Effects of Environmental Cadmium on Cadmium Accumulation,Oxidative Response, and Microelements Regulation in the Liver and Kidney of Hexagrammos otakii
HU Fawen, SUN Ming, LI Li, GAO Fengxiang, JIAN Yuxia, WANG Xue,WANG Xiaolong, and GUO Wen
Effects of Environmental Cadmium on Cadmium Accumulation,Oxidative Response, and Microelements Regulation in the Liver and Kidney of
HU Fawen#, SUN Ming#, LI Li, GAO Fengxiang, JIAN Yuxia, WANG Xue,WANG Xiaolong, and GUO Wen*
,,266104,
The effects of cadmium (Cd) on metal accumulation, microelements contents, and antioxidant responses inwere studied. The fish were exposed to 0.2, 2.5, or 10µgL−1Cd for 12 or 24 days. Then, the concentration of Cd and mi- croelements (Ca, Fe, Zn, and Se) were determined in the liver and kidney. Moreover, the activities of antioxidant enzymesincluding superoxide dismutase (SOD), catalase (CAT), and glutathione-S-transferase (GST), and the content of malondialdehyde (MDA) in the liver and kidney were also measured. A continuous accumulation of Cd was observed throughout the experimental period. Cd accumulation was higher in liver than that in the kidney, while Ca and Fe contents were lower in liver than those in the kidney. Cd exposure resulted in a decrease of Ca and Fe concentrations in the kidney, while there was no effect on the liver. Zn and Se remained unaffected with exposure to Cd. Cd exposure induced severe oxidative stress in, as indicated by significant induction of the activities of SOD, CAT, and GST, and a simultaneous increase of MDA content. These data show that antioxidant enzymes and microelements contents can be used as potential biomarkers to monitor environmental health in fish.
accumulation; oxidative response; ionoregulatory toxicity; waterborne cadmium;
1 Introduction
Heavy metals are hazardous to the marine environment due to their special chemical properties and toxicity, which has been a worldwide concern (Moyson., 2016; Sa- vorelli., 2017;Gobi., 2018;Vajargah., 2018, 2019; Vijayakumar., 2019; Mohsenpour., 2020). Heavy metals usuallyare not naturally degradable.They can be easily accumulated in marine organisms, and then enter aquatic food chains and pose long-term risks to aqua- tic animals (Singh and Chandel, 2006; Mendil., 2010). Among the metals, cadmium (Cd) is biologically non-es- sential and hazardous at low concentrations because it ex- erts various biological effects, threatens marine life (OSPAR, 2010), and induces cancer in humans (Benavides., 2005; Nordberg, 2010). The Cd concentrations in sea water and organisms have been reported. Wan. (2008) surveyed the trend in the spatial distribution and temporal change of dissolved metals in Jinzhou Bay and reported Cd concentrations of 1.65–2.01µgL−1. Gao. (2014) summarized the dissolved trace element concentrations in the Bohai Sea during 1996–2008 and reported that Cd con- centrations ranged from 0.007 to 5µgg−1. Zhao. (2016) collected 124 kinds of edible fish and measured Cd concentrations, and the Cd detection rate was 21.8%.
The accumulation of heavy metals in aquatic animals catalyzes the production of reactive oxygen species (ROS) that cause oxidative stress in aquatic animals. Defensive mechanisms, including various antioxidant defense enzymes are activated to balance ROS production (Tjalkens., 1998). Among the various antioxidant mechanisms, super- oxide dismutase (SOD) is the first oxidative stress defense system to counteract ROS production. SOD converts su- peroxide anions into hydrogen peroxide (Vlahogianni., 2007). Glutathione S-transferase (GST) is an antioxidant and phase II detoxification enzyme that is a reliable bio- marker of oxidative damage in aquatic animals (Regoli and Principato, 1995). Catalase (CAT) protects the cells from the toxic effects of H2O2by decomposing it to water. In its absence, H2O2accumulates resulting in an increase of hy- droxyl radical production. Excessive ROS production da- mages lipids. Malondialdehyde (MDA) is a secondary li- pid peroxidation (LPO) product, which is used as a common indicator of LPO in response to metal exposure (Fa- rombi., 2007). Various studies have been conducted in the last decade on the exposure to Cd2+and other heavy metals in different species (Capillo., 2018). The majority of these studies have focused on the antioxidant re- sponse induced by Cd2+in tissues of fish, such as(Firat., 2009; Atli and Canli, 2010), yellow perch (Defo., 2014), and(Liu., 2011). Antioxidant enzymes, such as SOD, CAT, and GST, can reduce the harmful effects of ROS.
Cd2+enters the animal via apical epithelial calcium chan- nels (Verbost., 1987;Galvez., 2006; Torre., 2013; Pagano., 2017). Consistent with its route of entry, the principal toxic effects of Cd are on calcium ho- meostasis. Ionic homeostasis plays a vital role in nutritional and metabolic diseases of organisms. Calcium, iron, zinc, and selenium are essential trace elements in the body, and are the prosthetic components of enzymes or activa- tors of enzymes that participate in immunity. A lack or in- sufficient content of trace elements causes various deficien- cies in animals.
Fat greenling,, is one of the most important commercial greenling species in China because of its high-quality meat, and its distribution along the Yel- low Sea, Bohai Sea, and East China Sea. However, Cd to- xicity inhas been insufficiently studied by using the antioxidant defense system and microelements contents as biomarkers to reflect the effects. The liver and kidney are endowed with an antioxidant defense system to protect them from oxidative stress caused by metals (Basha and Rani, 2003; Atlietal, 2006; Atli and Canli, 2008). There- fore, this study assessed the toxic effects of waterborne Cd onkidney and liver, the main sites of Cd ac- cumulation,as well as on the immune responses and ionic homeostasis, which will be critically important in the risk assessment of marine pollutants.
2 Materials and Methods
2.1 Ethics Statement
All animal experiments were conducted under the guide- lines and approval of the respective Animal Research and Ethics Committees of China. The field studies did not in- volve endangered or protected species.
2.2 Experimental Fish and Conditions
(weight, 51.37g±2.48g) were provided by the Marine Biology Institute of Shandong Province. The fish were acclimatized for 2 weeks under laboratory conditions. During the acclimation period, the fish were fed a Cd-free diet twice daily and maintained at a 12h:12h light:dark cy- cle and constant conditions at all times.
Cadmium (CdCl2;cadmium chloride anhydrous, CAS: 7790-78-5) was used to conduct the heavy metal exposure experiment. After 2 weeks of acclimation, 240were randomly divided into four groups with 60 fish pergroup, six tanks per group. Group I served as a control, while the other three groups were exposed to 0.2, 2.5, or 10µgL−1Cd2+for 24d.
The Cd exposure experiments were carried out in 150L PVC tanks containing 120L of exposure media (seawater) and 10 individuals per test unit. A complete water exchange was performed after 48h, and the water had been equili- brated for 24h. Water temperature was maintained at 24.5 ℃±0.5℃ under a photoperiodic regime of 12h light and 12h dark with dissolved oxygen of (6.37±0.32)mgL−1, pH of 7.31±0.34, and salinity of 32.
A 50mL aliquot of test medium was taken from each re- plicate tank in a polypropylene centrifuge tube to determine the chemical concentration of the medium in the three replicates on days 0, 12, and 24 of exposure. Fish were starved for 24h before terminating the experiment, and were euthanized in 0.02% tricaine methanesulfonate. The liversand kidneys were carefully dissected from three healthy fish as parallel samples, washed with distilled water, and stored at −20℃ prior to analysis.
2.3 Cadmium and Microelements Contents Analyses
and the water Cd analyses were conducted by inductively coupled plasma-mass spectrometry (ICP-MS) using methods similar to those of Gaw. (2012) and McRae. (2016). Water samples were acidified with 20μL of ultrapure 70% nitric acid (HNO3), and stored at 4℃ before being analyzed by ICP-MS. The kidney and liver tissues were weighed and placed in a freeze drier for 1 week. The freeze-dried tissue (0.2g) was placed in acid- washed polycarbonate vials. The tissues were digested by adding 5mL of 10% ultrapure HNO3and left for 24h be- fore refluxing at 85℃ for 1h. Volumes were adjusted to 20mL using Milli-Q water. If necessary, the samples were diluted using 2% ultrapure HNO3and placed in acid-wash- ed test tubes to be analyzed with ICP-MS (Agilent 7500cx;Agilent Technologies Inc., Palo Alto, CA, USA). Detection limits for the tissue analysis were calculated as three stan- dard deviations of the mean blank concentration (0.03μgg−1). Detection limits for the water analysis were calculated as three standard deviations of the mean blank concentration (0.05µgL−1).
2.4 Antioxidant Enzyme Analysis
Fish tissues (kidney and liver) were homogenized on ice with nine volumes of cold 0.86% physiological saline and then centrifuged at 600×and 4℃ for 15min. The super- natant was used to detect enzyme activities (SOD, CAT, and GST) and the levels of MDA using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, Chi- na). CAT activity was determined by measuring hydrogen peroxide based on the production of a stable complex with ammonium molybdate at 405nm. GST activity was mea- sured using 1-chloro-2, 4-dinitrobenzene (CDNB) as the substrate. Enzyme activity was determined by monitoring the changes of absorbance at 412nm. One unit of CAT ac- tivity was defined as the amount of enzyme consuming 1μmol of substrate or generating 1μmol of product per mi- nute per milligram soluble protein (U(mg protein)−1). One unit of GST activity was defined as the amount of enzyme that conjugates 1μmol of CDNB per min per mg protein (U(mg protein)−1). MDA content was determined using thiobarbituric acid as the reactive material. According to the manufacturer’s directions, a mixture of the kit reagents and the tissue homogenate was heated at 95℃ for 40min and cooled in running water. The mixture was centrifuged at 1200×for 10min and the absorbance of the superna- tant at 532nmwas recorded. All indicators in the homo- genates were normalized to the protein concentrations in the corresponding samples.
2.5 Statistical Analysis
Data are expressed as mean±standard deviation (SD). Differences in these data were detected by two-way ana- lysis of variance followed by Duncan’s multiple-range test. A-value≤0.05 was considered significant.
3 Results
3.1 Cadmium Accumulation
The exposure concentration of Cd in water was kept sta- ble during the accumulation and depuration phases and were within 93%–95% of the nominal values of 0.2, 2.5, and 10µgL−1. No significant variations were observed over time.
Exposure to Cd resulted in a significant dose-depen- dent accumulation in water and tissues (Fig.1). A signifi- cant increase in Cd accumulation was observed in the three treatments on days 12 and 24. After 12 days of exposure, Cd accumulation values in the kidney were 0.0262, 0.0564, 0.0693, and 0.0915μgg−1for the exposure concentrations of 0, 0.2, 2.5, and 10µgL−1, respectively. The Cd concen- tration values in the kidney after day 24 were 0.0236,0.0698, 0.0803, and 0.1127μgg−1for the exposure con- centrations of 0, 0.2, 2.5, and 10µgL−1, respectively. Cd concentration increased considerably in the liver on days 12 and 24. The highest Cd concentration in the liver was 1.11μgg−1on day 24 in the 10µgL−1exposure group.

Fig.1 Cadmium concentrations in kidney (A) and liver (B) of H. otakii exposed to 0, 0.2, 2.5, and 10µgL−1 for 12 and 24d. Values are mean±SD, n=10. Values with different superscript letters (a, b, c) are significantly different (P<0.05).
3.2 Antioxidant Enzyme Analysis
The activities of antioxidant enzymesincluding SOD, CAT, GST, and MDA were shown in Figs.2–5. Liver and kidney SOD activities were induced. SOD activity in the kidney increased along with increasing Cd exposure concentration on day 12. SOD activity in the kidney of the 10µgL−1Cd exposure groupwas lower on day 24 than those in the other two exposure groups. Liver SOD notably in- creased in the 10µgL−1Cd exposure group on day 12.
CAT activity increased in the kidney with exposure con- centration on day 12, but there was no significant difference among the three treatments on day 24. A considerable increase in liver CAT activity was observed in all three treatments on day 12. CAT activity increased considera- bly in the 0.2 and 2.5µgL−1Cd exposure groups, while it was significantly inhibited in the 10µgL−1Cd exposure group on day 24.
Kidney GST activity increased notably over the three treatments after 4 weeks, while GST decreased in the 10µgL−1Cd exposure group on day 24. Liver GST activity increased significantly in the 0.2µgL−1Cd exposure group on days 12 and 24.

Fig.2 Superoxide dismutase (SOD) activities in kidney (A) and liver (B) of H. otakii exposed to 0, 0.2, 2.5, and 10µgL−1 Cd for 12 and 24d. Values are mean±SD, n=10. Va- lues with different superscript letters (a, b, c) are significantly different (P<0.05).
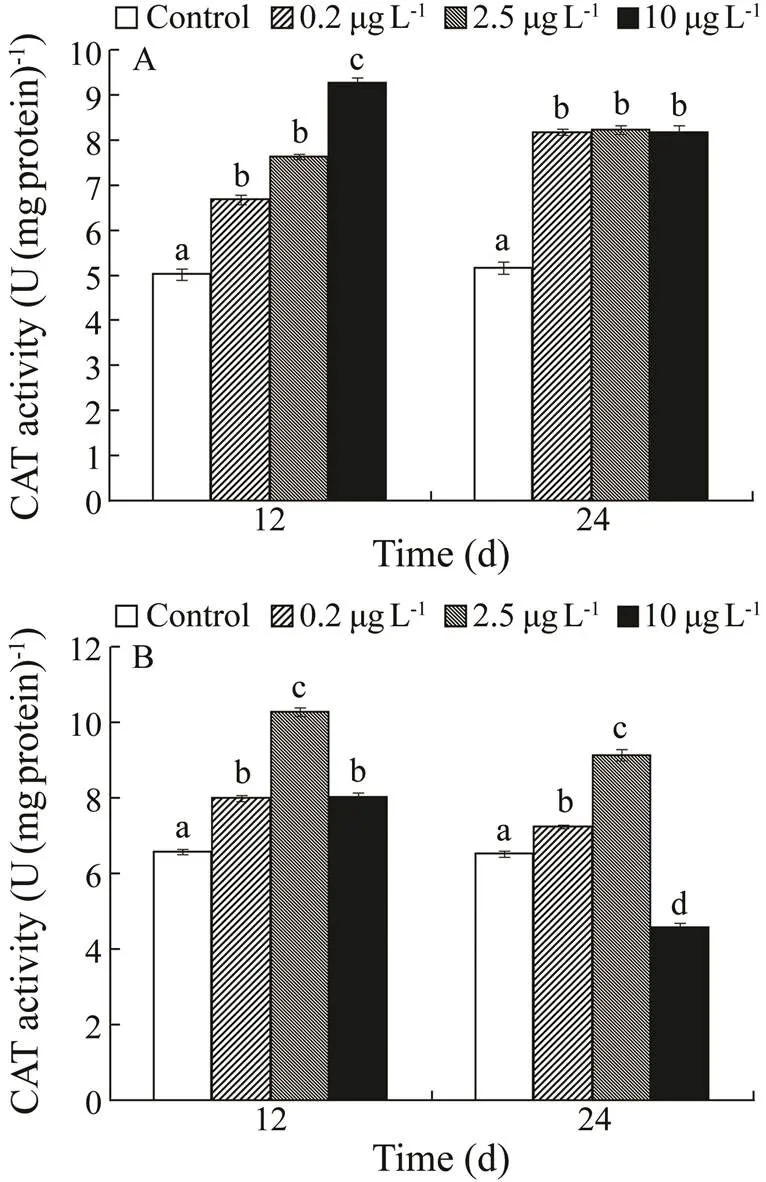
Fig.3 Catalase (CAT) activities in kidney (A) and liver (B) of H. otakii exposed to 0, 0.2, 2.5, and 10µgL−1 Cd for 12 and 24d. Values are mean±SD, n=10. Values with differ-ent superscript letters (a, b, c) are significantly different (P<0.05).
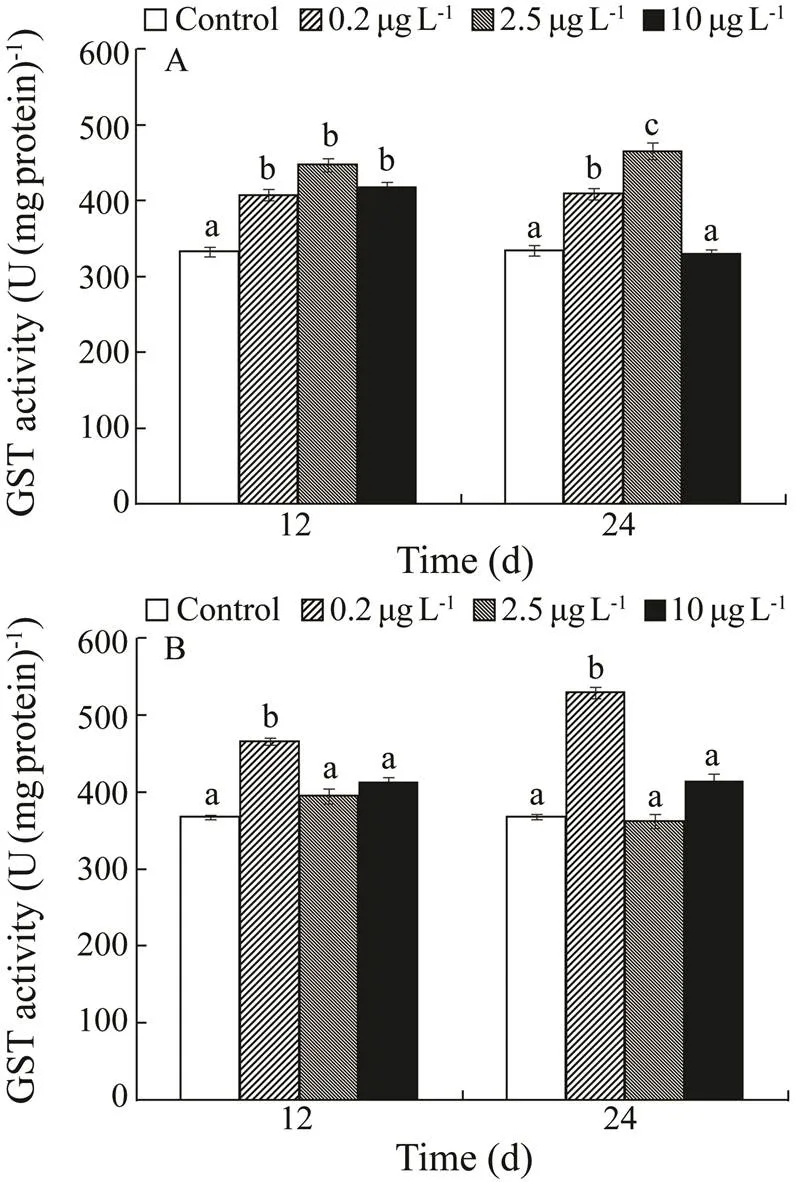
Fig.4 Glutathione-S-transferase (GST) activities in kidney (A) and liver (B) of H. otakii exposed to 0, 0.2, 2.5, and 10µgL−1 Cd for 12 and 24d. Values are mean±SD, n=10. Values with different superscript letters (a, b, c) are signifi- cantly different (P<0.05).
Induction of MDA in the kidney and liver was shown in Fig.5. MDA tended to increase in the kidney by day 12 as follows: 2.5>10>0.2µgL−1Cd exposure groups. MDA content was significantly higher in 0.2 and 10µgL−1Cd exposure groups, followed by the 2.5µgL−1Cd exposure group. MDA tended to increase in the liver in the following order 0.2=2.5>10µgL−1.
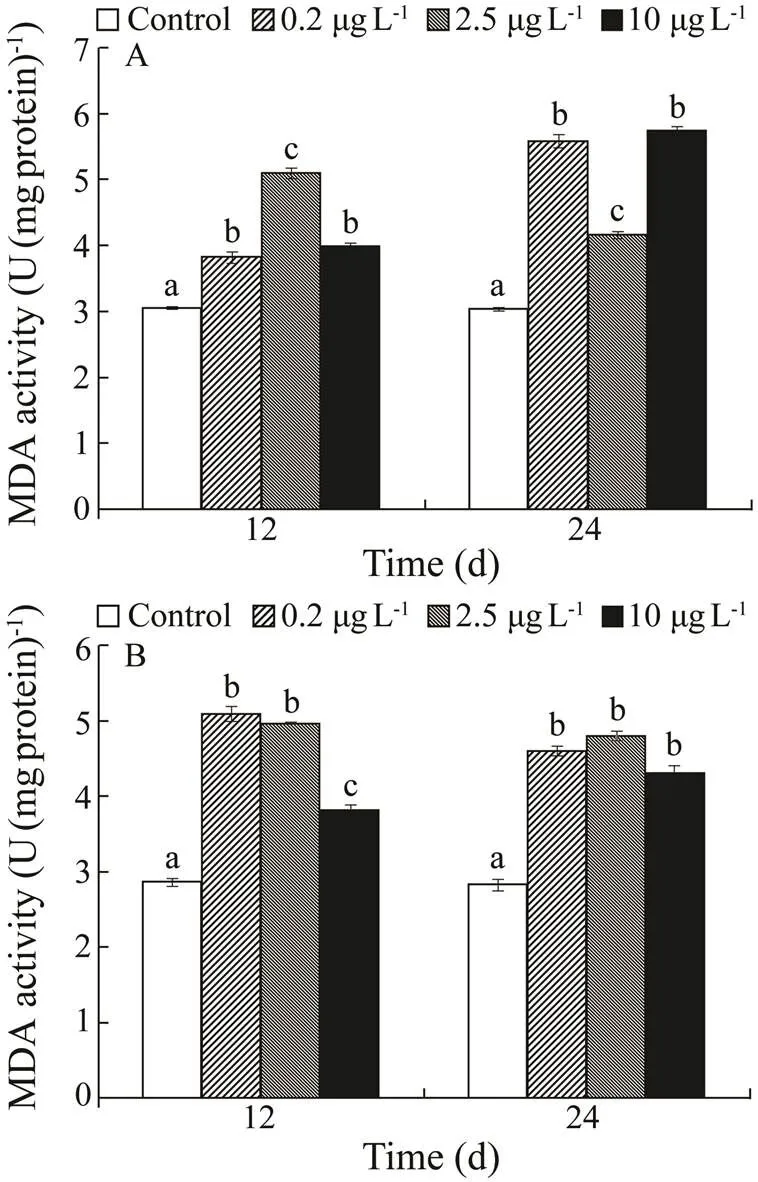
Fig.5 Malondialdehyde (MDA) contents in kidney(A) and liver (B) of H. otakii exposed to 0, 0.2, 2.5, and 10µgL−1 Cd for 12 and 24d. Values are mean±SD, n=10. Values with different superscript letters (a, b, c) are significantly different (P<0.05).
3.3 Microelements Contents
Microelements contents in the kidney and liver samples ofunder the different Cd exposure concentrations were shown in Table 1. Cd exposure resulted in decreased Ca and Fe concentrations in the kidney compared with control fish, but had no effect on the liver. Zn and Fe concentrations remained unaffected by exposure to Cd.
4 Discussion
In this study, we investigated the Cdaccumulation, antioxidant response, and microelements contents in liver and kidney ofafter chronic Cd exposure. Fish were exposed to 0.2, 2.5, and 10µgL−1waterborne Cd, according to the literature and environmentally relevant concentrations.exposed to Cd accumulated a sig- nificant amount of Cd, but the tissue concentrations of Ca and Fe decreased. A strong oxidative stress defense re- sponse was observed after exposed to Cd.
Cd concentrations in the kidney and liver increased with time and dose, which was consistent with findings in several other fish species, such as zebrafish,(0.022, 0.11, and 0.56mgL−1Cd2+; Matz., 2007), cat-fish (0.1, 0.2, and 0.4mgL−1; Asagba., 2008), Nile ti- lapia,(0.1 and 1.0mgL−1; Firat., 2009), and flounder,larvae (Cd; control, 0.01 and 0.15mgL−1). In this study, the greatest Cd accumula- tion inoccurred in the liver. Previous studies have shown that the fish liver is the primary tissue accumulating heavy metals, such as copper (Dang., 2012; Tsai., 2013; Zhou., 2017), lead (Reynders., 2006; Kim and Kang, 2015b), Cd (Kraal., 1995; Qu., 2014), and As (Roy and Bhattacharya, 2006; Kim andKang, 2015a). Metals accumulate in the liver because they are actively metabolized by this organ (Roy and Bhatta- charya, 2006). In addition, the liver is the most important detoxification organ (Das., 1998). Significant accu- mulation of Cd was also observed in the kidney ofat the higher levels of waterborne Cd exposure. A similar result was reported for As accumulation in, which was inferred to be the result from the detoxification function of the kidney (King and Kang, 2015). In addition, the kidney regulates the excretion of xe- nobiotics and toxic substances (Bodo., 2003). Rela- tively high concentrations of heavy metals have been found in the liver and kidney of different fish species. Kim and Kang reported high concentrations of Pb in the kidney and liver of juvenile rockfish,(2015b), and reported a high concentrationof chromium in the kidney and liver of(2016).
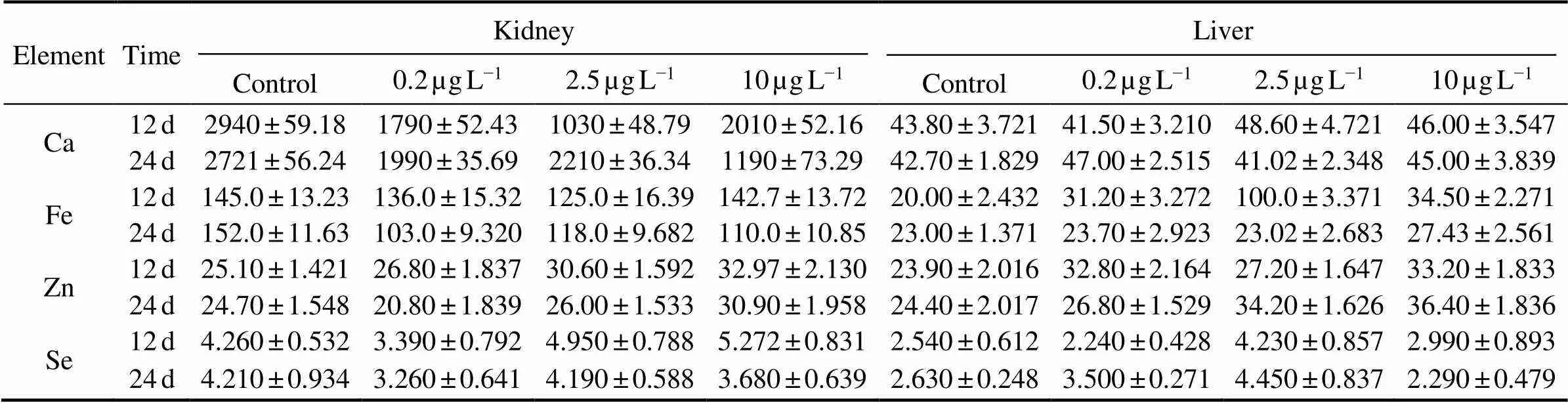
Table 1 Microelements contents in the liver and kidney of H. otakii after 12 and 24d exposure to 0, 0.2, 2.5, and 10µgL−1 Cd
Higher heavy metal tissue burdens lead to enhanced ac- cumulation of ROS, resulting in greater impairment of oxi-dative defense and/or damage. The defense mechanisms that detoxify and act as antioxidants help organisms survive in contaminated environments. Fishes have developed antioxidant defense mechanisms that commonly involve major antioxidant enzymes, such as SOD, CAT, and GST to protect against the detrimental effects of ROS (Brandão., 2015; Guardiola., 2016; Wu., 2018). These enzymes are induced as a compensatory response to a mild oxidative stress. However, excess ROS produced by xeno- biotics often overwhelm the detoxifying functions, which interferes with protein synthesis, resulting in suppressed antioxidant enzyme activities (Qu., 2014). SOD cata- lyzes superoxide anion radicals (O2−) and H+into the less toxic compound H2O2, which is subsequently decomposed into H2O by CAT. Together they constitute the first line of defense against oxidative stress induced by xenobiotics (Maulvault., 2017; Zhang., 2017; Wu., 2018). Therefore, SOD and CAT are induced simultaneously to remove ROS, as shown in gilthead seabream,(Guardiola., 2016), and European seabass,(Maulvault., 2017). SOD and CAT are critical enzymes to minimize oxidative injury in fish.
SOD activity increased significantly in the liver and kid- ney ofin response to all of the Cd concentrations. CAT was also induced by most of the Cd treatments. However, liver CAT activity was inhibited in the 10µgL−1treatment on day 24. The different responses of the two en- zymes were consistent with a previous study. For exam- ple, increased SOD and CAT activities have been detected in gilthead seabream exposed to 10µgL−1Cd for 2d, but were not found in fish exposed for 10 or 30d (Guardiola., 2016). CAT activity was inhibited when carp,, was exposed to 1.05 and 10.5μgg−1die- tary methyl mercury, while SOD activity wasnot affected significantly compared to the control (Mela., 2014).
GST has been recognized as a biomarker for oxidative stress caused by heavy metal exposure (Durou., 2007). In our study, GST activities in liver and kidneyincreased significantly inresponding to waterborne Cd, which might be caused by the accumulated Cd in the tis- sues. This enzyme regenerates GSH from its oxidized form,while glutathione disulfide plays a crucial role in the turn- over of GSH and in cellular antioxidant protection. How- ever, it can be inhibited by increased levels of reduced GSH in fishexposed to heavy metals (Ra- jeshkumar., 2013).
MDA is the product of lipid peroxidation (LPO), main- ly caused by the attack of excessive ROS on polyunsaturated fatty acids in cellular membranes (Freitas., 2016; Huang., 2018). Similar to the antioxidant enzymes, MDA content increased in the present study, indicating that oxidative stress occurred and led to LPO. The finding was consistent with previous studies. Qu. (2014) reported an increase of MDA content in the freshwater fishin response to Cd exposure. Cui. (2020) also reported an increase of MDA content in the larvae of flounder, which were exposed to seawater with acidification and Cd.
Ion homeostasis plays an important role in metabolism (Liew., 2013, 2015, 2020). Heavy metals exert their toxicity by altering ion homeostasis of the organism, such as iron deficiency, which may cause reproductive dysfunc- tion and affect immune function. In this study, Cd exposure decreased Ca and Fe contents in the liver and kidney of. In a previous study, Hou (2011) reported that Cd inhibits intestinal absorption of iron, resulting in iron deficiency and affecting normal development. Jiao (2019) also reported a decrease of Fe in common carp after exposure to Cd. No effect of Cd exposure on Zn and Se was observed in the present study, while Jiao (2019) reported a decrease of Zn in common carp. Cd exposure can affect microelements contents, which may have further aggravated the damage to the structure and function of liver and kidneyin.
5 Conclusions
We conclude that Cd exposure can affect the microelement and antioxidant defense system of. Cd preferentially accumulated in the liver tissue and increased with time and dose. Cd exposure resulted in a decrease of Ca and Fe concentrations in the kidney, while there was no effect on the liver. Cd exposure caused an antioxidant enzyme response at all three Cd exposure concentrations. Future toxicology studies should not only focus on the con- taminant concentration, but also consider the environment-relevant concentration. Such information will be critically important in the risk assessment of marine pollutants.
Acknowledgements
This study was supported by the Key Technology Research and Development Program of Shandong Province (Nos. 2019GHY112071 and 2019GHY112062), and the Major Agricultural Application Technology Innovation Pro- jects in Shandong Province (No. SD2019YY007).
Asagba, A. O., Eriyamremu, G. E., and Igberaese, M. E., 2008. Bioaccumulation of cadmium and its biochemical effect on se- lected tissues of the catfish ()., 34: 61-69.
Atli, G., Alptekin, Ö., Tükel, S., and Canli, M., 2006. Response of catalase activity to Ag+, Cd2+, Cr6+, Cu2+and Zn2+in five tissues of freshwater fish., 143: 218-224.
Atli, G., and Canli, M., 2008. Enzymatic responses to metal ex- posures in a freshwater fish., 145: 282-287.
Basha, P. S., and Rani, A. U., 2003. Cadmium-induced antioxi- dant defense mechanism in freshwater teleost(Tilapia)., 56: 218-221.
Benavides, M. P., Gallego, S. M., and Tomaro, M. L., 2005. Cad- mium toxicity in plants.,17: 21-34.
Brandão, F., Cappello, T., Raimundo, J., Santos, M. A., Maisano, M., Mauceri, A.,., 2015. Unravelling the mechanisms of mercury hepatotoxicity in wildfish () through a triad approach: Bioaccumulation, metabolomic profiles and oxida- tive stress., 7: 1352-1363.
Capillo, G., Silvestro, S., Sanfilippo, M., Fiorino, E., Giangrosso, G., Ferrantelli, V.,., 2018. Assessment of electrolytes and metals profile of the Faro Lake (Capo Peloro Lagoon, Sicily, Italy) and its impact on.,15 (5): e1800044, DOI: 10.1002/cbdv.201800044.
Cui, W. T., Cao, L., Liu, J. H., Ren, Z. H., Zhao, B., and Dou, S. Z., 2020. Effects of seawater acidification and cadmium on the antioxidant defense of flounderlar- vae., 718: 137234.
Dang, F., Wang, W. X., and Rainbow, P. S., 2012. Unifying prolonged copper exposure, accumulation, and toxicity from food and water in a marine fish., 46: 3465-3471.
Durou, C., Poirier, L., Amiard, J. C., Budzinski, H., Gnassia-Ba- relli, M., Lemenach, K.,., 2007. Biomonitoring in a clean and a multi-contaminated estuary based on biomarkers and chemical analyses in the endobenthic worm Nereis diversicolor., 148: 445-458.
Freitas, R., Pires, A., Velez, C., Almeida, Â., Moreira, A., Wrona, F. J.,., 2016. Effects of seawater acidification on(Polychaete, Onuphidae): Biochemical and regene- rative capacity responses., 60: 152-161.
Gao, X. L., Zhou, F. X., and Chen, C. T. A., 2014. Pollution sta- tus of the Bohai Sea: An overview of the environmental quali- ty assessment related trace metals., 62: 12-30.
Gaw, S., Northcott, G., Kim, N., Wilkins, A., and Jensen, J., 2012.Comparison of earthworm and chemical assays of the bioavail- ability of aged 1,1-dichloro-2,2-bis (p-chlorophenyl) ethylene 1,1,1-trichloro-2,2-bis (p-chlorophenyl) ethane, and heavy me- tals in orchard soils., 31: 1306-1316.
Gobi, N., Vaseeharan, B., Rekha, R., Vijayakumar, S., and Faggio, C., 2018. Bioaccumulation, cytotoxicity and oxidative stress of the acute exposure selenium in., 162: 147-159.
Grosell, M., McGeer, J. C., and Wood, C. M., 2001. Plasma cop- per clearance and biliary copper excretion are stimulated in copper-acclimated trout., 280: 796-806.
Guardiola, F. A., Chaves-Pozo, E., Espinosa, C., Romero, D., Me- seguer, J., Cuesta, A.,., 2016. Mercury accumulation, struc- tural damages, and antioxidant and immune status changes in the gilthead seabream (L.) exposed to methylmercury., 70: 734-746.
Hou, X. Y., 2011. Effect of zeolite on heavy metal accumulation, plasma indicators and liver metallothionein in carps () with water cadmium exposure. Master thesis. Nanjing Agricultural University (in Chinese with English abstract).
Huang, X. Z., Liu, Y. M., Liu, Z. K., Zhao, Z. H., Dupont, S., Wu,F. L.,., 2018. Impact of zinc oxide nanoparticles and oceanacidification on antioxidant responses of., 196: 182-195.
Jiao, W. Y., 2019. The study on the mechanism of immune injury induced by cadmium and chlorpyrifos exposure in common carp gills. Master thesis. Northeast Agricultural University (in Chinese with English abstract).
Kim, J. H., and Kang, J. C., 2015a. The arsenic accumulation andits effect on oxidative stress responses in juvenile rockfish,, exposed to waterborne arsenic (As3+)., 39: 668-676.
Kim, J. H., and Kang, J. C., 2015b. The lead accumulation and he- matological findings in juvenile rock fishexposed to the dietary lead (II) concentrations., 115: 33-39.
Kim, J. H., and Kang, J. C., 2016. The chromium accumulation and its physiological effects in juvenile rockfish,, exposed to different levels of dietary chromium (Cr6+) concentrations., 41: 152-158.
Kraal, M. H., Kraak, M. H. S., Degroot, C. J., and Davids, C., 1995.Uptake and tissue distribution of dietary and aqueous cadmium by carp ()., 31: 179-183.
Li, W., Wang, N. B., Li, Q. B., and Sun, B., 2008. Distribution of dissolved metals in seawater of Jinzhou Bay, China., 27 (1): 43-48.
Liew, H. J., Chiarella, D., Pelle, A., Faggio, C., Blust, R., and De Boeck, G., 2013. Cortisol emphasizes the metabolic strategies employed by common carp,at different feed- ing and swimming regimes.–, 166 (3): 449-464.
Liew, H. J., Fazio, A., Faggio, C., Blust, R., and De Boeck, G., 2015. Cortisol affects metabolic and ionoregulatory responses to a different extent depending on feeding ration in common carp,., 189: 45-47.
Liew, H. J., Pelle, A., Chiarella, D., Faggio, C., Tang, C. H., Blust, R.,., 2020. Common carp,, prefer branchial ionoregulation at high feeding rates and kidney ionoregulation when food supply is limited: Additional effects of cortisol and exercise., 46 (1): 451-469.
Matz, C. J., Treble, R. G., and Krone, P. H., 2007. Accumulation and elimination of cadmium in larval stage zebrafish following acute exposure., 66: 44-48.
Maulvault, A. L., Barbosa, V., Alves, R., Custódio, A., Anacleto, P., Repolho, T.,., 2017. Ecophysiological responses of ju- venile seabass () exposed to increased tem- perature and dietary methylmercury., 586: 551-558.
McRae, N. K., Gaw, S., and Glover, C. N., 2016. Mechanisms of zinc toxicity in the galaxiid fish,., 179: 184-190.
Mela, M., Neto, F. F., Yamamoto, F. Y., Almeida, R., Grötzner, S. R., Ventura, D. F.,., 2014. Mercury distribution in target organs and biochemical responses after subchronic and trophic exposure to Neotropical fish., 40: 245-256.
Mendil, D., Unal, O. F., Tuzen, M., and Soylak, M., 2010. Deter- mination of trace metals in different fish species and sediments from the River Yesilirmak in Tokat, Turkey., 48 (5): 1383-1392.
Mohsenpour, R., Mousavi-Sabet, H., Hedayati, A., Rezaei, A., Yalsuyi, A. M., and Faggio, C., 2020.effects of silver nanoparticles on gills morphology of female Guppy () after a short-term exposure., 83 (12): 1552-1557, DOI: 10.1002/jemt.23549.
Moyson, S., Liew, H. J., Fazio, A., Van Dooren, N., Delcroix, A., Faggio, C.,., 2016. Kidney activity increases in copper ex- posed goldfish ()., 190: 32-37.
Nordberg, G. F., 2010. Biomarkers of exposure, effects and suscep- tibility in humans and their application in studies of interactions among metals in China., 192 (1): 45-49.
OSPAR, 2010. Quality Status Report 2010. OSPAR Head of De- legation Meeting, London, UK, 12-13 November 2009, 176pp.
Pagano, M., Porcino, C., Briglia, M., Fiorino, E., Vazzana, M., Sil- vestro, S.,., 2017. The influence of exposure of cadmium chloride and zinc chloride on haemolymph and digestive gland cells from., 11 (2): 207-216.
Qu, R. J., Wang, X. W., Wang, Z. Y., Wei, Z. B., and Wang, L. S., 2014. Metal accumulation and antioxidant defenses in the fresh- water fishin response to single and combined exposure to cadmium and hydroxylated multi-walled carbon nanotubes., 275: 89-98.
Rajeshkumar, S., Mini, J., and Munuswamy, N., 2013. Effects of heavy metals on antioxidants and expression of HSP70 in dif- ferent tissues of Milk fish () of Kaattuppalli Is- land, Chennai, India., 98: 8-18.
Reynders, H., Campenhout, K. V., Bervoets, L., Coen, W. M. D., and Blust, R., 2006. Dynamics of cadmium accumulation and effects in common carp () during simultaneous exposure to water and food ()., 25: 1558-1567.
Roy, S., and Bhattacharya, S., 2006. Arsenic-induced histopatho- logy and synthesis of stress proteins in liver and kidney of., 65: 218-229.
Savorelli, F., Manfra, L., Croppo, M., Tornambè, A., Palazzi, D., Canepa, S.,., 2017. Fitness evaluation ofexposed to nickel., 177 (2): 384-393.
Torre, A., Trischitta, F., and Faggio, C., 2013. Effect of CdCl2on Regulatory Volume Decrease (RVD) indigestive cells., 27: 1260-1266.
Tsai, J. W., Ju, Y. R., Huang, Y. H., Deng, Y. S., Chen, W. Y., Wu, C. C.,., 2013. Toxicokinetics of tilapia following high ex- posure to waterborne and dietary copper and implications for coping mechanisms., 20: 3771-3780.
Vajargah, M. F., Yalsuyi, A. M., Hedayati, A., and Faggio, C., 2018. Histopathological lesions and toxicity in common carp (L. 1758) induced by copper nanoparticles., 81 (7): 724-729.
Vajargah, M. F., Yalsuyi, A. M., Sattari, M., Prokic, M., and Fag- gio, C., 2019. Effects of copper oxide nanoparticles (CuO-NPs) on parturition time, survival rate and reproductive success of guppy fish,., 31: 499-506,DOI: 10.1007/s10876-019-01664-y.
Vijayakumar, S., Vaseeharan, B., Sudhakaran, R., Jeyakandan, J., Ramasamy, P., Sonawane, A.,., 2019. Bioinspired zinc oxide nanoparticles usingfor antimi- crobial and anticancer applications., 30 (6): 1465-1479.
Wu, F. Z., Huang, W., Liu, Q., Xu, X. Q., Zeng, J. N., Cao, L.,., 2018. Responses of antioxidant defense and immune gene ex- pression in early life stages of large yellow croaker () under methyl mercury exposure., 9: 1436.
Zhang, C. N., Zhang, J. L., Ren, H. T., Zhou, B. H., Wu, Q. J., and Sun, P., 2017. Effect of tributyltin on antioxidant ability and immune responses of zebrafish ()., 138: 1-8.
Zhao, X., Wang, S., Ma, L., Yue, B., Zhao, Y. F., and Shang, X. H., 2016. Analysis of Pb and Cd pollution in marine fish in some sea areas of China., 45 (3): 474- 476 (in Chinese with English abstract).
Zhou, Y. Y., Zhang, W., Guo, Z. Q., and Zhang, L., 2017. Effects of salinity and copper co-exposure on copper bioaccumulation in marine rabbitfish., 168: 491-500.
March 4, 2021;
April 13, 2021;
April 27, 2021
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2022
#The two authors contributed equally to this work.
. E-mail: guowen1963@126.com
(Edited by Qiu Yantao)
 Journal of Ocean University of China2022年2期
Journal of Ocean University of China2022年2期
- Journal of Ocean University of China的其它文章
- Study of the Wind Conditions in the South China Sea and Its Adjacent Sea Area
- A Spatiotemporal Interactive Processing Bias Correction Method for Operational Ocean Wave Forecasts
- Characteristics Analysis and Risk Assessment of Extreme Water Levels Based on 60-Year Observation Data in Xiamen, China
- Underwater Target Detection Based on Reinforcement Learning and Ant Colony Optimization
- Polar Sea Ice Identification and Classification Based on HY-2A/SCAT Data
- Thermo-Rheological Structure and Passive Continental Margin Rifting in the Qiongdongnan Basin,South China Sea, China
