Glinus lotoides ethanolic extract alleviates LPS-induced anxiety and depression-like behavior by modulating antioxidant and inflammatory biomarkers in rats
Ambreen Mehmood Awan, Wafa Majeed,4, Faraza Javed, Bilal Aslam, Asra Iftikhar, Hafiza Arooj Kanwal,Sobia Fiaz
1Institute of Physiology and Pharmacology, University of Agriculture, Faisalabad, Pakistan
2Department of Pharmacology, Faculty of Pharmacy, the Islamia University of Bahawalpur, Bahawalpur, Pakistan
3Department of Pharmacy, University of Faisalabad, Faisalabad, Pakistan
4Department of Pharmacy, University of Agriculture, Faisalabad, Pakistan
ABSTRACT
KEYWORDS: Glinus lotoides; Biochemical markers; Depression;Traditional medicine; Nervous system; Anxiety; Antioxidant;Inflammation
1. Introduction
Depression is a common disorder and is considered as one of the leading causes of morbidity and mortality, affecting about 10%–15% of the population each year[1]. It is a mental disorder,mostly observed in people between 30 and 40 years old, and the symptoms of depression included loss of interest, low mood, low self-esteem and loss of pleasure in daily events, feeling hopeless,helpless, and anorexic, worried, and depressed[2]. Moreover,anxiety is an emotional response to psychological, physiological,and social stressors, characterized by madness, panic, impairment in mood, excessive activity of the autonomic nervous system,and reduction in learning. Physiological symptoms of anxiety include augmented heart rate, high blood pressure, and dizziness,breathing, and muscle contraction disturbance. Neurodegenerative factors have also been related to the pathogenesis of anxiety and depression[3]. Recent studies indicated that neuroinflammation has been considered to play a vital role in brain disorders, comprising anxiety and depression. Inflammatory mediators such as interleukin(IL)-6 and tumor necrosis factor (TNF)-alpha have been considered to play a vital role in anxiety and depression, as these cytokine levels have been known to be increased in anxiety and depression.Previous data suggested that systemic inflammation can affect brain function and produce inflammation in the central nervous system by stimulating the resident macrophages (microglia) of the central nervous system, resulting in the production of inflammatory cytokines[3,4]. Lipopolysaccharides (LPS) induced inflammation by over-production of inflammatory cytokines that triggers sickness behavior[5].
Recent evidence has shown that traditional remedies have many benefits in the treatment of depression such as improved tolerability and fewer side effects[6]. The safety and efficacy of natural plant extracts may be better than those of synthetic drugs. Interestingly,medicinal plants are always of great value due to their potential bioactivities and valuable constituents for the human population[7].They are important sources of natural products, which differ widely in terms of their mechanisms of action, biological properties, and structures. For various diseases, traditional remedies have been a vital part of treatment therapies like asthma, mental disorders,arthritis, cardiac disorders, digestive problems, inflammatory disorders, sexual diseases, and urinary infections. In recent decades,despite great advancements in modern medicines, herbal or folkloric remedies still provide important contributions to healthcare. In this modern era, people prefer herbal drugs because of their general approach, lower side effects, and qualitative action of the weather,seasonal requirement, and psychological dimensions[8]. In the South Asian desert belt area, Glinus lotoides (G. lotoides), a flowering plant,belongs to the family Molluginaceae and is commonly known as kotak, moghua, and okhrad. It is also known as damascisa and lotus sweet juice (theplantlist.org). G. lotoides is a branched natural herb with 30 cm-35 cm long stem covered with whitish hairs. Leaves are oval-shaped arranged in whorls around the stem. Traditionally, this herb is used as a purgative to treat diseases of the abdomen. Dried plant was used to treat diarrhea in native community. Fresh leave poultice is prepared and applied on wounds, inflamed and itchy skin to treat inflammatory disorders. Whole plant decoction is used as a blood purifier and anthelmintic as well as to manage arthritis,gastrointestinal disorders, and Kapha diseases (Loss of body’s balance due to lethargy, laziness, excessive sleep pattern, nausea,and depression). In carcinoma cell lines, G. lotoides seed extract exhibited differential growth inhibitory responses[9,10]. Therefore,the present study was planned to evaluate the anti-inflammatory and antidepressant effects of G. lotoides in animal models of depression.
2. Materials and methods
2.1. Plant material
The whole plant of G. lotoides was collected from the desert zone of Bahawalpur, Pakistan. After collection of plant material, a sample was identified, stored, and deposited in the herbarium of Institute of Physiology and Pharmacology, University of Agriculture, Faisalabad,Pakistan and the voucher number issued was 21, 156. The plant was washed with water, shade dried, and cautiously screened to remove any unnecessary matter.
2.2. Crude extract preparation
Plant material was exposed to coarse grinding using a grinder and was macerated in 70% ethanolic water for 3 d at room temperature.Then the plant material was filtered using a muslin cloth to obtain the filtrate. The resulting residue was soaked again and then filtered twice again. In the end, all filtrates were collected and evaporated under reduced pressure and at low temperature using a rotary evaporator (Heidolph Laborota 4000 efficient, Germany). Using a hot air oven, a thick sticky paste of plant extract was further dried at 40 ℃. Semisolid extract obtained was weighed, labeled, and stored in an air-tight container in a freezer below 0 ℃[11].
2.3. Qualitative phytochemical analysis
Qualitative phytochemical analysis of G. lotoides ethanolic extract was performed for the determination of alkaloids, amino acids,saponins, flavonoids, terpenoids, anthraquinones, cardiac glycoside,carbohydrates, tannins, coumarins, and phenols using standard procedures as described[12]. The determination of anthocyanins was done by the method of Savithramma et al[13]. Steroids and proteins were determined by the method of Guldiken et al[14]. Resins and fixed oil were determined by the method as prescribed[15,16].
2.4. Quantitative phytochemical analysis
2.4.1. High performance liquid chromatography (HPLC)analysis
HPLC analysis was performed by the following technique of Javed et al[11]. Using ethanol stock solutions, various standards were prepared to make a final concentration of 50 µg/mL. Ethanol was used as a solvent for polyphenols at the concentration of 10 mg/mL.SIL-20A autosampler and C-18 column were used to conduct the analysis. Solution system with linear gradient consisted of acetic acid with water and ethanol at an 1 mL/min flow rate. The absorbance was measured at 280 nm and the components of G. lotoides ethanolic extract were identified in comparison with the standard solutions.
2.4.2. Total alkaloid contents (TAC)
The method of Shamsa et al.[17] was followed with slight modifications. One gram of G. lotoides ethanolic extract was filtered and evaporated under a vacuum. Residues were dissolved in 2 N HCl and then filtered. Then 1 mL of solution was transferred to a separating funnel and washed three times with 10 mL chloroform.PH of this solution was adjusted with 0.1 mol/L NaOH. To this solution, 5 mL of Bromocresol green solution and 5 mL phosphate buffer were added. Then the mixture was shaken and extracted with 4 mL of chloroform. After that, the extract was collected in a 10 mL volumetric flask and then diluted with chloroform. The absorbance was found at 470 nm.
2.4.3. Total carbohydrate contents (TCC)
Standard and reagents were prepared by adopting the method of Kumar et al.[18] with slight modifications. To 1 mg of the extract (G.lotoides ethanolic extract), 2 mL of anthrone reagent was added into 25 mL of volumetric flask. Then the final volume of the mixtures was made with distilled water. The absorbance of samples at 750 nm was measured by using a spectrophotometer.
2.4.4. Total flavonoids contents (TFC)
TFC was estimated following the procedure of Amir et al.[19]with slight modifications. Briefly, 0.5 mg of extract (G. lotoides ethanolic extract) was dissolved in 1.5 mL of methanol, 0.1 mL of 1 M potassium acetate, 0.1 mL of 10% aluminum chloride, and 2.8 mL of distilled water. Different concentrations of rutin solution were prepared in methanol and treated with the same as described above. Reaction mixtures were kept for 30 min at room temperature.After the incubation period, absorbance was measured by a spectrophotometer at 415 nm.
2.4.5. Total phenolic contents (TPC)
Total phenolic content was estimated following the method of Amir et al.[19] with slight modifications. G. lotoides ethanolic extract (0.5 mg) and different concentrations of standard compound (gallic acid)were dissolved separately in 5 mL of Folin Ciocalteu reagent and 1 M aqueous sodium carbonate (4 mL). The mixtures were allowed to stand for 15 min and absorbance was measured at 765 nm.
2.4.6. Total tannin contents (TTC)
TTC was estimated following the method of Tamilselvi et al[20]. To 0.1 mg G. lotoides ethanolic extract, 0.5 mL of Folin Denis reagent,1 mL of 35% sodium carbonate solution, and 7.5 mL of distilled water were added and diluted up to 10 mL. The mixture was shaken properly and allowed to stand for 30 min at room temperature.Absorbance was measured at 725 nm using a spectrophotometer.
2.5. Experimental animals
Male Wistar albino rats, 12 weeks old and weighing (240 ± 10) g as well as Swiss albino mice of either gender weighing 25-35 g were kept in the animal house of Institute of Physiology and Pharmacology,University of Agriculture, Faisalabad, Pakistan. Animals were housed under standard protocols and provided with a standard rodent diet and water ad libitum. Normal room temperature i.e., 25 ℃ was maintained in the animal house with a 12-hour period of light and dark cycle.
2.6. Acute toxicity study
According to the guidelines of the Organization for Economic Cooperation and Development, acute toxicity studies were performed. Mice weighing 25-35 g were grouped into 4 groups,with 5 mice in each group, and were observed for normal behavioral parameters. Group 1 received vehicle (normal saline 10 mL/kg p.o.)and the other 3 groups received the G. lotoides ethanolic extract at the doses of 1, 2, and 3 g/kg p.o., respectively. Response parameters and behavioral changes; i.e. hyperactivity, lacrimation, alertness,sweating, grooming, convulsion, urination, corneal reflex, righting reflex, gripping strength, pain response, and mortality were observed and recorded at 0.5, 1, 2, 4, 6, 12, 24 and 48 h[21].
2.7. Experimental design
Rats were separated into six groups (n = 6 rats/group) as follows:GroupⅠ: Normal control group received normal saline (5 mL/kg p.o.); GroupⅡ: Intoxicated group received normal saline (5 mL/kg p.o.) and LPS (0.83 mg/kg i.p.); Group Ⅲ: Standard group received imipramine (20 mg/kg p.o.) and LPS (0.83 mg/kg i.p.); Group Ⅳ,Ⅴ, Ⅵ: The treatment groups received G. lotoides ethanolic extract at the doses of 100, 300 and 500 mg/kg p.o. and LPS (0.83 mg/kg i.p.).All groups received the respective treatments once daily for 14 d. LPS (0.83 mg/kg) was given i.p. after 30 min of respective treatments on the 14th day to all groups except the normal control group. To evaluate the anxiety and depression-like behavior in rats, behavioral tests including forced swimming test (FST), tail suspension test (TST), open field test (OFT), and sucrose preference test (SPT) were performed after 24 h of LPS administration[22-25].Following the behavioral studies, rats were decapitated, under ether anaesthesia and blood samples were collected. Blood samples were centrifuged for 10 min at 4 000 rpm and the resultant serum was stored at −80 ℃ for antioxidant and biochemical investigation. Total antioxidant capacity (TAC), total oxidant status (TOS)[26,27], catalase(CAT)[28], superoxide dismutase (SOD), malondialdehyde (MDA),and glutathione (GSH) activities were estimated in the serum samples. TNF alpha and IL-6 were also analyzed by using serum samples[29,30].
2.8. Behavioral tests
2.8.1. OFT
Anxiety and locomotor activity were observed using an open field arena with dimensions of 100 cm × 100 cm. In the center of the field,each rat was released and monitored for 5 min by using a digital camera. The total number of crossing and rearing was reflected indexes of locomotor activity[22].
2.8.2. FST
FST was used to evaluate depression-associated behavior. For this purpose, a cylindrical reservoir (60 cm height and 38 cm width)made of glass and loaded with water at the temperature of 24-25 ℃was used. Animals were placed in the water-filled cylinder and their mobile and immobile time were measured by using a stopwatch.When rats stopped making attempts to escape out from water, they were considered in an immobile state and vice versa[23].
2.8.3. TST
For this test, a 70 cm high wooden chamber was used. At the center of the sidewalls of the chamber, a rod of 60 cm high from the ground was fitted properly. Adhesive tape was placed one inch from the tip and then animals were suspended with the rod for 5 min. The duration of immobility of each animal for 5 min was noted. When rats made no effort to escape from the position, they were considered in an immobile state and vice versa[24].
2.8.4. SPT
For evaluation of anhedonia, SPT was used. Before LPS administration, all rats were adapted to the drinking water and 2% sucrose solution to create a baseline sucrose preference for 5 d before testing. The drinking bottle filled with sucrose solution having a closure valve was positioned in the animal’s home cage. To avoid place preference development, the relative position of bottles was changed daily. On the day of the test, rats were deprived of food and water 2 h before the procedure. Later, after 24 h of LPS administration, fluid contents were measured and sucrose preference was calculated[25].
2.9. Hematological studies
Rats were anesthetized and blood samples were taken from the retro-orbital sinus. To prepare the blood sample, Turk solution was used for counting. This solution was comprised of equal amounts of gentian violet solution, glacial acetic acid 1%, and 100 mL of distilled water. Using a hemocytometer, the total white blood cells(WBC) count was measured carefully by following the procedure as described[26].
2.10. Measurement of oxidative stress and antioxidant capacity
2.10.1. Determination of TAC
TAC was measured spectrophotometrically by the method developed by Erel[26]. This test was dependent on the 2,2’-azinodi-3-ethylbenzthiazoline sulfonate (ABTS)+and the reduced ABTS molecule was oxidized to ABTS using hydrogen peroxide in an acidic medium. In the acidic medium, deep green ABTS+molecules became more stable for a long time. The reaction rate was calibrated with Trolox which was a standard for TAC measurement assays and results were expressed in mmol Trolox equivalent/L.
2.10.2. Determination of TOS
TOS was determined by using the method described by Erel[27].This procedure was based on the oxidation of ferrous ion to ferric ion in the presence of various oxidative species under acidic conditions as ferric ion makes a colored complex with xylene orange. The color intensity was measured spectrophotometrically and it was associated with the total amount of oxidant molecules present in the sample. Hydrogen peroxide was used for calibration and results were depicted as µmol H2O2equivalent/L.
2.10.3. Measurement of CAT
CAT is widely distributed in animals, plants, microorganisms, and culture cells, and is the most important hydrogen peroxide (H2O2)species scavenger. The working solution was incubated at 37 ℃for 10 min and 10 µL of sample and the working solution were added to micro plate and fully mixed. CAT level was measured by colorimetric method and absorbance was measured at 240 nm[28].
2.11. Measurement of biochemical parameters
The levels of MDA, GSH, SOD, TNF-alpha, and IL-6 were determined by colorimetric and ELISA kit methods, respectively.The level of MDA was estimated by TBA method. MDA reacts with TBA to form MDA-TBA adduct which was quantified by measuring OD at 450 nm. SOD and GSH are antioxidant enzymes that scavenge free radicals and maintain the physiological process.SOD was measured by WST1-method. Xanthin oxidase cleaves WST-1 that reacts with oxygen to produce formazan dye and SOD correlates with the production of formazan dye, hence SOD activity was measured colorimetrically by the quantification of foramzan dye. IL-6 and TNF-α levels were determined by sandwich ELISA immunoassay techniques[29,30].
2.12. Statistical analysis
Results were expressed as mean ± SEM. The analysis of the results was made using one-way analysis of variance (ANOVA) followed by Bonferroni’s test. Graph Pad Prism software version 8 was used for analysis and P<0.05 was considered significantly different.
2.13. Ethical statement
Studies were carried out according to the recommendations approved by Institutional Biosafety and Bioethics Committee,University of Agriculture, Faisalabad, Pakistan with letter no. 1729/ORIC.
3. Results
3.1. Qualitative phytochemical analysis
Qualitative analysis of G. lotoides ethanolic extract showed the presence of alkaloids, carbohydrates, glycoside, coumarins, fixed oil,flavonoids, phenols, and tannins.
3.2. HPLC analysis
HPLC fingerprinting of G. lotoides ethanolic extract showed the presence of gallic acid, benzoic acid, caffeic acid, chlorogenic acid,and quercetin as shown in Figure 1.

Figure 1. HPLC chromatogram of Glinus lotoides (G. lotoides) ethanolic extract.
3.3. Quantitative phytochemical analysis
In G. lotoides ethanolic extract, TAC was (2.72 ± 0.02) mg/g, TCC(279.1 ± 1.66) mg/g, TFC (15.57 ± 0.36) mg rutin equivalent/100 g,TPC (34.74 ± 1.37) mg gallic acid equivalent/100 g and TTC (1.63 ±0.09) mg tannic acid equivalent/100 g.
3.4. Acute toxicity study
G. lotoides ethanolic extract was found safe up to the dose of 3 g/kg with no mortality observed during the experiment, confirming its low toxicity.
3.5. Behavioral tests
3.5.1. OFT results
In OFT, the LPS administered group showed a significant decrease in the crossing and rearing movements when compared to the normal control group (P<0.001). Pre-treatment with imipramine and G.lotoides ethanolic extract (100, 300, and 500 mg/kg) significantly mitigated the decreased movements and increased the number of crossing and rearing along with improved mobility time (P<0.001),as shown in Figure 2.

Figure 2. Effects of different doses of G. lotoides ethanolic extract in open field test. Values are given as mean ± SEM (n=6) and analyzed by one-way ANOVA followed by Bonferroni test. Values are statistically significant at#P<0.001 between the normal and the intoxicated groups, and *P<0.001 between the intoxicated and the treated groups. C: control, Int: intoxicated,Imp: imipramine.

Figure 3. Effects of different doses of G. lotoides ethanolic extract in (A) forced swim test and (B) tail suspension test. Values are given as mean ± SEM (n=6)and analyzed by one-way ANOVA followed by Bonferroni test. Values are statistically significant at #P<0.001 between the normal and the intoxicated groups,and *P<0.001 between the intoxicated and the treated groups.
3.5.2. FST results
In FST, LPS markedly increased the time in the immobile phase compared with the normal control group (P<0.001). The time spent in the immobile phase was significantly decreased in the imipramine and G. lotoides ethanolic extract-treated groups, as compared to the LPS challenged group (P<0.001) (Figure 3A).
3.5.3. TST results
In this test, the imipramine treated group significantly increased the duration of time spent in the mobile phase, while the time spent in the immobile phase was decreased as compared to the LPS treated groups (P<0.001). G. lotoides ethanolic extract at all the doses also showed a significant increase in the mobile phase in comparison to the LPS administered group (P<0.001) (Figure 3B).
3.5.4. SPT results
There was a marked increase in the sucrose preference (%) in the imipramine and G. lotoides ethanolic extract treated groups (100, 300,and 500 mg/kg), as compared to the LPS treated group (P<0.001). In addition, among all the doses, 500 mg/kg G. lotoides ethanolic extract showed a maximal increase in the sucrose preference. Results are shown in Figure 4.
3.6. Hematological result
Hematological study showed a significant increase in the WBC count in the LPS administered group when compared to the normal control group (P<0.001). The imipramine and G. lotoides ethanolic extract-treated groups at the doses of 100, 300 and 500 mg/kg showed significant decreases in the WBC count, when compared to the LPS administered group (P<0.001) (Figure 5).

Figure 4. Effects of different doses of G. lotoides ethanolic extract in sucrose preference test. Values are given as mean ± SEM (n=6) and analyzed by oneway ANOVA followed by Bonferroni test. Values are statistically significant at #P<0.001 between the normal and the intoxicated groups, and *P<0.001 between the intoxicated and the treated groups.
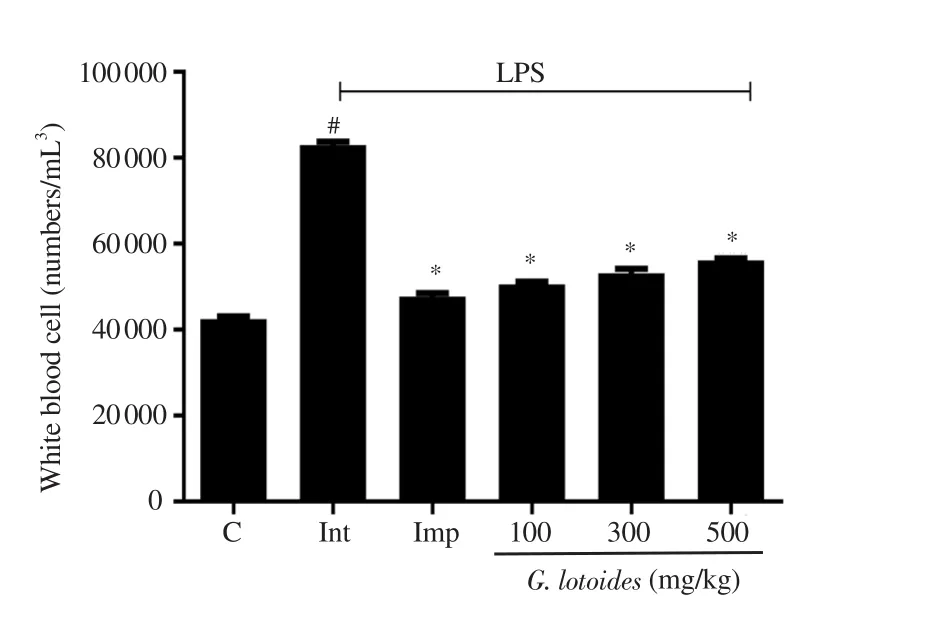
Figure 5. Effects of different doses of G. lotoides ethanolic extract on white blood cell count. Values are given as mean ± SEM (n=6) and analyzed by one-way ANOVA followed by Bonferroni test. Values are statistically significant at #P<0.001 between the normal and the intoxicated groups, and*P<0.001 between the intoxicated and the treated groups.
3.7. Oxidative stress and antioxidant capacity
TOS level was significantly increased while TAC level significantly decreased in the LPS administered group as compared to the normal control group (P<0.001). Treatment with standard drug imipramine and G. lotoides ethanolic extract at the doses of 100, 300, and 500 mg/kg reversed the LPS-induced changes in TOS and TAC levels(P<0.001) (Table 1).

Table 1. Effects of G. lotoides ethanolic extract on total oxidant status (TOS),total antioxidant capacity (TAC), and catalase (CAT) activity against LPS-induced anxiety and depression-like behavior in rats.
3.8. CAT activity
CAT level was decreased significantly in the LPS administered group in comparison to the normal control group (P<0.001). The imipramine and G. lotoides ethanolic extract treated groups (at the doses of 100, 300, and 500 mg/kg) mitigated the decreased levels significantly when compared to the LPS challenged group (P<0.001)(Table 1).
3.9. Biochemical parameters
LPS caused a significant decrease in the antioxidant enzymes;i.e. SOD and GSH (P<0.001). G. lotoides ethanolic extract at all doses significantly mitigated the LPS-induced decreased levels of SOD and GSH (P<0.001). The levels of MDA, IL-6, and TNF-α were elevated significantly in the LPS challenged group (P<0.001),when compared to the normal control group. However, G. lotoides ethanolic extract at all doses and imipramine significantly (P<0.001)decreased the levels of MDA, IL-6, and TNF-α (P<0.001). Results are summarized in Table 2.
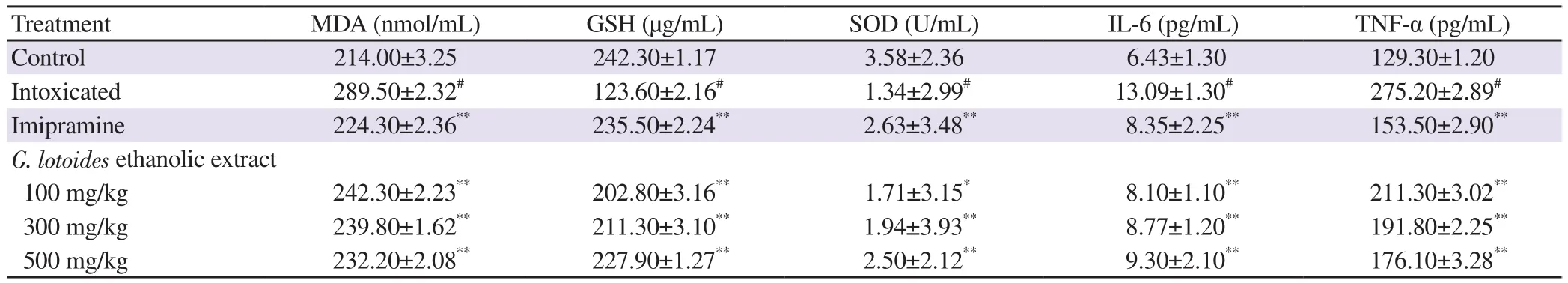
Table 2. Effects of G. lotoides ethanolic extract on malondialdehyde (MDA), glutathione (GSH), superoxide dismutase (SOD), interleukin (IL-6), and tumor necrosis factor (TNF-α) levels against LPS-induced anxiety and depression-like behavior in rats.
4. Discussion
Previous studies have indicated that anxiety and depression induced by LPS are associated with stimulation of inflammatory responses[24]. Depression is a complex interaction between social,physiological, and biological factors. Various studies revealed that several ailments may provoke depression; i.e. diabetes, stroke,cardiovascular disorders, cancer, Huntington, and Parkinson’s disease. Although dysfunction of monoaminergic neurotransmitter systems plays a significant role in mediating some aspects of the pathophysiology of depression, there are other probable mechanisms involved too[31]. Anti-anxiety and antidepressant activities were evaluated by OFT, FST, TST, and SPT. For the estimation of antidepressant activity, FST and TST are the most commonly used tests due to their high prognostic validity[32].These are reliable behavioural tests for the screening of new antidepressant drugs. In FST, duration of immobility is a major parameter related to depression-like behavior in rats. Previous research studies have demonstrated that systemic administration of LPS induces anxiety or depressive behavior in rats[5]. In previous studies, imipramine diminishes stress-induced inflammation[33]and is used as a reference drug[34]. We found that, in FST and TST tests, LPS challenged animals showed a significant increase in time spent in the immobile phase as compared to the normal control group, whereas, pre-treatment with imipramine and G. lotoides ethanolic extract significantly reversed the LPS-induced increase in the immobile time. Oxidative stress pathways are crucial in the pathophysiology of depression. Lipid peroxidation, high cytokine levels, and low antioxidant enzymes are predisposing variables in neuroprogression during the depression. Antioxidant enzymes are potent free radical scavengers and oxidative damage inhibitors.Between oxidative and antioxidative systems in an organism, there is a balance in physiological circumstances. Imbalance between these systems has been concerned with the pathophysiology of several neuropsychiatric diseases, like major depressive disorders. In the last decade, researchers worked and focused on the relationship between the oxidant and antioxidant systems in depression[35]. SOD, CAT,and GSH are antioxidant enzymes that work together to scavenge ROS[36]. We observed a significant reduction in antioxidant enzyme levels (SOD; CAT; GSH) in LPS-induced rats. Inflammatoryand neurodegenerative processes also play a vital role in the pathogenesis of depression[37]. In the present study, elevated levels of inflammatory indicators such as IL-6 and TNF-α accompanied by depression-linked behaviors were observed in the LPS administered group[24]. Our results are in accordance with previous research studies, which confirmed the direct connection of oxidative stress with depression[31], through a significant decrease in the levels of SOD, CAT, GSH, and a significant increase in MDA levels[2,6]. In contrast, we found that G. lotoides crude extract reversed the LPS-induced changes.
Phytochemical investigations confirmed the presence of alkaloids,carbohydrate, cardiac glycoside, coumarins, flavonoids, fixed oil,phenols, and tannins in the G. lotoides crude extract. Flavonoids have potent antioxidant activity against the superoxide radical.Phenols and polyphenolic compounds have been shown to possess significant antioxidant and neuroprotective activities. HPLC analytical technique is used for the isolation of various natural products. In the present investigation, HPLC technique was used to study the secondary metabolites[38,39]. Phenolic compounds and their derivatives (gallic acid, benzoic acid, caffeic acid, chlorogenic acid) and flavonoids (quercetin) were quantified in the G. lotoides crude extract. Gallic acid has several beneficial effects including antioxidant, anti-inflammatory, and antineoplastic properties. It has characteristics of strong antioxidant and free radical scavenging activities[40]. In biological systems, caffeic acid and its combination with chlorogenic acid showed potent antioxidant activity. Phenolic acids and their derivatives have antioxidant, anti-inflammatory, and anti-carcinogenic activities[41,42]. Chlorogenic acid has antioxidant,anti-inflammatory, antibacterial, cardioprotective, neuroprotective,hepatoprotective, anti-hypertension, antipyretic, and free radicals scavenging activities[43,44]. Flavonoids are major bioactive compounds that have been used against many chronic diseases such as cancer, neurodegenerative inflammation, viral infections,and cardiovascular disorders[45]. Quercetin (flavonoid) has potent antioxidant and anti-inflammatory activities[14]. Antioxidant and biochemical findings support the traditional uses of G. lotoides and the antidepressant mechanism of the tested plant via modulation of cytokine and ROS level cannot be ruled out. Hence, this plant may be a good candidate for the management of inflammatory and depressive disorders, however, further studies are required to isolate the pure compounds to verify the antidepressant activities and underlying mechanisms of actions.
Conflict of interest statement
Authors declare no conflict of interest.
Acknowledgments
The authors are grateful to Institute of Physiology and Pharmacology, University of Agriculture, Faisalabad, Pakistan for providing research facilities for this study.
Authors’ contributions
AMA designed the work and was involved in data collection. Both WM and BA were involved in data analysis and interpretation. FJ and AI were involved in drafting the article and critical revision of the article. HAK and SF finally approved the version to be published.
 Asian Pacific Journal of Tropical Biomedicine2022年2期
Asian Pacific Journal of Tropical Biomedicine2022年2期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Protein extract of kenaf seed exhibits anticoagulant, antiplatelet and antioxidant activities
- Schisandrae Fructus oil-induced elevation in serum triglyceride and lipoprotein concentrations associated with physiologic hepatomegaly in mice
- Anti-tumor effects and cellular mechanisms of Pistacia atlantica methanolic extract against Ehrlich solid tumor in mice
- Barrientosiimonas humi ethyl acetate extract exerts cytotoxicity against MCF-7 and MDA-MB-231 cells via induction of apoptosis and cell cycle arrest
