Angiography findings and endovascular management of acute nonvariceal gastrointestinal bleeding: A pictorial essay
Haiyang Yu, Junhao Mei, Lihao Qin, Zhongzhi Jia
Department of Interventional and Vascular Surgery, Changzhou No. 2 People's Hospital, Changzhou, 213003, China
Keywords:Gastrointestinal Bleeding Angiography Management Embolization
ABSTRACT Patients with acute nonvariceal gastrointestinal bleeding (GIB) not treatable via endoscopic therapy require angiography and endovascular management. If the source of the bleeding can be identified on angiography, the bleeding can be controlled with minimal complications by endovascular treatments such as intra-arterial infusion of vasopressin, embolization,covered stent placement,or a combination thereof.This pictorial essay reviews the angiographic findings for and the endovascular management of acute nonvariceal GIB.
1. Introduction
Acute nonvariceal gastrointestinal bleeding (GIB) is a common problem,resulting in as many as 390,000 hospital admissions per year in the United States.The mortality rate for GIB,which ranges from 3.0%to 8.8%, has not changed substantially over the past five decades.1Endoscopic therapy remains the primary modality for nonvariceal GIB management.2,3However, in a subset of patients with GIB, it is ineffective.4For these patients, angiography (which is considered the gold-standard imaging technique for nonvariceal GIB) with an alternative intervention such as endovascular therapy is necessary to control bleeding.5,6
To help clinicians better manage acute nonvariceal GIB,this pictorial essay illustrates the angiographic features of this condition as well as the various endovascular therapies available.
2. Etiology
Upper GIB develops in the esophagus,stomach,or duodenum and has an incidence of 47/100,000.7The most common cause of acute upper GIB is a gastric or duodenal peptic ulcer, followed by mucosal erosive diseases of the upper gastrointestinal tract and malignancy.7Lower GIB develops in the small bowel,colon,or anorectum and has an incidence of 33/100,000.7The most common cause of acute lower GIB is diverticular bleeding,which accounts for more than 20%of acute lower GIB-related hospital admissions, followed by anorectal diseases, colitis, radiation proctitis, iatrogenic-induced bleeding, vascular malformations, and colorectal cancer.8
3. Angiographic evaluation
Angiographic evaluation of a patient with acute nonvariceal GIB should begin with superselective catheterization of the artery supplying the most likely site of the bleeding as determined via clinical,endoscopic,and imaging data. A road map providing real-time guidance for catheterization can be created by overlaying the angiographic image on live fluoroscopic images.
Transfemoral arterial access is used in most patients with GIB who undergo angiography. To successfully identify the bleeding source via angiography,the bleeding must be active at the time of the examination.Bleeding rates of 0.5-1.0 mL/min have traditionally been considered necessary to angiographically demonstrate contrast extravasation9;however, digital subtraction angiography may be far more sensitive in this regard than was previously thought.10
The main limitation of angiography is its failure to detect the bleeding source in a large number of cases.Low sensitivity may be due to a variety of factors, including intermittent bleeding, vascular spasm, administration of vasoconstrictor drugs, and slow arterial, venous, or small vessel bleeding. Ways of improving sensitivity include altering the timing of angiography,using superselective catheterization,increasing the amount of iodinated contrast medium,foregoing the use of vasoconstrictor drugs and enlarging the image during the procedure.11Use of various magnification views and alternative projections can also be helpful in identifying the bleeding source.Another limitation of angiography is the high incidence of rebleeding after embolization, with rates of approximately 33%in cases of upper GIB and 21%in cases of lower GIB.10,12
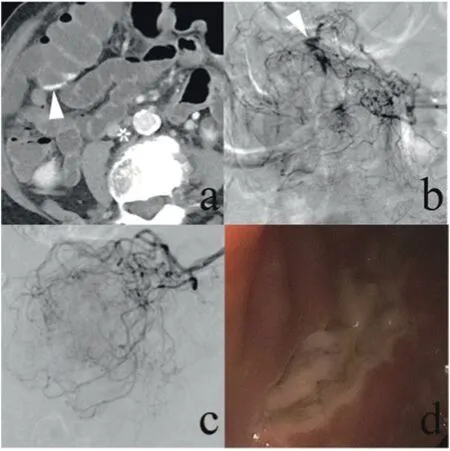
Fig. 1. A 97-year-old woman reported multiple episodes of bright red blood from the rectum. (a) A contrast-enhanced computed tomography scan demonstrated small amounts of high-density fluid within the colon (arrow). (b) Conventional angiography demonstrated contrast extravasation (arrow). The bleeding was being supplied by multiple small branches of the middle colic artery, so superselective embolization was not an option. Vasopressin infusion was therefore initiated via a microcatheter at a rate of 0.03 U/h. (c) Focal contrast extravasation was no longer seen on angiography one day after the procedure. (d) When endoscopy was performed one week later, an ulcer of the colon was identified.
4. Positive angiographic findings
Positive angiographic findings for GIB include direct and indirect signs. Contrast extravasation into the bowel lumen is a direct, although infrequently observed,sign of active bleeding in patients with GIB.13-15Commonly encountered indirect angiographic signs include delayed focal contrast stasis, intramural pooling, aneurysm, pseudoaneurysm,arteriovenous shunt,a submucosal vessel,and early venous drainage due to angiodysplasia, neovascularity, mucosal or extramucosal hyperemia,arterial wall abnormalities, vessel caliber changes or sudden truncations.16,17Mass lesions can be identified by the presence of tumor vascularity,encasement or narrowing of regional arteries,or mass effects such as draping and displacement. Calcification may indicate the formation of an aneurysm.
5. Endovascular therapies
Endovascular management of acute nonvariceal GIB takes three forms: 1) infusion of a vasoconstricting medication, 2) mechanical occlusion of the artery responsible for the bleeding, and 3) endovascular repair of the artery responsible for the bleeding.5,18-20Some patients may need a combination of these techniques.
5.1. Vasopressin infusion
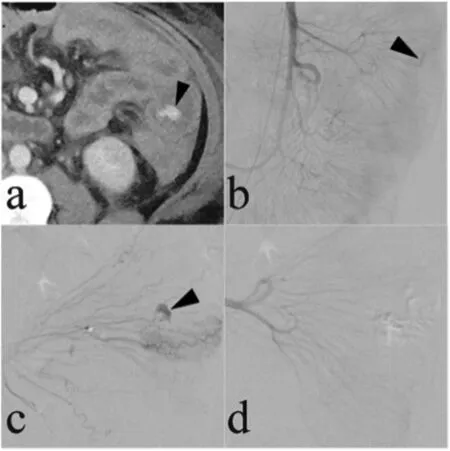
Fig.2. A 57-year-old woman presented with bright red blood in the rectum.(a)A contrast-enhanced computed tomography scan demonstrated small amounts of high-density fluid within the intestine (arrow). (b, c) Superior mesenteric artery arteriogram and superselective arteriogram demonstrated contrast extravasation(arrows).(d)Superselective arteriogram showed that the contrast extravasation was no longer present after superselective embolization with gelatin sponge particles was performed.
Vasopressin is generally considered a safe vasoconstrictor for the control of GIB. Superselective transcatheter infusion of vasopressin proximal to a mesenteric arterial bleeding source reduces blood flow,thereby lowering the perfusion pressure and permitting clot formation at the lesion. Vasopressin infusion has some disadvantages, including numerous side effects, high rebleeding rates, and prolonged catheterization times (up to several days). Additionally, it should not be used when the bleeding originates from a large-diameter artery or in patients with severe coronary artery disease, extreme hypertension, limb ischemia,or cardiac arrhythmias.Nevertheless,vasopressin can be used to treat microcatheter-inaccessible lesions and diffuse mucosal oozing(Fig. 1) and to control multiple bleeding sites in high-risk surgical patients.
5.2. Embolization
Embolization is an effective means of interrupting blood flow to the bleeding source while maintaining bowel viability.4Although there is a risk of bowel ischemia and infarction, the coaxial catheter systems and embolic agents currently used for embolotherapy allow for selective and precise treatment, thus decreasing the risk of other complications.Research has shown that major adverse events occur in less than 2% of GIB patients treated via embolization.
Empiric embolization should be considered when the bleeding is localized but cannot be controlled via endoscopy and when extravasation is not observed on angiography.20,21The clinical outcomes of empiric embolization are similar to those of non-empiric embolization.22,23In general, embolization should not be attempted unless a microcatheter can be advanced close to the bleeding point so that the embolic agent can be deployed as selectively as possible.
Various agents can be used for embolization. The most commonly used agents include absorbable gelatin sponge particles, particulate agents(polyvinyl alcohol),spherical agents(embospheres),glue(N-butyl cyanoacrylate glue and Onyx glue), coils (microcoils, detachable coils),and the Amplatzer vascular plug.16,18,24
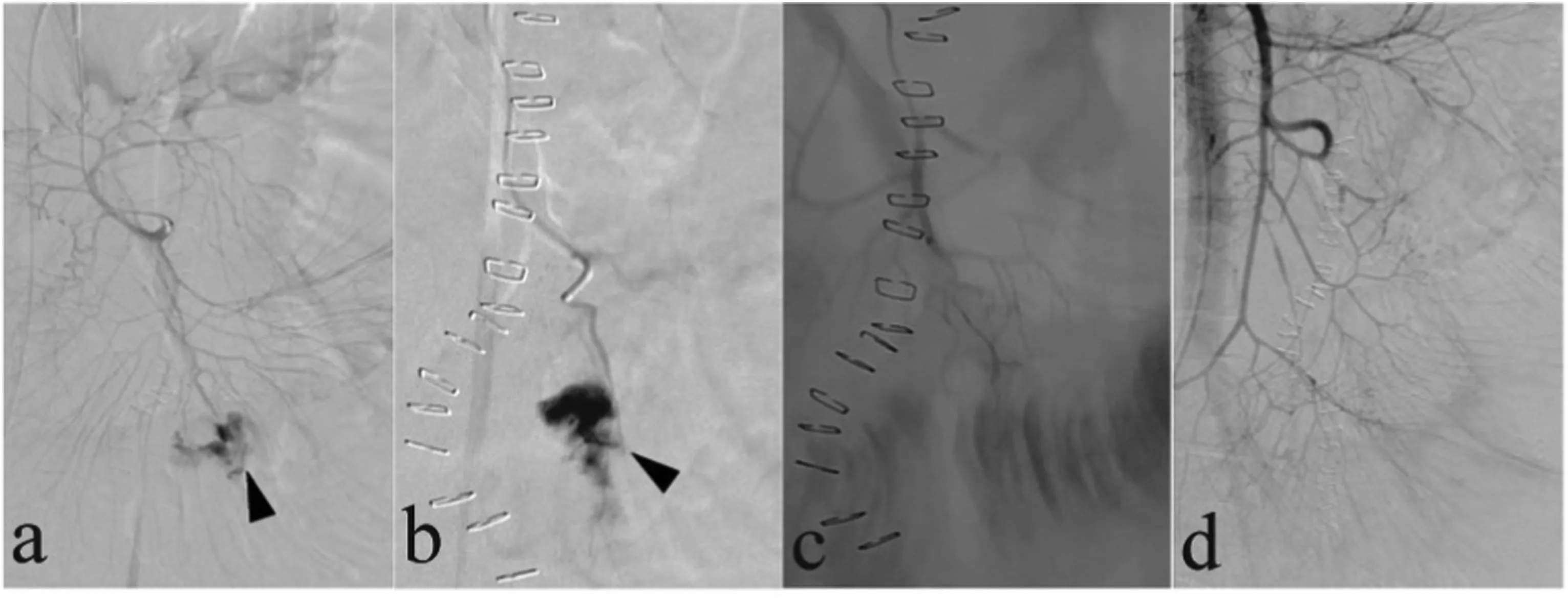
Fig. 3. A 43-year-old woman with a history of Crohn's disease who had undergone ileostomy surgery presented with massive bleeding from the ostomy. (a, b)Superior mesenteric artery arteriogram and superselective arteriogram demonstrated contrast extravasation (arrows). (c) Superselective embolization with gelatin sponge particles was performed.(d)Superior mesenteric arteriogram obtained after embolization demonstrated that the contrast extravasation was no longer present.
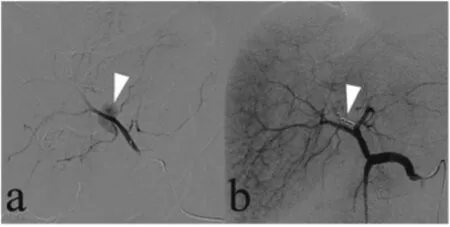
Fig. 4. One day after undergoing biliary lithotomy for gallstones,a 77-year-old man presented with approximately 200 mL of bright red blood that had drained via a T-tube placed in the common bile duct during the lithotomy. (a) Superselective hepatic artery arteriogram demonstrated the presence of a pseudoaneurysm(arrow).(b)The pseudoaneurysm was no longer present(arrow)on the hepatic artery arteriogram after embolization with microcoils was performed.
Absorbable gelatin sponge particles and particulate agents are frequently used for embolization(Figs.2 and 3).A disadvantage common to all particulate agents is that some particles may reach the tissue parenchyma distal to collateral vessels or may reflux into nontarget arteries. A disadvantage of absorbable gelatin sponge particles is the high incidence of rebleeding after embolization. Additionally, most particulate embolic agents must be mixed with iodinated contrast medium to be visible during administration. In cases with high rates of extravasation,particulate agents and absorbable gelatin sponge particles may be ineffective if they pass directly into the bowel lumen or another extravascular space.
The coils used for embolization have good radiopacity,which allows for precise deployment.Consequently,the arterial perfusion pressure to the bleeding source is reduced while sufficient collateral flow is preserved. Intra-arterial coil placement is analogous to the placement of a surgical ligature. The coils physically occlude the vascular lumen and decrease perfusion pressure, while the attached synthetic fibers maximize thrombogenicity (Fig. 4). Placement of the coil distal to the proximal occlusion prevents the continued bleeding that can occur if retrograde perfusion of the site is performed. Alternatively, separate collateral vessels feeding the site of extravasation can be selectively catheterized and embolized sequentially(Fig.5).
Liquid embolic agents,such as glue,have also been used to treat acute GIB(Fig.6).19,25An advantage of liquid agents is that they can be used in small caliber vessels.However,the operator must be familiar with their use to achieve optimal outcomes while minimizing the risk of complications.
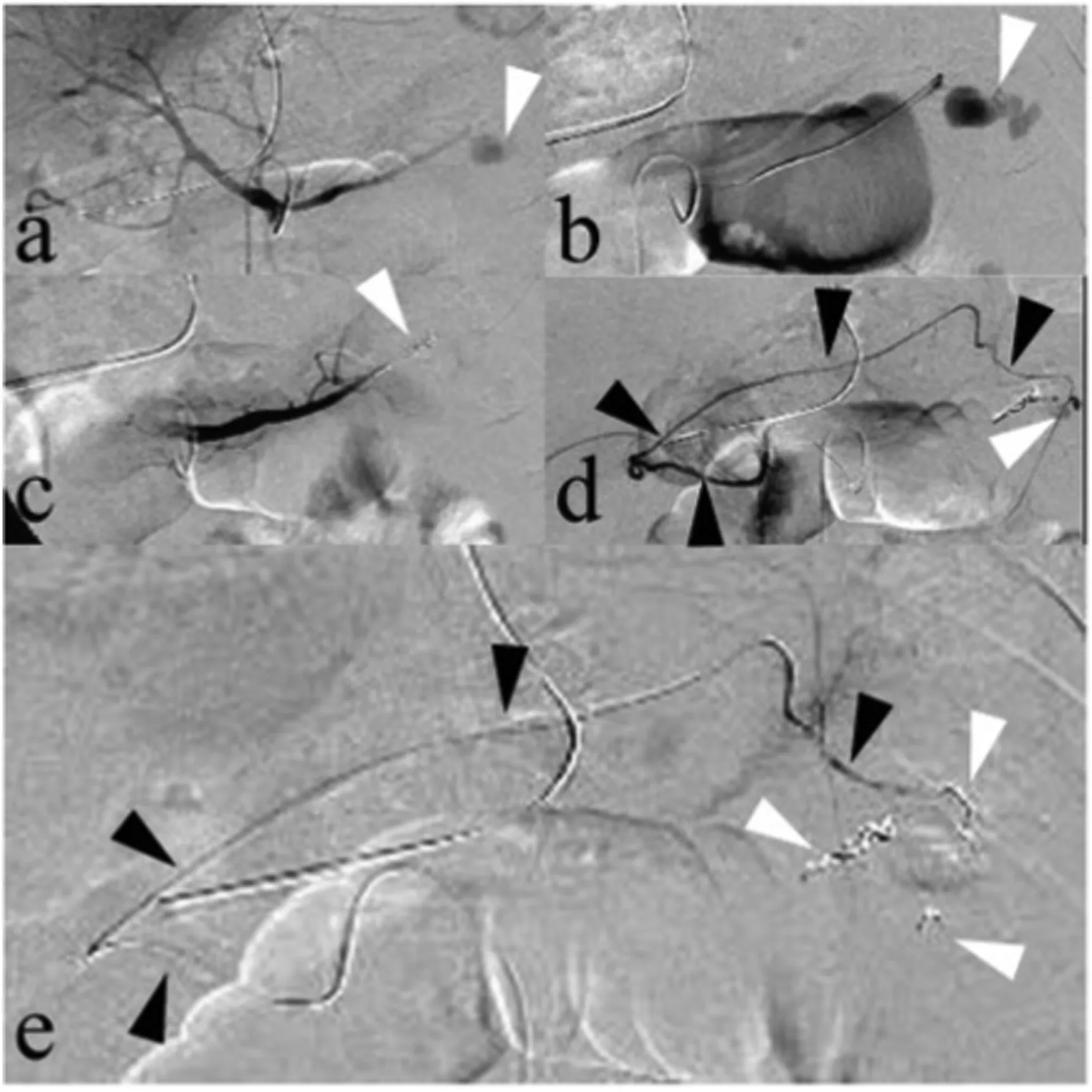
Fig. 5. A 47-year-old woman presented with recurrent massive upper gastrointestinal bleeding after undergoing a gastrectomy. (a, b) Celiac artery arteriogram and superselective left gastric artery arteriogram showed an area of contrast extravasation of the left gastric artery (arrows); this was identified as the site of bleeding. (c) The left gastric artery was embolized with microcoils(arrow). (d) Superselective right gastric artery (black arrows) arteriogram demonstrated an area of contrast extravasation (white arrow), and separate collateral vessels were found to be feeding the site of extravasation. (e) The distal and proximal areas near the site of extravasation of the right gastric artery(black arrows) were embolized with microcoils (white arrows).
5.3. Covered stent placement
Covered stent placement is commonly used to repair the artery responsible for the bleeding.26It has also been used to repair vascular lacerations, pseudoaneurysms, and arteriovenous fistulae.26,27Covered stents can be inserted in aneurysms with a narrow neck(Fig.7)and after coil failure(Fig.8).
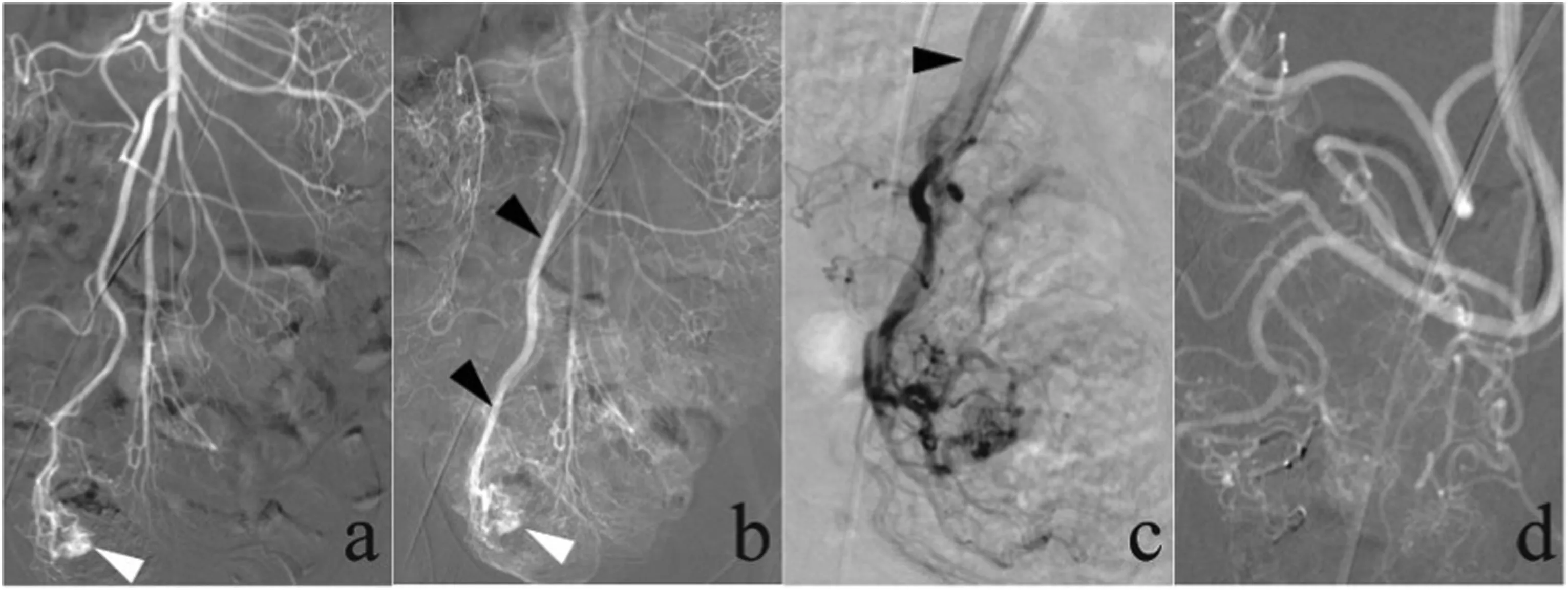
Fig.6. A 27-year-old man presented with a 5-year history of melena.Computed tomography angiography demonstrated evidence of cecal angiodysplasia(not shown).(a,b)Superior mesenteric arteriogram demonstrated the presence of an arteriovenous malformation,a large angiodysplasia in the cecum with an abnormal tangle of arterial vessels (white arrows), and an early draining vein (black arrows, b). (c) The early draining vein (black arrow) was identified on superselective ileocolic arteriograms, and the presence of an arteriovenous malformation was confirmed. (d) After embolization with Onyx glue was performed, an ileocolic arteriogram demonstrated that the arteriovenous malformation was no longer present.
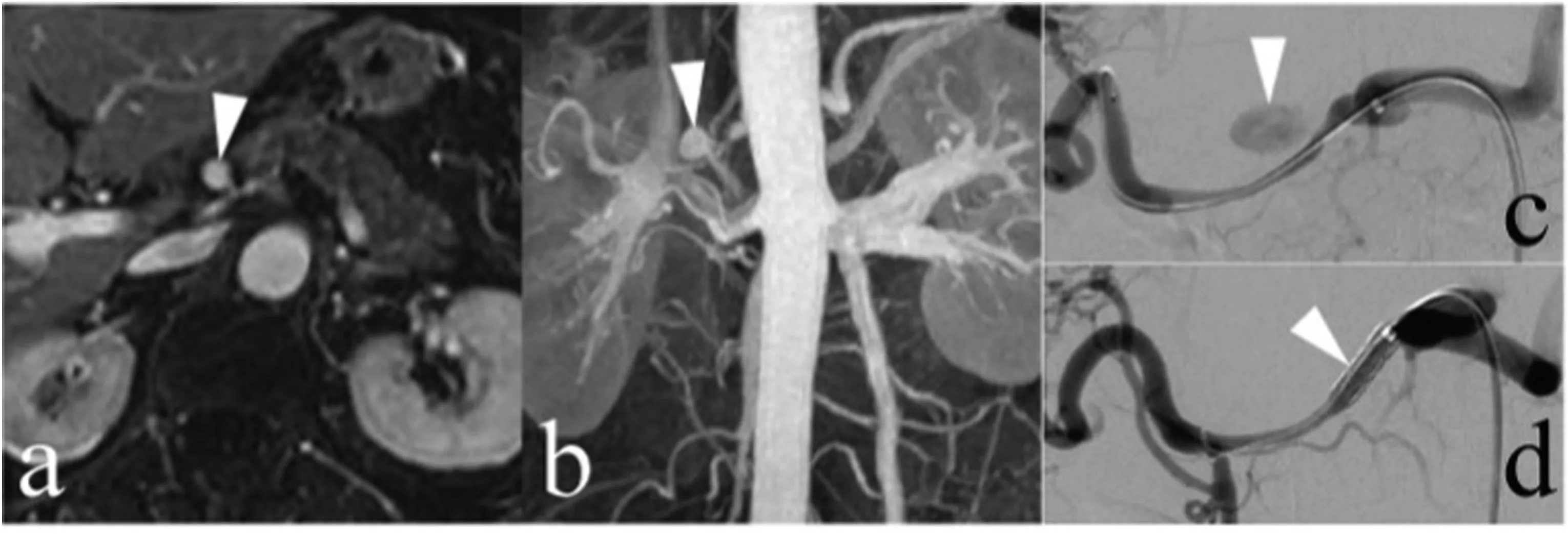
Fig.7. A 74-year-old woman with a saccular aneurysm in the common hepatic artery presented for endovascular treatment with a covered stent because of aneurysm growth. (a, b) A saccular aneurysm in the common hepatic artery (arrows) was demonstrated on a contrast-enhanced magnetic resonance imaging scan. (c) The presence of this aneurysm was confirmed on an arteriogram (arrow). (d) After covered stent placement was performed, the aneurysm was no longer present on the common hepatic arteriogram.
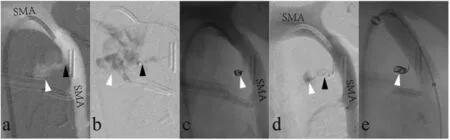
Fig. 8. Three days after undergoing pancreaticogastrostomy, a 47-year-old man was diagnosed with hemoperitoneum based on computed tomography results (not shown).(a)Superior mesenteric artery(SMA)arteriogram demonstrated the presence of rupture of SMA(black arrow)and contrast extravasation (white arrow).(b)Superselective arteriogram with the tip of the microcatheter placed in the pseudoaneurysm (black arrow) confirmed the presence of contrast extravasation (white arrow).(c,d)Coil embolization was unsuccessful(white arrow),and SMA arteriogram demonstrated the continued presence of contrast extravasation.(e)A covered stent was used to seal the rupture in the SMA.
6. Summary
Diagnostic angiography and endovascular management are excellent options for evaluating and managing acute nonvariceal GIB that is refractory to endoscopic therapy.The most common angiographic finding is contrast extravasation.Endovascular management,which may include intra-arterial infusion of vasopressin, embolization, covered stent placement, or a combination thereof, is minimally invasive and has a high success rate in patients with GIB. Clinicians should be familiar with the angiographic features of acute nonvariceal GIB as well as the various endovascular therapies available.
Funding sources
None.
Declaration of competing interest
The authors declare that they have no conflicts of interest regarding this work.We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Acknowledgements
We thank Qiao Chen for his help with revising the manuscript.
 Journal of Interventional Medicine2022年1期
Journal of Interventional Medicine2022年1期
- Journal of Interventional Medicine的其它文章
- Safety and efficacy of Endovascular Management of high-grade blunt renal injury
- Molecular targeted therapy causes hepatic encephalopathy in patients after Transjugular intrahepatic portosystemic shunt (TIPS): A case report and literature review
- A low-grade cerebral arteriovenous malformation suspected of being a metastatic tumor: A case report and literature review
- Comparison of tumor response following conventional versus drug-eluting bead transarterial chemoembolization in early- and very early-stage hepatocellular carcinoma
- Complications from port-a-cath system implantation in adults with malignant tumors: A 10-year single-center retrospective study
- Paclitaxel-coated balloons angioplasties for extra-long femoropopliteal artery atherosclerotic lesions(>30 cm):12 months outcomes from a single center
