抑制G蛋白偶联受体40通过RhoA/ROCK1信号通路缓解小鼠过敏性哮喘*
林西西, 王丽可, 万锦贻, 张维溪, 赵伟
抑制G蛋白偶联受体40通过RhoA/ROCK1信号通路缓解小鼠过敏性哮喘*
林西西1, 王丽可2, 万锦贻2, 张维溪2, 赵伟3△
[1温州医科大学附属第二医院,育英儿童医院药剂科,浙江 温州 325027;2温州医科大学附属第二医院,育英儿童医院儿童变态反应(过敏)与免疫科,浙江 温州 325027;3温州医科大学,浙江 温州 325027]
探究抑制G蛋白偶联受体40(GPR40)减轻过敏性哮喘小鼠症状的作用及机制。将28只雄性C57BL/6小鼠随机分为正常对照组、哮喘模型组(用卵清蛋白建立过敏性哮喘模型)、低剂量GPR40抑制剂DC260126干预组(3 mg/kg DC260126组)和高剂量DC260126干预组(10 mg/kg DC260126组),每组7只。通过小鼠肺功能仪检测各组小鼠气道高反应性;对各组小鼠支气管肺泡灌洗液(BALF)炎症细胞分类并予以计数;肺组织切片进行HE染色评估炎症细胞浸润程度和炎症评分;Western blot检测肺组织中GPR40、GTP-RhoA和Rho相关激酶1(ROCK1)蛋白表达水平。与哮喘模型组相比,10 mg/kg DC260126组小鼠气道阻力显著降低(<0.05),BALF中的炎症细胞显著减少(0.05),肺组织嗜酸性粒细胞和淋巴细胞浸润显著减少(0.01),肺组织中GTP-RhoA和ROCK1蛋白水平显著降低(0.01)。抑制GPR40可能通过Rho/ROCK1信号通路减轻小鼠过敏性哮喘气道炎症和气道高反应性。
哮喘;G偶联受体蛋白40;Rho/ROCK1信号通路;气道高反应性
哮喘是一种严重的慢性异质性气道炎症性疾病,其核心特征包括气道高反应性(airway hyperresponsiveness,AHR)、可逆性气道阻塞和气道重塑[1]。目前,使用皮质类固醇抑制气道炎症,或联合支气管舒张剂和抗胆碱能药物缓解气道狭窄是哮喘治疗的主流[2]。虽然哮喘对标准治疗的反应一般较好,但是仍有相当一部分患者出现严重和(或)持续的症状[3]。世界许多地区的哮喘患病率仍在增加,全世界大约有3亿哮喘患者[4]。因此,迫切需要寻找能够有效延缓哮喘进展和避免哮喘发作的药物。
G蛋白偶联受体(G-protein-coupled receptors,GPCRs)是构成人类最大药物靶点家族的7次跨膜受体[5]。大量临床试验提供了GPCR靶向治疗途径治疗哮喘的安全性和有效性[6]。G蛋白偶联受体40(G-protein-coupled receptor 40,GPR40)又名游离脂肪酸受体1(free fatty acid receptor-1,FFAR-1),是以短链和长链脂肪酸为配体的一种孤儿GPCR[7]。Mizuta等[8]鉴定了GPR40在气道平滑肌中的表达。GPR40激活会引起胞内Ca2+浓度升高[9],然而Ca2+浓度升高会引起气道平滑肌收缩。气道平滑肌过强的收缩是气道高反应性的主要表现之一[10]。有研究显示,GPR40激活可引起气道平滑肌收缩,引起人气道平滑肌细胞增殖[11]。气道平滑肌细胞的过度增殖是支气管哮喘的重要病理生理学基础之一。基于这些事实,GPR40很可能在哮喘的发病机制中起关键作用,因此可能是一个潜在和有效的哮喘治疗靶点。
RhoA是Rho家族的小GTP酶的成员,可以通过GDP-RhoA(非活动状态)和GTP-RhoA(活动状态)交替转换,发挥其分子开关的作用[12]。RhoA蛋白下游信号分子——Rho相关激酶(Rho-associated kinase,ROCK)与Rho蛋白共同参与调节平滑肌细胞的黏附、增殖和分化[13]。重要的是,RhoA/ROCK1信号通路与哮喘的病理生理过程密切相关[14]。抑制RhoA/ROCK1信号通路可减轻气道平滑肌收缩、气道重塑和气道高反应性[15-16]。因此,阻断RhoA/ROCK1信号通路可能是哮喘治疗的潜在干预措施。
本研究对卵清蛋白(ovalbumin,OVA)诱导的过敏性哮喘小鼠腹腔注射GPR40小分子抑制剂DC260126,评估其呼吸道症状、肺部炎症及病理变化情况,从而探讨GPR40是否通过Rho/ROCK1通路减轻过敏性哮喘小鼠气道炎症和AHR。
材料和方法
1 材料
1.1动物SPF级C57BL/6小鼠,雄性,6~8周龄,体重(20±2) g,购自浙江省实验动物中心[许可证号SCXK(浙)2019-0002],饲养于温州医科大学实验动物中心SPF级动物房。所有动物饲养和动物实验均按照温州医科大学动物实验伦理委员会批准的指导方针进行。实验前,小鼠在标准实验室条件下进行适应性饲养1周。
1.2药品试剂与仪器DC260126购于MedChemExpress;OVA购于Sigma;RhoA和ROCK1抗体购于Abcam,GPR40抗体购于Affinity;苏木素-伊红(hematoxylin-eosin,HE)染色试剂盒购于北京索莱宝科技有限公司;BCA蛋白试剂盒购于碧云天生物技术研究所;化学发光成像系统和酶标仪均购自Bio-Rad。
2 方法
2.1动物分组及模型建立将28只SPF级C57BL/6小鼠随机分为4组(每组7只):正常对照(control)组:给予生理盐水;哮喘模型(OVA)组:用OVA建立过敏性哮喘模型,雾化激发前用生理盐水;低剂量DC260126组(3 mg/kg DC260126组)和高剂量DC260126组(10 mg/kg DC260126组):用OVA建立过敏性哮喘模型,雾化激发前分别用3 mg/kg和10 mg/kg DC260126进行腹腔注射。过敏性哮喘模型的建立是参照先前的研究[17]。OVA组、3 mg/kg DC260126组和10 mg/kg DC260126组小鼠在第0天和第14天腹腔注射0.1 mL致敏液(含有OVA 10 μg和氢氧化铝20 mg的生理盐水混合液),以达到致敏效果,control组注射等体积生理盐水。从第21~28天以含1% OVA的生理盐水每天进行雾化激发,每次30 min;control组使用生理盐水。DC260126治疗组在雾化激发前0.5 h分别腹腔注射不同浓度的DC260126,其浓度的选择参考Sun等[18]的研究方法,control组和OVA组腹腔注射等体积、同比例的DMSO生理盐水混合液,见图1。
Figure 1.Establishment of allergic asthma mouse model. OVA: ovalbumin; Alum: aluminum hydroxide adjuvant.
2.2气道高反应性(airway hyperresponsiveness,AHR)测定末次激发后24 h内,用1 %戊巴比妥钠(50 mg/kg)腹腔注射麻醉小鼠。使用浓度分别为 3.125、6.25、12.5、25和50 g/L呈浓度梯度的乙酰甲胆碱(methacholine,MCh)对小鼠进行雾化10 s,并且测定依次气道阻力(air way resistance,aw)和肺动态顺应性(dynamic compliance,dyn),再分别计算各浓度上的aw和dyn与基线的百分比。
2.3支气管肺泡灌洗液(bronchoalveolar lavage fluid,BALF)的制备和细胞计数结扎左肺,用含1%牛血清白蛋白和5 000 U/L肝素的PBS 0.5 mL灌洗右肺3次,获得BALF。在光镜下计算BALF中总细胞数。1 000×离心10 min,收集细胞涂片进行瑞氏-吉姆萨染色,光镜下分类计数炎症细胞,结果表示为×107L-1。
2.4肺组织病理学检查小鼠的肺组织固定在4%多聚甲醛中,石蜡包埋,切片厚度为3~4 μm。按照HE染色试剂盒说明书步骤进行HE染色。光镜下半定量判定肺部炎症严重程度:无炎症细胞(0分);少许炎症细胞(1分);较多分布不均的炎症细胞(2分);大量炎症细胞,分布较均匀,少见聚集成团(3分);大量炎症细胞聚集成团(4分)。
2.5免疫组织化学染色法石蜡切片常规脱蜡后水化,置于修复盒内并用柠檬酸抗原修复缓冲液进行抗原修复,PBS洗涤5 min×3次,滴加阻断内源性过氧化物酶室温孵育10 min,PBS洗涤5 min×3次,滴加10%山羊血清封闭,30 min后吸去再加抗GPR40抗体,4 ℃孵育过夜,37 ℃复温30 min,PBS洗涤5 min×3次,加HRP标记聚合物室温孵育30 min,PBS洗涤5 min×3次,DAB显色镜下观察显色反应,纯水终止显色,苏木素复染,常规脱水,透明,干燥,封片。
2.6Western blot分析各组小鼠肺组织样本匀浆裂解于含1% PMSF的RIPA缓冲液,BCA蛋白试剂盒检测蛋白浓度,样品蛋白(40 µg)在10% Tris/glycine SDS-PAGE分离后转移至PVDF膜上。随后在室温下5%脱脂牛奶封闭1 h,与抗体共同于4 ℃孵育过夜。TBST冲洗后,用辣根过氧化物酶标记的山羊抗兔Ⅱ抗(1∶5 000)与PVDF膜在室温孵育2 h,再采用ECL检测试剂和化学发光成像系统检测蛋白条带。
2.7RhoA pulldown活化检测GTP-RhoA蛋白采用RhoA pulldown活化试剂盒。肺组织碎片化,裂解缓冲液中裂解,离心。每份样品上清液分别用40μg rhotekin-RBD或PAK-PBD琼脂糖珠结合,4 ℃缓慢搅拌孵育1 h。琼脂糖珠经离心、沉降、洗涤3次后重悬,使用抗RhoA抗体进行Western blot检测沉淀的GTP-RhoA表达。
3 统计学处理
所有统计计算均采用SPSS 18.0软件。结果以均数±标准差(mean±SD)表示。采用单因素方差分析进行组间比较,然后采用Student-Newman-Keuls检验进行多重比较。以<0.05为差异有统计学意义。
结果
1 GPR40在过敏性哮喘小鼠肺组织中表达的变化
免疫组织化学结果显示,与control组小鼠相比,OVA诱导的过敏性哮喘小鼠肺组织中GPR40表达显著增加(<0.01),见图2。
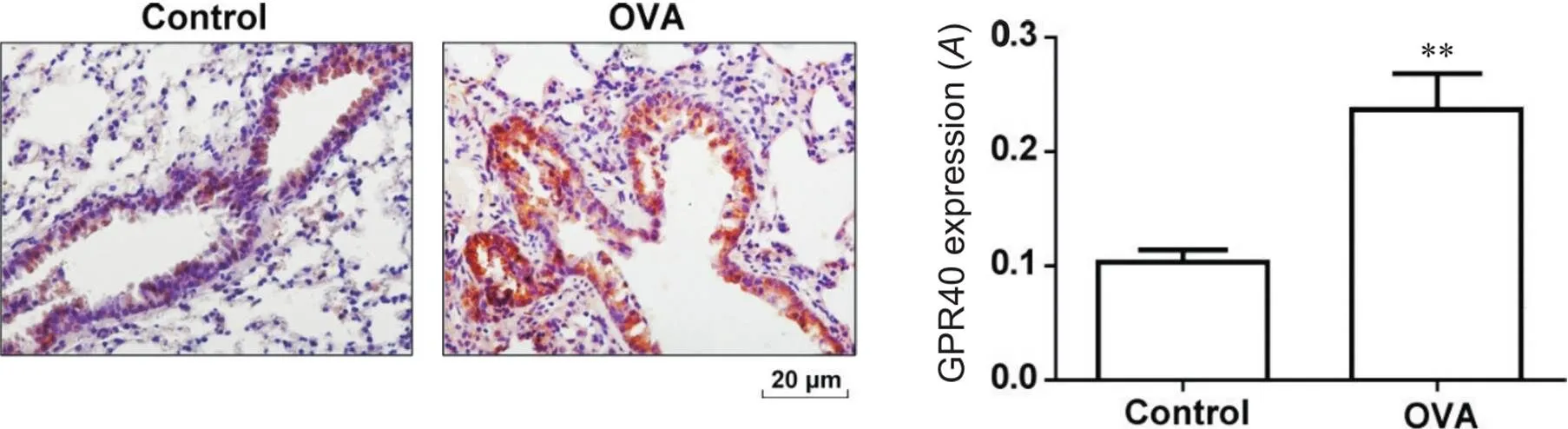
Figure 2.Expression of GPR40 protein in the lung tissue of mice with allergic asthma (immunohistochemical staining,scale bar=20 μm). Mean±SD. n=7. **P<0.01 vs control group.
2 抑制GPR40对小鼠AHR的影响
小鼠肺功能仪结果显示了3.125~50 mg/L MCh雾化后小鼠aw的变化。随着吸入MCh浓度的升高,各组小鼠aw增大。与control组小鼠相比,OVA组小鼠从25~50 g/L MCh开始,aw显著升高(<0.05),dyn显著降低(<0.01);而10 mg/kg DC260126组aw升高幅度显著降低(<0.01),dyn显著升高(<0.01),肺顺应性显著改善,见图3。
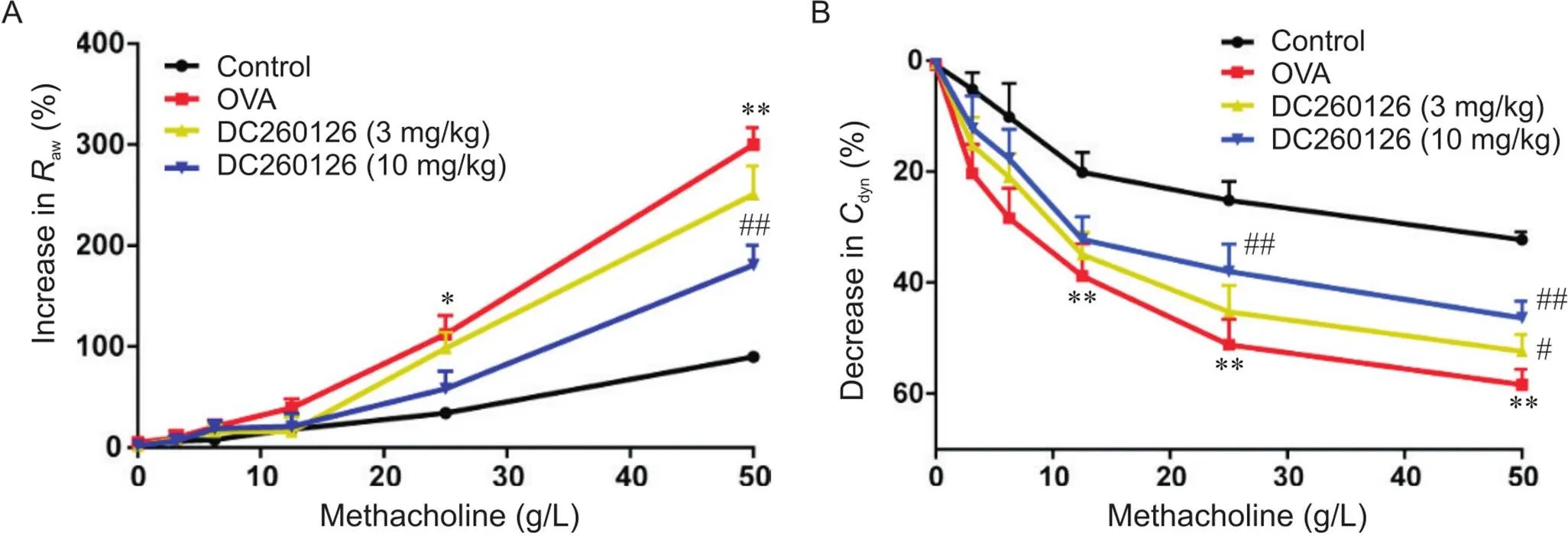
Figure 3.Results of airway resistance (Raw) and dynamic compliance (Cdyn) in the mice of each group. The changes of Raw(A) and Cdyn(B) were determined using a whole-body plethysmography. Mean±SD. n=7. *P<0.05,**P<0.01 vs control group; #P<0.05,##P<0.01 vs OVA group.
3 抑制GPR40对小鼠肺组织病理学的影响
HE染色结果显示,OVA组小鼠在小鼠肺部气道和小血管周围炎症细胞浸润显著(0.01),黏膜及黏膜下层水肿,气管壁显著增厚,肺毛细血管水肿;而control组小鼠肺部气道的黏膜无明显水肿,周围未见炎性细胞的浸润;10 mg/kg DC260126组小鼠的肺部气管周围炎症细胞浸润较OVA组显著减少(0.01),支气管上皮基本完整,毛细血管水肿减轻,见图4。
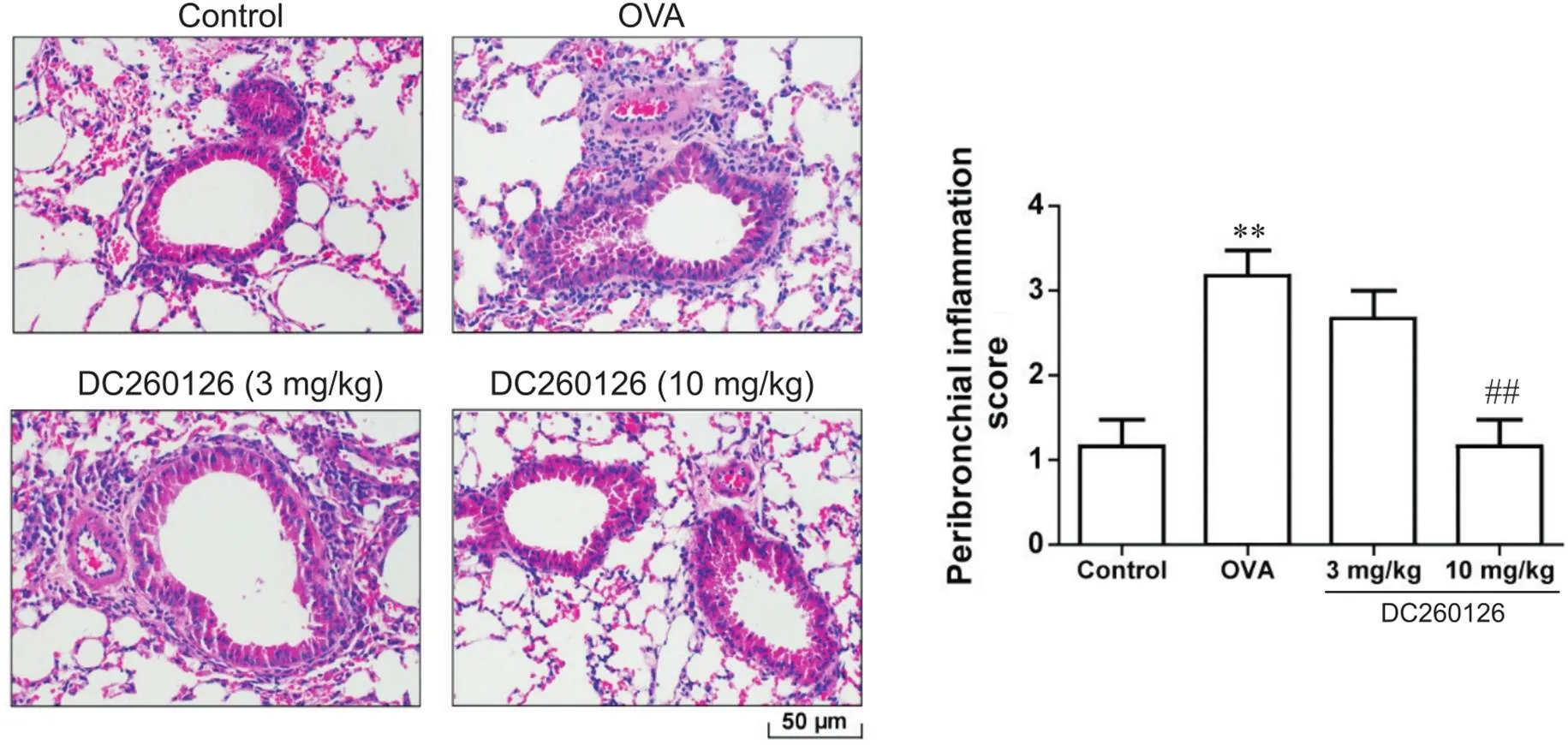
Figure 4.HE staining results of lung tissues of mice in each group (scale bar=50 μm) and the severity of inflammation graded based on the 5-point scoring system. Mean±SD. n=7. **P<0.01 vs control group; ##P<0.01 vs OVA group.
4 抑制GPR40对BALF内细胞分类的影响
BALF细胞计数结果显示,与control组相比,OVA组细胞总数、嗜酸性粒细胞数量、淋巴细胞数量和巨噬细胞数量均显著增多(<0.01),而10 mg/kg DC260126组这些指标均显著下降(0.01),趋于正常,见图5。

Figure 5.Classification and counting of cells in bronchoalveolar lavage fluid (BALF) from the mice in each group. The number of total inflammatory cells in BALF (A) were calculated,and a minimum of 200 cells were employed to classify eosinophils (B),lymphocytes (C) and macrophages (D) after the last OVA challenge. Mean±SD. n=7. **P<0.01 vs control group; ##P<0.01 vs OVA group.
5 抑制GPR40对RhoA/ROCK1信号通路的影响
Western blot分析显示,与control组相比,OVA组小鼠肺组织GPR40表达显著上升(<0.01),10 mg/kg DC260126组小鼠肺组织内由OVA诱导表达的GPR40蛋白显著下降(<0.01),见图6A。此外,OVA组小鼠肺组织内RhoA活化(<0.01),然而10 mg/kg DC260126显著下调GTP-RhoA的表达(<0.01),见图6B。同时,10 mg/kg DC260126也抑制过敏性哮喘小鼠肺组织内由OVA诱导产生的ROCK1表达(<0.05),见图6C。
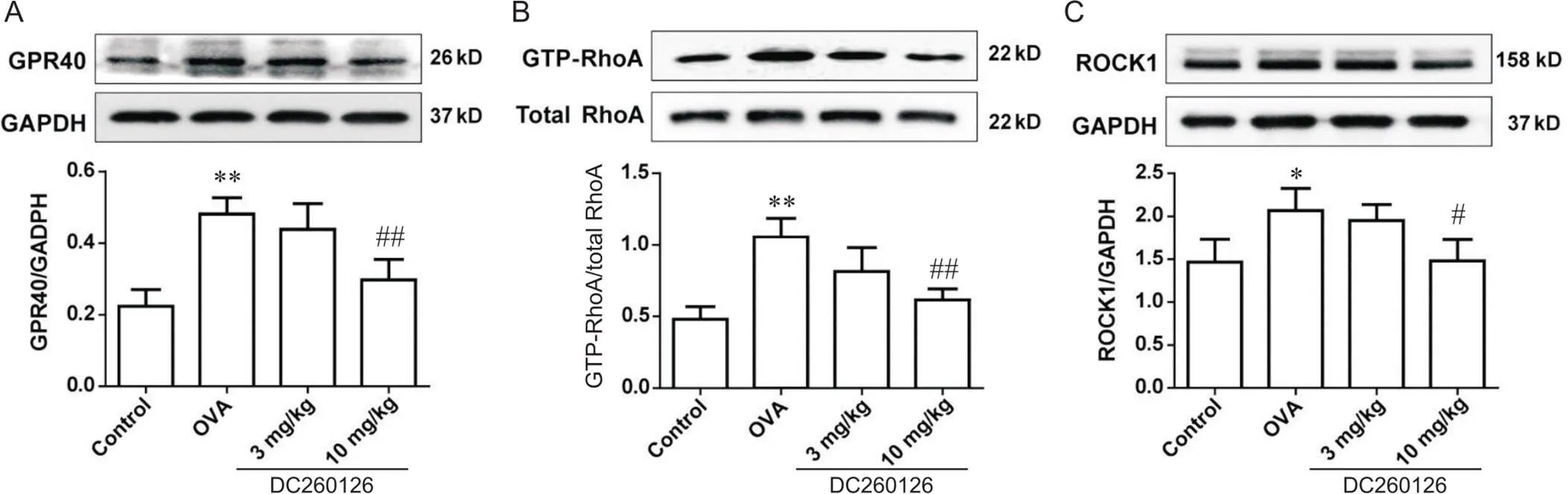
Figure 6.The protein levels of GPR40 (A),GTP-RhoA (B) and ROCK1 (C) in the lung tissues of mice in each group were measured by Western blot. Mean±SD. n=4 to 6. *P<0.05,**P<0.01 vs control group; #P<0.05,##P<0.01 vs OVA group.
讨论
本研究通过建立OVA诱导的过敏性哮喘小鼠模型,在此基础上给予不同浓度的GPR40小分子抑制剂——DC260126,观察并研究抑制GPR40对过敏性哮喘小鼠的炎症浸润、AHR等的影响,研究结果显示抑制GPR40能够有效降低过敏性哮喘模型中炎症细胞浸润及AHR。此外,我们观察到RhoA/ROCK1信号通路可能共同参与了哮喘的发病机制。据我们所知,这是首次明确GPR40抑制剂在过敏性哮喘的作用,研究结果提示GPR40可能是哮喘治疗的分子靶点。
哮喘是一种全球常见的慢性气道疾病,其病理生理复杂,具有支气管收缩、炎症、AHR等多种致病特点[19]。有研究证明,GPCRs在调节气道平滑肌收缩和气道炎症方面发挥了重要作用[20-21]。激活或失活GPCRs是哮喘的主要治疗手段,因此,寻找新型GPCR激动剂或拮抗剂成为开发新型、有效的抗哮喘药物的重要途径[22]。一般认为,气道平滑肌细胞是形成气道高反应性的主要细胞类型[23],其收缩特性的改变在哮喘气道高反应性的发生发展中起重要作用[24]。近来,研究人员显示GPR40在气道平滑肌细胞上表达,在介导气道平滑肌收缩中发挥重要作用[11,25]。与上述研究结果一致,我们证明哮喘小鼠肺内GPR40表达水平明显升高。
AHR是过敏性哮喘的临床症状之一,持续的AHR会引起肺功能的下降,气道阻力升高,肺顺应性下降,影响过敏性哮喘患者的生活。本实验显示,OVA组小鼠对MCh的反应性显著高于control组,说明OVA诱导的过敏性哮喘小鼠表现出较强的AHR;而GPR40抑制剂高剂量组小鼠的aw值显著下降,dyn值显著升高,提示抑制GPR40能够有效降低OVA诱导过敏性小鼠产生的AHR,减轻哮喘症状。
过敏性哮喘病理特征一般表现为反复发作的Th2免疫反应及其导致的嗜酸粒细胞性气道炎症和气道重塑变窄。本研究通过肺组织HE染色发现,与OVA诱导的过敏性哮喘小鼠相比,10 mg/kg DC260126能显著缓解肺内支气管、血管及其周围的炎症细胞浸润。另外。抑制GPR40能够显著减轻过敏性哮喘小鼠BALF中炎症细胞蓄积,尤其是嗜酸性粒细胞。嗜酸性粒细胞是过敏性哮喘气道黏膜最具特征性的炎性细胞,与哮喘症状、气流阻塞和AHR密切相关[26]。嗜酸性粒细胞在肥大细胞释放的过敏性嗜酸性粒细胞趋化因子作用下,在气道及肺组织内聚集。嗜酸性粒细胞的产生和活化受控于Th2细胞,活化的嗜酸性粒细胞会分泌血小板活化因子、白三烯等炎症介质,引起支气管平滑肌收缩,微血管通透性增加等引起气道炎症和高反应。有大量研究证实GPCRs在嗜酸性粒细胞中的功能和调控作用[27-28],减少嗜酸性粒细胞能抑制或减轻过敏性哮喘的发生发展[29],结合本研究BALF中细胞分类计数及HE染色结果,提示抑制GPR40表达具有减轻过敏性哮喘炎症细胞浸润的作用。
为了探讨GPR40调控AHR和炎症的分子机制,我们重点研究了RhoA/ROCK1信号通路。RhoA及其下游的ROCK最近被认为是哮喘的治疗靶点[30]。RhoA的激活已被证实在平滑肌收缩中起重要作用,包括大鼠和小鼠气道[31]。阻断RhoA/ROCK1信号可预防过敏性气道炎症,也可逆转蟑螂过敏原引起的气道重塑[32]。重要的是,RhoA已被报道定位在众多GPCRs的下游,并作为近端效应分子调节多种基本细胞功能[33],这促使我们探讨GPR40在OVA诱导的哮喘中是否调节RhoA活性。本研究结果表明,过敏性哮喘小鼠肺组织中GTP-RhoA和ROCK1表达显著升高,而DC260126显著抑制OVA诱导的小鼠肺组织中GTP-RhoA和ROCK1的表达。这表明GPR40调控过敏性哮喘小鼠RhoA/ROCK1信号通路。
综上所述,我们证明抑制GPR40能有效缓解OVA诱导过敏性小鼠的病理生理,包括肺部炎症浸润和AHR。此外,抑制GPR40明显下调RhoA和ROCK1的活化,从而提示GPR40与过敏性哮喘小鼠RhoA/ROCK1信号通路的调控有关。本研究为GPR40抑制剂治疗哮喘的炎症、AHR等提供了科学的药理实验基础,但GPR40抑制剂用于临床治疗哮喘仍需要进一步的研究。
[1] Saglani S,Lloyd CM. Novel concepts in airway inflammation and remodelling in asthma[J]. Eur Respir J,2015,46(6):1796-1804.
[2] Pavord ID,Beasley R,Agusti A,et al. After asthma: redefining airways diseases[J]. Lancet,2018,391(10118):350-400.
[3] Schoettler N,Strek ME. Recent advances in severe asthma: from phenotypes to personalized medicine[J]. Chest,2020,157(3):516-528.
[4] Zaidan MF,Ameredes BT,Calhoum WJ. Management of acute asthma in adults in 2020[J]. JAMA,2020,323(6):563-564.
[5] Weis WI,Kobilka BK. The molecular basis of G protein-coupled receptor activation[J]. Annu Rev Biochem,2018,87:897-919.
[6] Wendell SG,Fan H,Zhang C. G protein-coupled receptors in asthma therapy: pharmacology and drug action[J]. Pharmacol Rev,2020,72(1):1-49.
[7] Sharma N,Bhagat S,Chundawat TS. Recent advances in development of GPR40 modulators (FFA1/FFAR1): an emerging target for type 2 diabetes[J]. Mini Rev Med Chem,2017,17(11):947-958.
[8] Mizuta K,Zhang Y,Mizuta F,et al. Novel identification of the free fatty acid receptor FFAR1 that promotes contraction in airway smooth muscle[J]. Am J Physiol Lung Cell Mol Physiol,2015,309(9):L970-L982.
[9] Shapiro H,Shachar,S,Sekler I,et al. Role of GPR40 in fatty acid action on the beta cell line INS-1E[J]. Biochem Biophys Res Commun,2005,335(1):97-104.
[10] 张景鸿,李超乾. 支气管哮喘气道高反应性机制的研究进展[J]. 中国呼吸与危重监护杂志,2011,10(3):304-307.
Zhang JH,Li CQ. Research progress on the mechanism of airway hyperresponsiveness in bronchial asthma[J]. Chin J Respir Crit Care Med,2011,10(3):304-307.
[11] Matoba A,Matsuyama N,Shibata S,et al. The free fatty acid receptor 1 promotes airway smooth muscle cell proliferation through MEK/ERK and PI3K/Akt signaling pathways[J]. Am J Physiol Lung Cell Mol Physiol,2018,314(3):L333-L348.
[12] Chiba Y,Matsusue K,Misawa M. RhoA,a possible target for treatment of airway hyperresponsiveness in bronchial asthma[J]. J Pharmacol Sci,2010,114(3):239-247.
[13] Biro M,Munoz MA,Weninger W. Targeting Rho-GTPases in immune cell migration and inflammation[J]. Br J Pharmacol,2014,171(24):5491-5506.
[14] Xu C,Wu X,Lu M,et al. Protein tyrosine phosphatase 11 acts through RhoA/ROCK to regulate eosinophil accumulation in the allergic airway[J]. FASEB J,2019,33(11):11706-11720.
[15] Amison RT,Momi S,Morris A,et al. RhoA signaling through platelet P2Y1receptor controls leukocyte recruitment in allergic mice[J]. J Allergy Clin Immunol,2015,135(2):528-538.
[16] Bhattacharya M,Sundaram A,Kudo M,et al. IQGAP1-dependent scaffold suppresses RhoA and inhibits airway smooth muscle contraction[J]. J Clin Invest,2014,124(11):4895-4898.
[17] Zeng Z,Lin X,Zheng R,et al. Celastrol alleviates airway hyperresponsiveness and inhibits Th17 responses in obese asthmatic mice[J]. Front Pharmacol,2018,9:49.
[18] Sun P,Wang T,Zhou Y,et al. DC260126: a small-molecule antagonist of GPR40 that protects against pancreatic β-cells dysfunction in/mice[J]. PLoS One,2013,8(6):e66744.
[19] Sharma P,Yi R,Nayak AP,et al. Bitter taste receptor agonists mitigate features of allergic asthma in mice[J]. Sci Rep,2017,7:46166.
[20] Billington CK,Penn RB. Signaling and regulation of G protein-coupled receptors in airway smooth muscle[J]. Respir Res,2003,4:2.
[21] Deshpande DA,Penn RB. Targeting G protein-coupled receptor signaling in asthma[J]. Cell Signal,2006,18(12):2105-2120.
[22] Madigan LA,Wong GS,Gordon EM,et al. RGS4 overexpression in lung attenuates airway hyperresponsiveness in mice[J]. Am J Respir Cell Mol Biol,2018,58(1):89-98.
[23] Janssen LJ. Airway smooth muscle as a target in asthma and the beneficial effects of bronchial thermoplasty[J]. J Allergy (Cairo),2012,2012:593784.
[24] Black JL,Panettieri RA Jr,Banerjee A,et al. Airway smooth muscle in asthma: just a target for bronchodilation?[J]. Clin Chest Med,2012,33(3):543-558.
[25] Mizuta K,Matoba,A,Shibata S,et al. Obesity-induced asthma: Role of free fatty acid receptors[J]. Jpn Dent Sci Rev,2019,55(1):103-107.
[26] Brussino L,Heffler E,Bucca C,et al. Eosinophils target therapy for severe asthma: critical points[J]. Biomed Res Int,2018,2018:7582057.
[27] Ilmarinen P,James A,Moilanen E,et al. Enhanced expression of neuropeptide S (NPS) receptor in eosinophils from severe asthmatics and subjects with total IgE above 100IU/ml[J]. Peptides,2014,51:100-109.
[28] Tamaki M,Konno Y,Kobayashi Y,et al. Expression and functional roles of G-protein-coupled estrogen receptor (GPER) in human eosinophils[J]. Immunol Lett,2014,160(1):72-78.
[29] 张翊玲,曹颖,郑梦凝,等. 删除嗜酸性粒细胞对支气管哮喘模型小鼠sPLA2-X的影响[J].中国病理生理杂志,2020,36(10):1818-1824.
Zhang YL,Cao Y,Zheng MN,et al. Effects of eosinophils deletion on sPLA2-X in bronchial asthma model mice[J]. Chin J Pathophysiol,2020,36(10):1818-1824.
[30] Yu OM,Brown JH. G protein-coupled receptor and RhoA-stimulated transcriptional responses: links to inflammation,differentiation,and cell proliferation[J]. Mol Pharmacol,2015,88(1):171-180.
[31] Chiba Y,Uchida T,Sakai H,et al. Acetylcholine-induced translocation of RhoA in freshly isolated single smooth muscle cells of rat bronchi[J]. J Pharmacol Sci,2004,95(4):479-482.
[32] Ke X,Do DC,Li C,et al. Ras homolog family member A/Rho-associated protein kinase 1 signaling modulates lineage commitment of mesenchymal stem cells in asthmatic patients through lymphoid enhancer-binding factor 1[J]. J Allergy Clin Immunol,2019,143(4):1560-1574.e6.
[33] Zhang Y,Saradna A,Ratan R,et al. RhoA/Rho-kinases in asthma: from pathogenesis to therapeutic targets[J]. Clin Transl Immunol,2020,9(5):e01134.
Inhibition of GPR40 alleviates allergic asthma in mice through RhoA/ROCK1 signaling pathway
Lin Xi-xi1,Wang Li-ke2,Wan Jin-yi2,Zhang Wei-xi2,Zhao Wei3△
(1,,325027,;2,,325027,;3,325027,)
To investigate the effect of inhibition of G-protein-coupled receptor 40 (GPR40) on the symptoms of asthmatic mice,and to explore the mechanism.Healthy male C57BL/6 mice (=28) were randomly divided into normal control group,model group,low-dose GPR40 inhibitor DC260126 group (3 mg/kg DC260126 group) and high-dose DC260126 group (10 mg/kg DC260126 group). The allergic asthma mouse model was induced by ovalbumin. The airway hyperresponsiveness was detected by the mouse lung function instrument. The pathological changes of the lung tissues were observed by HE staining. The protein levels of GPR40,GTP-RhoA and Rho-associated kinase 1 (ROCK1) in lung tissues were determined by Western blot.Compared with model group,DC260126 at the dose of 10 mg/kg significantly reduced the airway resistance and the accumulation of inflammatory cells. The inhibition of GPR40 decreased the infiltration of eosinophils and lymphocytes in lung tissues. In addition,both GTP-RhoA and ROCK1 were significantly decreased after the treatment with 10 mg/kg DC260126.Inhibition of GPR40 attenuates the airway inflammation and airway hyperresponsiveness of allergic asthma mice through the Rho/ROCK1 signaling pathway.
Asthma; G-protein-coupled receptor 40; Rho/ROCK1 signaling pathway; Airway hyperresponsiveness
R562.2+5; R363.2
A
10.3969/j.issn.1000-4718.2022.02.002
1000-4718(2022)02-0202-07
2021-09-23
2021-11-23
[基金项目]国家自然科学基金资助项目(No. 81803544)
Tel: 0577-88002944; E-mail: wzhao@hotmail.com
(责任编辑:林白霜,罗森)

