An n-n type heterojunction enabling highly efficient carrier separation in inorganic solar cells
Gang Li(李刚) Yuqian Huang(黄玉茜) Rongfeng Tang(唐荣风) Bo Che(车波) Peng Xiao(肖鹏)Weitao Lian(连伟涛) Changfei Zhu(朱长飞) and Tao Chen(陈涛)
1Hefei National Laboratory for Physical Sciences at Microscale,CAS Key Laboratory of Materials for Energy Conversion,Department of Materials Science and Engineering,School of Chemistry and Materials Science,University of Science and Technology of China,Hefei 230026,China
2Institute of Energy,Hefei Comprehensive National Science Center,Hefei 230026,China
Keywords: n-n junction,carrier separation,solar cell,antimony selenosulfide,thin film
1. Introduction
Solar cells convert light directly into electricity.[1]In the device operation,the absorber materials harvest light to generate either electron-hole pairs or excitons.[2,3]In any case,the separation of opposite charge carriers is enabled by a junction,either homojunction or heterojunction.[4,5]Conventionally,the efficient separation is conducted by the semiconductor with different conductivity types, i.e., p- and n-type semiconductors,since which generates an internal electric field within the p-n junction.[6-8]In fact,the high efficiency solar devices usually rely on the p-n junction.[9-15]
In this study, we demonstrate a device configuration based on n-n type inorganic semiconductor heterojunction,where Sb2(S,Se)3and Sb2Se3are applied as absorber and interfacial materials, respectively. Both the optical and electrical characterizations indicate n-type conductivity of the as-synthesized Sb2(S,Se)3and Sb2Se3films. Interestingly,Sb2(S,Se)3and Sb2Se3form a heterojunction, instead of the formation of gradient light harvesting layer. This n-n type heterojunciton is found to generate highly efficient carrier separation and in turn high power conversion efficiency(PCE).
2. Experiments
The FTO glass was ultrasonically cleaned with deionized water, isopropyl alcohol, acetone and ethanol for 30 min respectively. It was cleaned by ultraviolet ozone for 15 min to remove residual organic species. The CdS buffer layer was grown on the FTO substrate about 60 nm by the chemical bath deposition(CBD)method(66°C,16 min). The solution contains cadmium nitrate (Cd(NO3)2), thiourea (CH4N2S), and ammonia (NH3·H2O) with concentrations of 0.15 mol·L-1,1.50 mol·L-1, and 1.56 mol·L-1, respectively. Afterwards,the sample was taken out and cleaned with water and alcohol and dried with nitrogen. Finally,the CdS film was treated by spin-coating of cadmium chloride (CdCl2) methanol solution(20 mg·mL-1) at 4000 rpm for 30 s and annealed (400°C,10 min) in air. The samples were cooled down naturally to room temperature.
Deposition of the Sb2(S,Se)3and Sb2Se3layer The Sb2(S,Se)3absorber layer was deposited by hydrothermal deposition according to our reported method.[16]The Sb2Se3was deposited on Sb2(S,Se)3absorption layer via thermal evaporation under 10-4Pa about 150 nm,where there are two evaporation sources. After the deposition of Sb2Se3, another evaporation source was used to evaporate the Se film about 20 nm on the Sb2Se3surface. The samples were then transferred to a nitrogen glove box and annealed on a heating plate at 175°C for 30 min.
Fabrication of solar cell devices Au electrode was deposited onto the Sb2(S,Se)3or Sb2Se3layer under a pressure of 5.0×10-4Pa to complete the device fabrication. The effective area of the device was defined as 0.12 cm2and the photovoltaic performance measurement was conducted using a mask with an area of 0.04 cm2.
Characterizations and measurements The surface and cross-section scanning electron microscopy were taken by the cold field-emission SEM (SU8200). The x-ray diffraction(XRD)of the samples was tested via the Bruker Advance D8 diffractometer (Cu,Kα,λ=1.5406 °A). The ultraviolet photoelectron spectroscopy(UPS)was measured through Thermo Scientific K-Alpha+. The ultraviolet-visible absorption spectra were obtained by the ultraviolet-visible spectrophotometer(UV-vis DRS, SOLID 3700). Meanwhile, the capacitancevoltage (C-V) curves were obtained from the same electrochemical workstation with a frequency of 50 kHz and the AC amplitude of 5 mV.The devices were tested using a Keithley 2400 source-measure unit under AM 1.5 G.The external quantum efficiency(EQE)was measured using a single source illumination system(halogen lamp)combined with a monochromator. The EQE values of the devices were measured using an illumination system with a single source (halogen lamp)in connection with a monochromator (Model SPIEQ. 200).Optical deep-level transient spectroscopy (ODLTS) measurement was performed to detect the defects of the devices using a Phystech FT-1230 HERA DLTS system equipped with a 10 mW laser with a 635 nm wavelength. The capacitance was measured using a Boonton 7200 capacitance meter with an upper limit frequency of 1 MHz. In the actual process to record the ODLTS spectra of the solar cell devices, the frequency was varied in a range of 1 Hz to 75 kHz to measure the capacitance.The samples were placed in a liquid helium cryostat with a temperature scan ranging from 120 K to 425 K at 2 K intervals.The reverse bias,optical pulse width,and period width were 0.3 V,10 ms,and 100 ms,respectively.
3. Results and discussion
In specific,the Sb2(S,Se)3thin film was fabricated by the hydrothermal deposition method onto a CdS substate, which generated flat, compact surface morphology (Figs. 1(a) and 1(b)),this film is labeled as SbSSe film for the convenience of presentation.[16]The atomic ratio of S/(S+Se)is measured as 0.68(Fig.S1). Afterwards,the Sb2Se3film was fabricated by thermal evaporation deposition,this approach allows the generation of flat surface morphology onto the Sb2(S,Se)3film with high surface coverage (Fig. 1(c)). The thicknesses of Sb2(S,Se)3and Sb2Se3in the optimized device are 250 nm and 150 nm,respectively(Fig.1(a)).

Fig.1. (a)Schematic illustration of the device structure and SEM cross section of a typical device,(b)SEM image of the as-synthesized Sb2(S,Se)3 film, (c)SEM image of the as-synthesized Sb2Se3 film onto the surface of Sb2(S,Se)3, (d)XRD patterns of the Sb2(S,Se)3 and Sb2Se3 films, (e)energy level alignment of each layer in the device. The Sb2(S,Se)3 and Sb2Se3 are abbriviated as SbSSe and SbSe,respectively.
XRD characterization was firstly applied to determine the crystal structure of the as-obtained film (Fig. 1(d)). Both the Sb2(S,Se)3and Sb2Se3display similar diffraction patterns.The diffractions at 15.508°, 17.368°, 24.848°, 28.228°and 28.948°can be indexed as (020), (120), (130), (230) and(211) of stibnite crystal structures. In addition, there is a slight peak shifting towards low diffraction angle, owing to the larger atomic radius of Se than that of S.Furthermore,because of the presence of Sb2Se3film, this film (denoted as SbSSe-SbSe film) exhibits diffraction peaks of both Sb2Se3and Sb2(S,Se)3. The light absorption spectra show that the corresponding bandgaps of Sb2(S,Se)3and Sb2Se3are 1.50 eV and 1.24 eV,respectively(Figs.S2(a)and S2(b)).
We then measured the energy level of the assynthesized film by ultraviolet photoelectron spectroscopy(UPS)(Figs.S2(c)and S2(b)). The valence band edge(VBE)and Fermi level (EF) of Sb2(S,Se)3are obtained as-5.6 eV and-4.44 eV, respectively. For Sb2Se3, the VBE andEFare respectively changed to-5.1 eV and-4.26 eV. Combining with the bandgap value measured from the light absorption spectra,the conduction band edges are-4.10 eV and-3.86 eV for Sb2(S,Se)3and Sb2Se3,respectively. In this regard, we are able to depict the energy alignment of the interfacial and absorber materials in the device(Fig.1(e)). Clearly,the Fermi levels are much closer to the conduction band in both Sb2(S,Se)3and Sb2Se3. We can readily conclude that both of the Sb2(S,Se)3and Sb2Se3display n-type conductivity.
We fabricated a complete device based on the above films to examine the photovoltaic performance. The control device was fabricated by depositing Au film directly onto the Sb2(S,Se)3surface, forming FTO/CdS/Sb2(S,Se)3/Au device structure, abbreviated as SbSSe device. Figure 2(a) shows the current density-voltage(J-V)characteristic curves of the best performing device under standard one Sun (AM 1.5 G,100 mW·cm-2) illumination. The control device appears SshapeJ-Vcurve, which may be due to the influence of the large valence band offset or surface defect states of Sb2(S,Se)3.The barrier formed between Sb2(S,Se)3and Au electrode affects the charge transfer.[17,18]The correspondingJ-Vparameters are summarized in Table S1. The control device delivers a PCE of 4.86% with open-circuit voltage (VOC) of 0.517 V,short-circuit current density (JSC) of 18.19 mA/cm2, and fill factor(FF)of 51.07%.After the deposition of Sb2Se3,it forms FTO/CdS/Sb2(S,Se)3/Sb2Se3/Au structure and is denoted as SbSSe-SbSe device.The PCE is strikingly increased to 7.75%,where theVOC,JSCand FF are 0.647 V, 18.46 mA/cm2and 64.86%, respectively. TheVOCand FF show essential improvement. The n-n type heterojunction was also demonstrated in organic solar cells and showed efficiency of 3%,[19]here the utilization of inorganic absorber and interfacial materials is promising for further improving the device performance.
In the control device, the difference between theEFof Au electrode and the valence band edge (EV) for Sb2(S,Se)3is 0.5 eV (Fig. 1(e)), such a large energy offset is not conducive to hole extraction. However, theEVof Sb2Se3in the SbSSe-SbSe device is 0.5 eV higher than that of the control device(Fig.1(e)),which is closer to the work function of Au.This energy alignment enables more efficient carrier transport,rendering the FF enhancement.

Fig.2. (a)The J-V curves,(b)EQE,(c)C-V,and(e)light intensity dependent VOC of the SbSSe and SbSSe-SbSe devices.
In the SbSSe device, obviously, the charge separation should be due to the extraction of Au electrode.In the SbSSe-SbSe solar cell, one can also consider that the Sb2(S,Se)3/Sb2Se3/Au semiconductor-metal contact is at work for the carrier separation, where a gradient Sb2(S,Se)3/Sb2Se3absorber facilitates the carrier transport.The formation of gradient absorber would enhance the carrier transport for high FF. At the same time, this gradient band structure would result in reducedVOCdue to the lowered bandgap. However, the fact is that theVOCexhibits essential increase from 0.517 V to 0.647 V.This contradiction implies new operational mechanism of the SbSSe-SbSe device. In this regard, we characterize the EQE of the devices (Fig. 2(b)).Surprisingly, both of the devices display nearly identical onset wavelength at~850 nm, in accordance with the spectral response of the Sb2(S,Se)3film. If the absorber material of the device is Sb2(S,Se)3/Sb2Se3,the onset wavelength should be extended to>1000 nm since the band gap of Sb2Se3is 1.2 eV. In fact, there is no red shift in the response spectrum of the SbSSe-SbSe solar cell. In this case, the Sb2Se3layer was post-annealed at low temperature(175°C),this annealing process is not able to generate crystalline Sb2Se3for serving as light harvesting material. Instead,it plays a role as interfacial material. Therefore,on the ground of the changes inVOCand EQE, we conclude that the Sb2Se3facilitates the formation of n-n type heterojunction where Sb2(S,Se)3serves as the light-harvesting material.
A possible process of electron and hole transport is shown in Fig.3. For the SbSSe device, Sb2(S,Se)3/Au forms Ohmic contact (Fig. 3(a)), the carrier separation is enabled by the work function difference in the two electrodes.[19]For the SbSSe-SbSe device, the Fermi level alignment between Sb2(S,Se)3and Sb2Se3would form an electric field,which facilitates the carrier separation and transport and impedes the interface recombination(Fig.3(b)).

Fig. 3. The schematic illustration of the electron and hole transport in (a)SbSSe and(b)SbSSe-SbSe devices.
To examine the heterojunction quality of the device,capacitance-voltage (C-V) profiling was measured at room temperature and the frequency of 50 kHz in dark condition(Fig. 2(c)). TheVbiof the devices can be estimated by the point where the straight extension of the descending part of the curve intersects theX-axis. TheVbiof the SbSSe control device and SbSSe-SbSe device are 0.527 V and 0.676 V,respectively. This result is in good agreement with theVOCchanges,indicating that the n-n heterojunction between Sb2(S,Se)3and Sb2Se3increases the internal electrical field.
Furthermore, to analyze how the junction influences the recombination kinetics of two devices under working conditions,the relationship betweenVOCand light intensity(I)was tested(Fig.2(d)),in which theVOCand logarithm ofIsatisfy the equation[20,21]
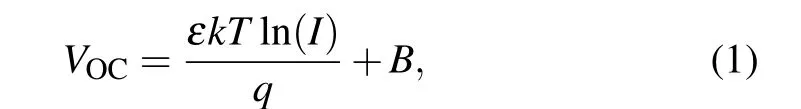
whereε,k,T,qandBrepresent the ideal factor, Boltzmann constant, absolute temperature, fundamental charge and constant, respectively. Fitting the lines of ln(I) andVOC, we can get the slopeεkT/q. Compared to the control device(2.73kT/q), the SbSSe-SbSe device shows the smaller slope(1.39kT/q), which indicates that the n-n type heterojunction can also alleviate the trap-assisted recombination under lighting condition.
To understand how the formation of n-n type junction influences the defect properties of the absorber materials in the device, we conducted deep level transient spectroscopy(DLTS) analysis (Fig. 4(a)). DLTS is a powerful tool for detecting the defect level,type and concentration in photovoltaic devices.[22,23]A voltage or light pulse is applied to fill the device trap and change the relevant capacitance. The transient capacitance is monitored under different temperatures,which exhibits the charge state at the deep level defect center.It should be pointed out that the signal of DLTS is seriously distorted in the FTO/CdS/Sb2(S,Se)3/Au device (Fig. S3), so that the result is not valid,due to the abrupt energy barrier between Sb2(S,Se)3and Au.[24,25]Instead,a conventional Spiro-OMeTAD hole transporting material based SbSSe device(i.e.,FTO/CdS/Sb2(S,Se)3/Spiro-OMeTAD/Au) is used here as a comparison. According to this characterization and Arrhenius plots(Fig.4(b)),the absorber materials display n-type conductivity.
The defect energy level, concentrations (NT), and crosssections(σ)are calculated and summarized in Table S2. Obviously, the two devices exhibit different types of defects.There are three kinds of electron trap states detected in the n-n heterojunction-based device, which are denoted as E1,E2, and E3 (donor defects), respectively. The corresponding energy levels(ET)are 0.237 eV,0.560 eV and 0.774 eV below the conduction band edge(CBE).The energy level alignment(Fig. 4(d)) shows that trap E1 is less than 0.3 eV away from CBE and away fromEF,so it is not a major defect deteriorating the device efficiency.[23]In this case,the E2 and E3 are detrimental defects in the device. The Sb2(S,Se)3/Spiro-OMeTAD based device,on the other hand,exhibits two hole traps which are denoted as H1 and H2 (acceptor defects) with energy levels of 0.465 eV and 0.699 eV above the VBM (Fig. 4(c)).Both of them are deep-level defects. Furthermore,there is no significant difference of capture cross-sectionσin both devices. However, the trap density (NT) in SbSSe-SbSe device is essentially lower than that in Sb2(S,Se)3/Spiro-OMeTAD based device. Therefore, the SbSSe-SbSe device is less defective than that of the SbSSe device,although it exhibits one more defect type.
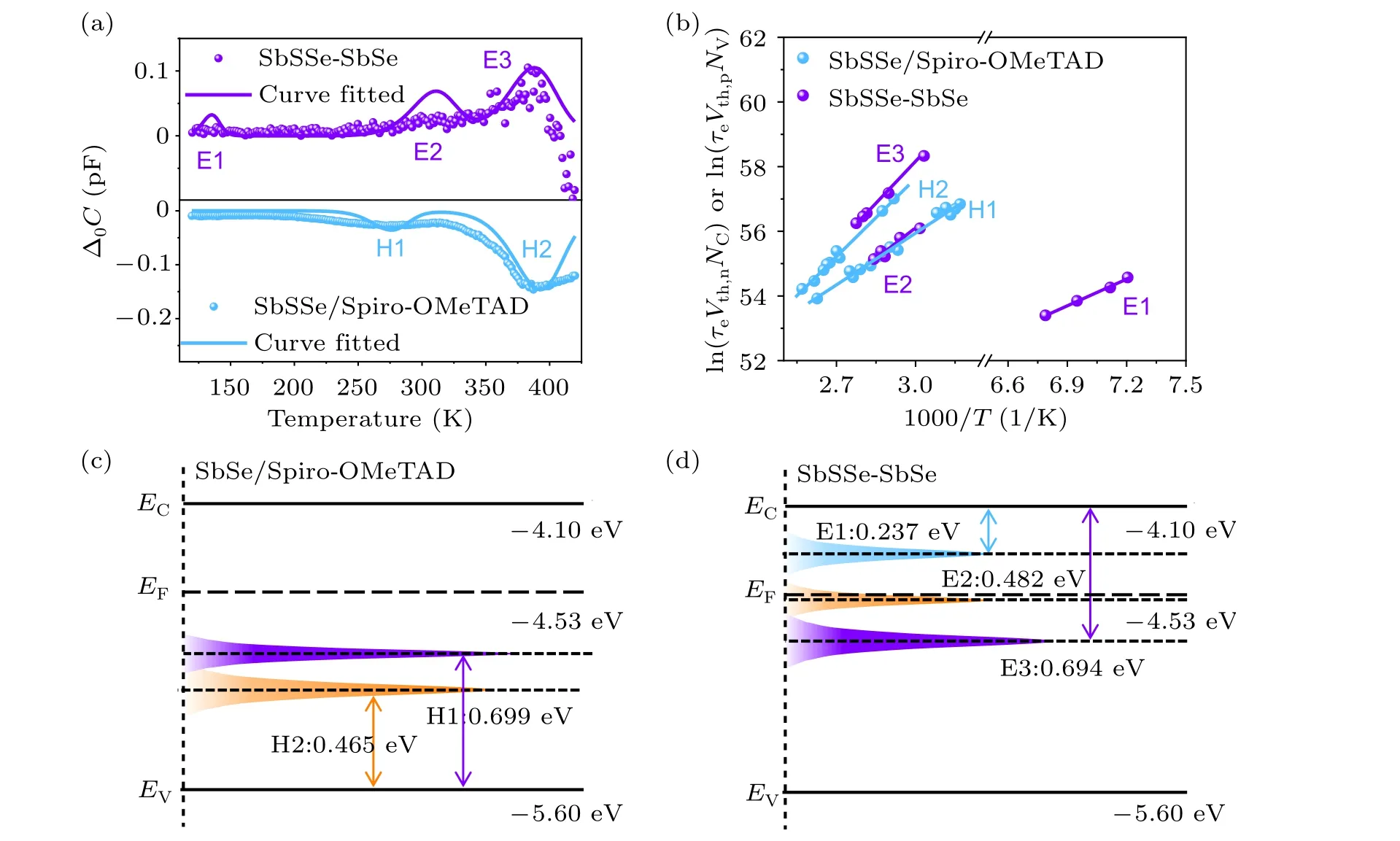
Fig.4. (a)O-DLTS signals from SbSSe and SbSSe-SbSe devices,(b)Arrhenius plots obtained from O-DLTS signals. (c)-(d)Schematic diagrams of the defect energy levels of the absorber materials.
The fabrication of Sb2Se3onto the surface of Sb2(S,Se)3is through thermal deposition followed by a brief annealing in selenium vapor,this post-deposition and thermal treatment would induce the change in materials composition and defect properties. In addition, the fabrication of organic Spiro-OMeTAD hole transporting layer is via spin-coating approach with a mild annealing, which does not influence the absorber layer considerably.[16]In this case,the utilization of inorganic material possesses triple advantages: (1) facilitating the formation of optimized interface due to the identical crystal structure between Sb2(S,Se)3and Sb2Se3,(2)forming junction that leads to more efficient carrier separation and transport and(3)modifying the defect properties of the pre-formed absorber layer.
4. Conclusion
In summary, we have demonstrated inorganic solar cells with n-n type heterojunction. We found that this device configuration is able to efficiently drive the carrier separation.Compared with semiconductor-metal contact, this n-n type heterojunction exhibits remarkably stronger capability of carrier separation and transport. This proof-of-concept study enables versatile materials utility,interfacial engineering as well as the defect manipulation.The study in the n-n type solar cell is also expected to bring about more fundamental findings in the photovoltaic energy conversion process.
Acknowledgments
Project supported by Institute of Energy, Hefei Comprehensive National Science Center (Grant No. 21KZS212),the National Key Research and Development Program of China (Grant No. 2019YFA0405600), the National Natural Science Foundation of China (Grant Nos. U19A2092 and 22005293),the China Postdoctoral Science Foundation(Grant No.2021M693045),and Collaborative Innovation Program of Hefei Science Center,Chinese Academy of Sciences.
- Chinese Physics B的其它文章
- Surface modulation of halide perovskite films for efficient and stable solar cells
- Graphene-based heterojunction for enhanced photodetectors
- Lithium ion batteries cathode material: V2O5
- A review on 3d transition metal dilute magnetic REIn3 intermetallic compounds
- Charge transfer modification of inverted planar perovskite solar cells by NiOx/Sr:NiOx bilayer hole transport layer
- A low-cost invasive microwave ablation antenna with a directional heating pattern

