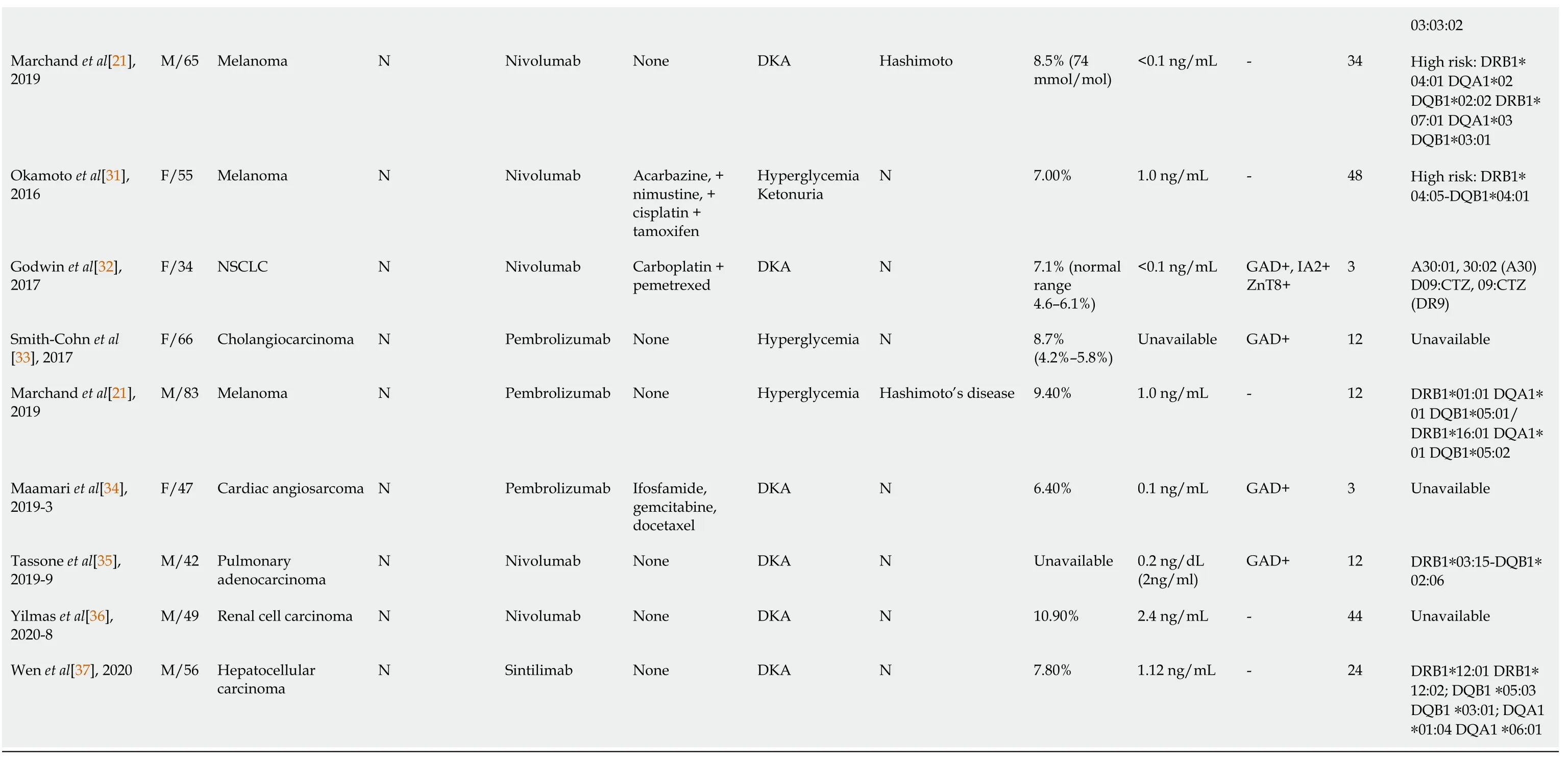
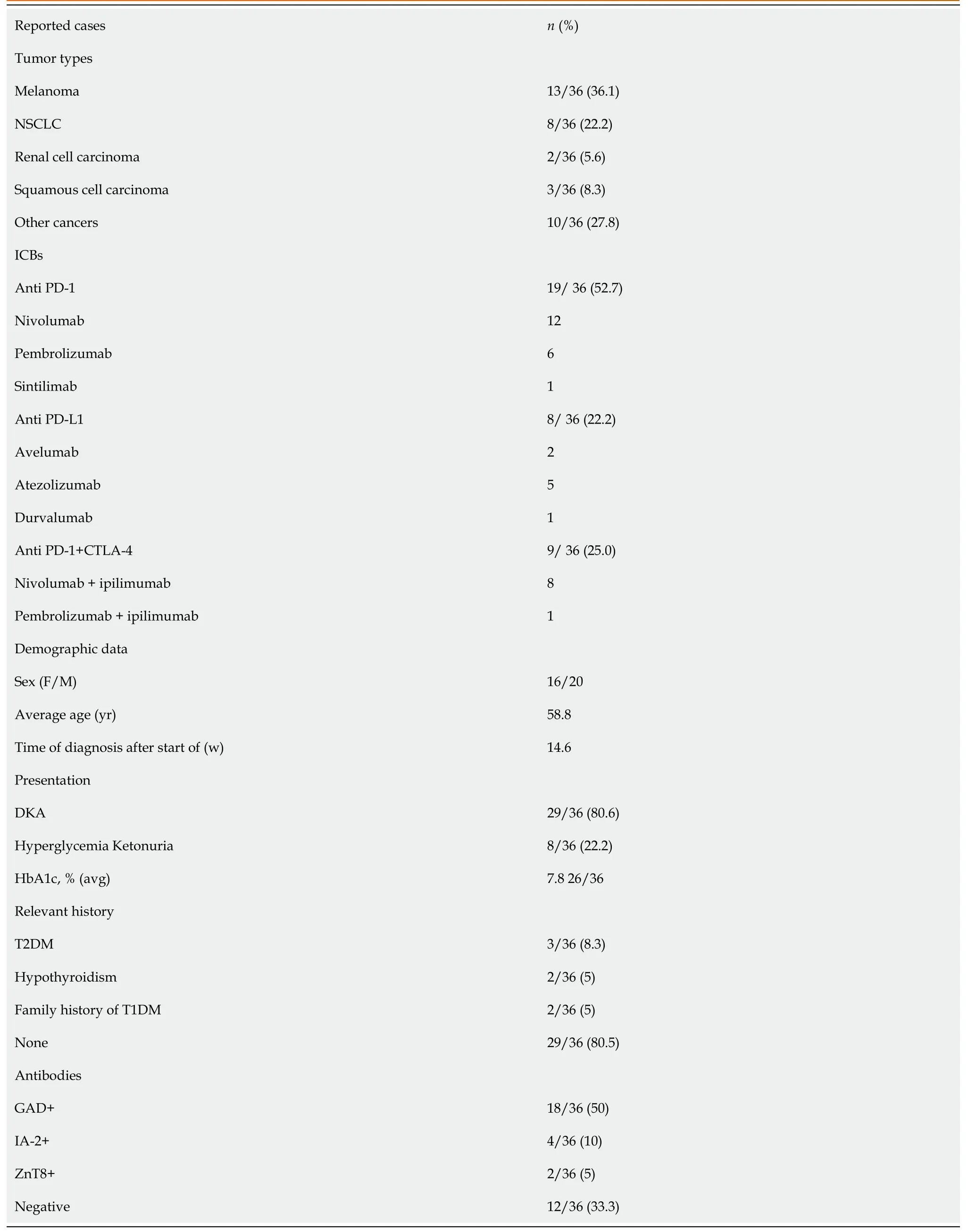
By the time the manuscript was completed,the patient was without evidence of SCLC recurrence with no further treatment since sintilimab (Figure 3).Other endocrine adverse effects such as thyroiditis and hypophysitis did not occur.We retrieved 36 relevant case reports from 2016 to 2020 in PubMed to determine the common features of ICI-DM (Table 3)[6-37].Table 4 summarizes the key features.
DISCUSSION
Sintilimab is a fully humanized IgG4 monoclonal antibody that binds to PD-1,then interferes with the interaction of PD-1 and its ligands (PD-L1 and PD-L2),thus activating and restoring the function of T cells,which contributes to an obvious antitumor effect.Accordingly,some normal tissues have been damaged in this process by the increase of cytokines.ICI-DM has rarely been reported as an irAEs of anti-PD1/PD-L1 therapy,and primarily in case reports.
In our review of case reports,the main tumor types were melanoma (13/36,36.1%) and non-SCLC (8/36,22.2%).The different treatment regimens included monotherapy with anti-PD-1 (19/ 36,52.7%) anti-PD-L1 (8/36,22.2%) or a combination of anti-CTLA-4 with anti-PD-1 (9/36,25.0%).Diabetic ketoacidosis (DKA) was the first sign of diabetes in 29 of 36 (80.5%) case reports,which is similar to 85.7% in another study[38],and the average time from initiation of anti-PD-1/PD-L1 therapy to diagnosis of ICI-DM was 14.6 wk (range 2–48 wk).Low C-peptide levels were present at diagnosis in 82% (23/28) of cases.In addition,26 of 36 patients presented with a median glycated hemoglobin level of 7.6% (average:7.8%;range:6.4%–10.9%),which is the same as in other studies[39,40].Most of the patients did not have relevant autoimmune history,which was only seen in 18.3% of our reviewed cases.These case reports have different definitions for ICI-DM.Since the syndrome has similarities with classic type 1 (T1)DM,most reports simply classified ICI-DM as T1DM,but ICI-DM has its own features.We found several significant features of ICI-DM:(1) Abrupt onset of hyperglycemia,and low to absent insulin C-peptide levels;(2) Rapid destruction of islets β cells,leading to endogenous insulin deficiency;and (3) High risk of DKA[39].In addition to the above features,ICI-DM does not have a “honeymoon period” like juvenile T1DM,nor does it have GADA as in latent autoimmune DM in adults[41].
Similar to a previous study[39],our autoantibody analysis was positive in 50% of patients for GADA.Some studies have demonstrated that GADA-positive patients developed ICI-DM in the first 2 mo after initiation of therapy,and GADA-negative patients developed ICI-DM after 2 mo of treatment[42].Patients with any positive diabetes autoantibodies at the time of presentation of ICI-DM have fewer cycles than those with negative autoantibodies.Our results showed that GADA-positive patients had ICI-DM onset at an average of 8 wk after immunotherapy compared with 22.8 wk in GADA-negative patients.It has been demonstrated that the interval from initiation of anti-PD-1/PD-L1 therapy and onset of ICI-DM is related to the presence/absence of GADA.Serological examination of GADA prior to anti-PD-1/PD-L1 therapy might be helpful for predicting the development of ICI-DM.In addition,several major histocompatibility complex and HLA molecules are associated with increased susceptibility to T1DM,especially HLA-DRB1,-DQB1 and –DQA1[43].Different combinations of DRB1,DQB1 and DQA1 determine the extent of haplotypic risk.The most susceptible HLA haplotypes are DRB1*0405–DQA1*0301–DQB1*0302,followed by DRB1*0401–DQA1*0301–DQB*0302,DRB1*0301–DQA1*0501–DQB1*0201,and DRB1*0402–DQA1*0301–DQB1*0302.Subsequently,DQB1*0302 allele is the key susceptibility allele[44].However,another study confirmed that DPB1*0301 and DPB1*0202 are also susceptible haplotypes for T1DM.Hence,the HLA typing of our patient (Table 2) showed a high risk of T1DM.Based on this evidence,there were seven patients with high-risk genes for T1DM among 13 patients tested.Accordingly,understanding the association between HLA and the development of ICI-DM by anti-PD-1/PD-L1 therapy is significant in predicting susceptible patients.When clinical features are discordant with the results of autoantibody testing,genetic risk score (GRS) could be an important addition to diagnosis of ICI-DM.This GRS summarizes risk-associated variation across the genome of T1DM[45].One limitation of our case was the lack of information before sintilimab,such as autoimmune antibodies and genetic factors like HLA genotypes that may predispose to endocrine irAEs.
Multiple studies have indicated both the PD-1 and CTLA-4 pathways in the pathogenesis of T1DM and suggest a synergistic effect between these two negative regulatory receptors to enhance autoimmune disorders.Furthermore,the incidence of ICI-induced endocrine irAEs is significantly higher in patients treated with combination immunotherapy compared with single immunotherapy.The incidence of thyroid dysfunction is high in patients treated with single PD-1 antibodies.In contrast,the incidence of hypophysitis is highest in patients treated with ipilimumab[46].Similarly,our review also found that combination of PD-1 inhibitor and anti-CTLA-4 therapy causes endocrine dysfunction.The most common combination was nivolumab and ipilimumab.An animal study found that single CTLA-4 blockers in nonobese diabetic (NOD) mice only induced diabetes in baby mice,while PD-1 blocked secondary diabetes in NOD mice at any age[47].A recent case reported that ipilimumab induced T1DM.The mechanism by which single anti-CTLA-4 therapy induced ICI-DM was unclear and needs further study[48].However,a randomized,double-blind,phase 3 study suggested that combination of immunotherapy significantly increases progression-free survival more than monotherapy does[49].Therefore,it is important for clinicians to consider whether to continue to use combination therapy when endocrine irAEs appear.
T1DM is caused by destruction of pancreatic β cells by virus infection,genetic factors and autoimmune disorders[32].Accordingly,the main mechanism of ICI-DM may be islet cell damage.There is an active interaction between β cells and immune cells during insulitis.This kind of interaction usually has a largely negative effect on β cells.An animal study has shown that PD-1 deficiency accelerates the occurrence and frequency of T1DM in NOD mice,and infiltration of pancreatic islets by T cells with strong T helper 1 polarization[50].In addition,animal and human experiments have shown that PD-L1 in insulin-positive cells of T1DM,but absent in nondiabetic individuals and type 2 DM,is mainly due to islet β cell expression[6].The present data indicate that interferon (IFN)-α and IFN-γ are the main regulators of PD-L1 expression in human pancreatic β cells,especially IFN-γ.IFN-γ suppresses autoreactive T cells by upregulating PD-L1.In other words,PD-L1 protects islet β cells to delay progression of DM and even prevent its onset[50-52].Yet,IFN-α and IFN-γ induce proinflammatory responses.For instance,HLA class I upregulation,cytokine production and endoplasmic reticulum stress are harmful to the human body,including the pancreas.Inhibition of signal transducer and activator of transcription 2 can prevent IFNαinduced HLA class I expression,and at the same time allow PD-L1 upregulation[53],but this lacks clinical validation.Therefore,the level of PD-L1 expression may serve as an additional criterion for irAEs after ICI treatment.PD-L1 expression can also be used as a prognostic marker of immunotherapy[54].In our patient,in spite of tumor necrosis factor-α,IL-1β and IFN-γ,all of the cytokines were normal during treatment with sintilimab,which suggest the particular pathogenic mechanism of ICI-DM.However,the precise mechanism mediating ICI-DM is still unclear.Further studies are required to elucidate the pathogenesis and background factors for this form of DM.
CONCLUSION
This is the second case of sintilimab-induced autoimmune DM.The first one was a recently published case report of autoimmune DM diagnosed in a patient with hepatocellular carcinoma[37].What makes our case different from others is that there was no DKA in the process of DM.This may be because patients were regularly monitored for plasma glucose level.This illustrates the importance of regular monitoring of glucose during immunotherapy for inhibiting progression of DM.Furthermore,based on the information collected in our review,we recommend measuring PD-L1 expression,HLA typing,islet cell antibody testing,C peptide measurement,or even determining T1DM-associated GRS for clinicians before or during immunotherapy.We can compare symptom severity and therapeutic efficacy in DM patients with or without a history of DM after treatment with PD-1/PD-L1 inhibitors in the future,so as to evaluate whether patients with potential risk of DM are suitable for treatment with PD-1/PD-L1 inhibitors.
 World Journal of Clinical Cases2022年4期
World Journal of Clinical Cases2022年4期
- World Journal of Clinical Cases的其它文章
- Surgical treatment of acute cholecystitis in patients with confirmed COVID-19:Ten case reports and review of literature
- Rituximab as a treatment for human immunodeficiency virusassociated nemaline myopathy:What does the literature have to tell us?
- Eustachian tube involvement in a patient with relapsing polychondritis detected by magnetic resonance imaging:A case report
- Endoscopic clipping for the secondary prophylaxis of bleeding gastric varices in a patient with cirrhosis:A case report
- Inflammatory myofibroblastic tumor after breast prosthesis:A case report and literature review
- Langerhans cell histiocytosis presenting as an isolated brain tumour:A case report
