The Roles of Nuclear Energy in Hydrogen Production
Shinji Kubo
Japan Atomic Energy Agency, Ibaraki-ken 311-1393, Japan
1. Nuclear energy as primary energy and hydrogen energy as secondary energy
Fossil resources are unevenly distributed on the earth and are a finite source of primary energy that is widely used in industry(factories, etc.), transportation (automobiles, etc.), and energy conversion (power generation, etc.). It must be said that the mass consumption of fossil resources is non-sustainable. Primary energies that can replace fossil-fuel-based energy include renewable energy and nuclear energy, while hydrogen energy has the potential to be a secondary energy source that can be widely used in industry for various purposes,including the use of hydrogen as raw material for chemical products,a reducing agent,and fuel.For example, the International Energy Agency (IEA) has provided a roadmap[1]for achieving net-zero emissions by 2050,stating that about 530 Mt∙a-1of global hydrogen is needed. This is about six times the hydrogen demand in 2020(about 90 Mt∙a-1).
Nuclear energy can provide a stable power supply without using fossil resources. It can also complement fluctuations in renewable energy output and produce hydrogen that can be used for various purposes.It is becoming increasingly important to convert primary energies into chemical energy in hydrogen.This paper describes the roles that nuclear energy, as a primary energy, can play in hydrogen production from the basis of energy-form conversions.
2. Hydrogen production methods using nuclear energy
Fig. 1 provides a diagram of the energy-form conversions involved in hydrogen production using nuclear energy. As a secondary energy source, hydrogen can be produced by adding nuclear energy (primary energy) to water or hydrocarbons (fossil resources) as raw materials. That is, this process converts nuclear energy into the chemical energy of hydrogen.Nuclear reactors that generate thermal energy include the following reactor types, with the available temperature of the thermal energy of each reactor ordered from lowest to highest: light-water reactors (coolant:water); fast breeder reactors (coolant: sodium); and hightemperature gas-cooled reactors (coolant: helium).
The direct heat energy or the electrical energy converted by power generation is supplied to energy form conversion methods in order to convert raw materials into hydrogen. The generated hydrogen can be stored,and the hydrogen delivered to consumers is used in a wide range of applications (as fuel, a chemical raw material, a reducing agent, etc.).
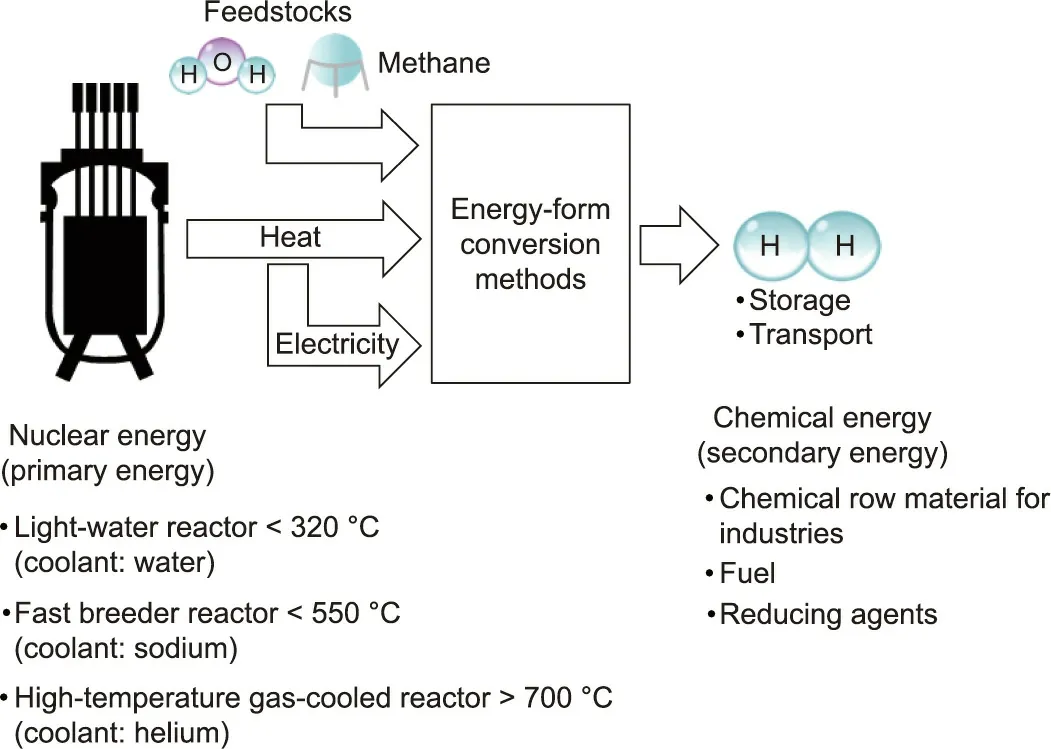
Fig. 1. Energy-form conversions of hydrogen production using nuclear energy.
Fig. 2 summarizes the hydrogen production methods, required raw materials,and required driving energy forms that can harness nuclear energy.
The top two hydrogen production methods shown in Fig. 2 involve the electrolysis of water.The low-temperature electrolysis of liquid water can be done via alkaline water-electrolysis or the use of a polymer electrolyte membrane (PEM), which uses electrical energy.The other method is high-temperature steam electrolysis, which uses heat and electricity.
The two methods shown in the middle of Fig.2 involve thermochemical cycles. Thermochemical water-splitting produces hydrogen by combining exothermic chemical reactions in a lowtemperature region and endothermic chemical reactions in a high-temperature region. Hybrid thermochemical water-splitting uses electricity for some part of the whole chemical reactions comprising the cycle.
The lower two hydrogen production methods shown in Fig. 2 involve endothermic chemical reactions that use hydrocarbons as raw materials.By supplementing the required heat of the chemical reactions with nuclear power,fossil resources consumption can be reduced in hydrogen production.The steam reforming method that produces hydrogen from hydrocarbons and water is a mature industrial technology,and the pyrolysis of methane,which is under development, converts methane into hydrogen and solid carbon.
3. Electrolysis of water
Hydrogen can be obtained by decomposing water.The chemical equations for water splitting are shown below:
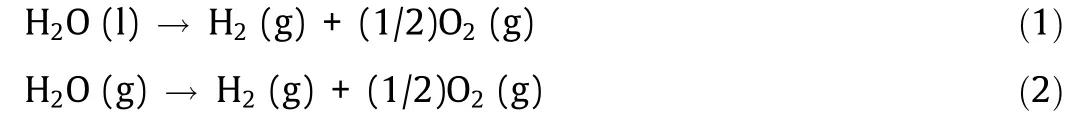
where ‘‘l” denotes the liquid phase, and ‘‘g” denotes the gaseous phase.Fig.3(a)shows a ΔH–T and ΔG–T diagram of water decomposition reactions,where T is the reaction temperature and ΔH and ΔG are the enthalpy difference and free energy difference between the reactants and the products,respectively(thermochemical data of liquids and gases [2]).
In order to decompose liquid water to obtain gaseous hydrogen(1 mol)and oxygen(0.5 mol),a total energy(286 kJ)corresponding to the energy of (i) + (ii) + (iii), as marked in Fig. 3(a), is required.Free energy (237 kJ) corresponding to at least the energy of (iii)must be added as electrical energy.In low-temperature water electrolysis, all of the energy, (i) + (ii) + (iii), is provided by electrical energy.
On the other hand, in high-temperature steam electrolysis, the latent heat of vaporization of water (i)can be supplied by thermal energy so that the required energy is reduced accordingly.In principle, the energy of ΔH (ii) can be supplied from the outside with thermal energy; in practice, a mainstream method is to convert electricity into Joule heat (while tolerating this exergy loss) by energizing electrolytic cells, which is called the thermal neutral condition [3].
Since the low-temperature water electrolysis method can be driven by electric energy alone, a light-water reactor, fast breeder reactor, or high-temperature gas-cooled reactor can be used as an energy source. The heat of vaporization of water (i) required for high-temperature steam electrolysis can also be supplied by a light-water reactor, fast breeder reactor, or high-temperature gas breeder reactor.
Fig.3(b)shows an energy conversion diagram[4]that converts nuclear thermal energy into hydrogen using high-temperature steam electrolysis. The energy conversion diagram compares the amount of enthalpy and amount of exergy before and after energy conversion using the exergy ratio as an index (vertical axis).The exergy ratio of heat at a certain temperature indicates the percentage of potential work (relative to enthalpy) available as the temperature drops to the ambient temperature (25 °C). In principle, the hydrogen production efficiency becomes more advantageous as the efficiency of the electricity generaion increases, resulting in a higher exergy ratio; that is, the reactor temperature can be ordered as follows: light-water reactor < fast breeder reactor < high-temperature gas-cooled reactor.
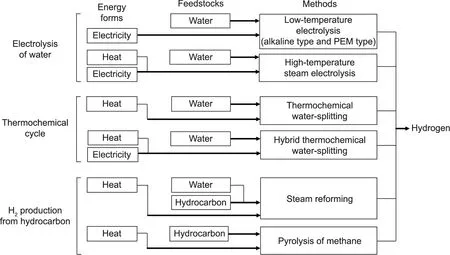
Fig. 2. Hydrogen production methods using nuclear energy. PEM: polymer electrolyte membrane.
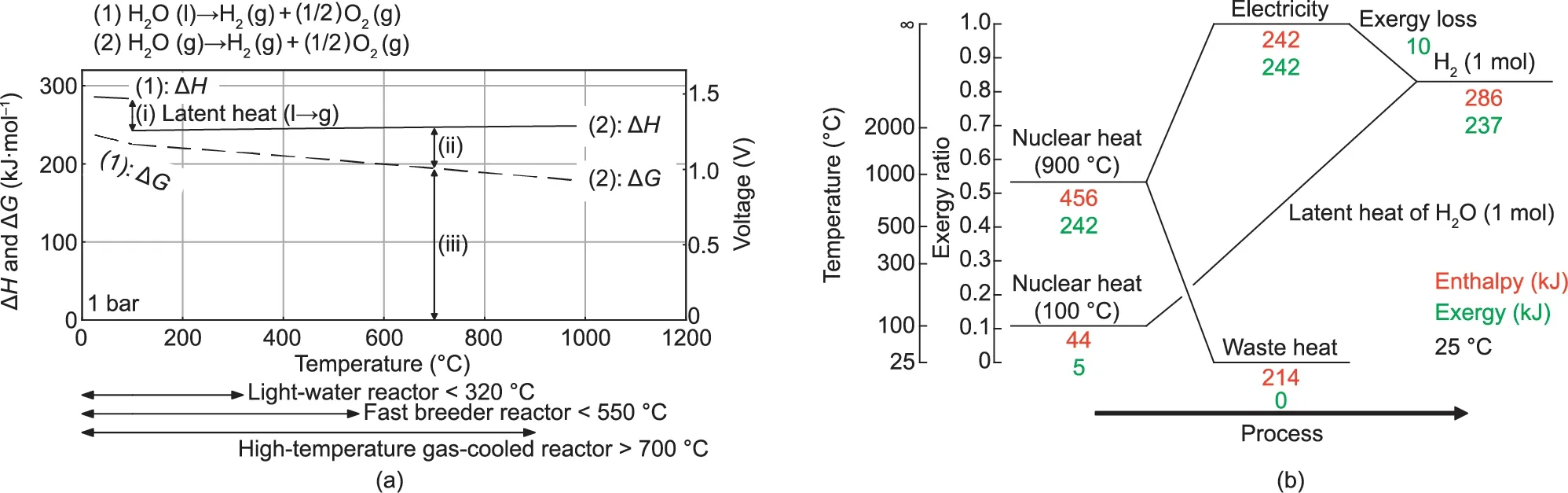
Fig. 3. (a)ΔH–T and ΔG–T diagram of water decomposition reactions; (b) energy conversion diagram of high-temperature steam electrolysis.
Fig. 3 illustrates the production of 1 mol of hydrogen using a heat at the temperature of 900 °C (as an example) obtained using a high-temperature gas-cooled reactor. The exergy ratio of heat at 900°C is 0.53.Therefore,in principle,242 kJ of electrical energy is obtained from the heat with an enthalpy of 456 kJ, and 214 kJ must be discharged to a low-temperature environment as exhaust heat. In addition, 1 mol of water vapor can be obtained from the heat with an enthalpy of 44 kJ at 100 °C. Since a standard exergy ratio of hydrogen is 0.83, the conversion of electrical energy and water vapor yields hydrogen with an enthalpy of 286 kJ and an exergy of 237 kJ (an exergy loss of 10 kJ).
In this way, the high-temperature steam electrolysis method,which starts from nuclear thermal energy and converts it into hydrogen energy, can be understood as follows: In principle,1 mol of hydrogen can be obtained from 456 kJ of heat (900 °C)and 44 kJ of heat (100 °C). Since nuclear thermal energy with an exergy ratio of about 0.5(900°C)is converted to hydrogen energy,which has a higher exergy ratio of about 0.8, the process must involve exhaust heat of nearly half of the thermal energy. Like a heat pump for improving the quality of energy, low-temperature thermal energy can be effectively utilized by transforming the nuclear thermal energy, which has an exergy ratio of about 0.1(100 °C) into hydrogen energy, which has a high exergy ratio.
4. Thermochemical cycles
Direct thermal decomposition of water requires a high temperature of several thousand degrees. Thermochemical cycles are methods of thermally decomposing water at a more practical temperature level of 1000°C or lower by combining various chemical reactions. As examples of thermochemical cycles, the sulfur family cycles of the iodine–sulfur (IS) process (also known as the SI process) and a hybrid sulfur process are described below.
The IS process consists of the following three chemical reactions:
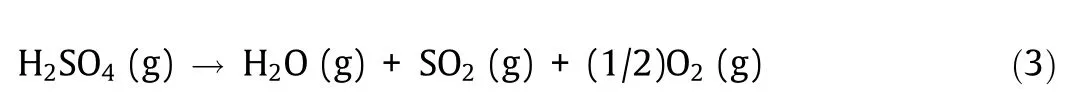
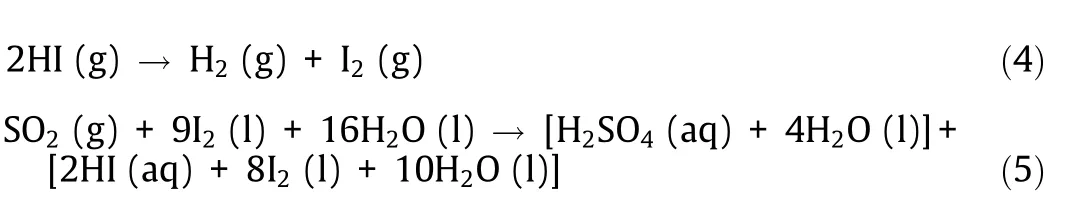
where ‘‘aq” denotes an aqueous solution. Reaction (3) is a sulfuric acid (H2SO4) decomposition reaction that thermally decomposes H2SO4in the gas phase to generate oxygen, and reaction (4) is a hydrogen iodide (HI) decomposition reaction that thermally decomposes HI in the gas phase to produce hydrogen. Reaction(5) is called the Bunsen reaction and is a liquid phase reaction in which water, sulfur dioxide, and iodine are reacted to produce H2SO4and HI. The H2SO4and HI generated in the Bunsen reaction can be separated into an upper liquid phase and a lower liquid phase by the liquid–liquid phase separation phenomenon.
Fig. 4(a) shows a ΔH–T and a ΔG–T diagram of the chemical reactions constituting the IS process (thermochemical data of liquids and gases [2], the dilution enthalpy, and the hydration entropy(infinite dilution)of H2SO4and HI[5]). The H2SO4decomposition reaction (3) is operated at above 600 °C where ΔG is negative, accompanied by large endothermic heat. The HI decomposition reaction (4) is a slightly endothermic reaction and is operated at about 500 °C. Since ΔG is small but positive, the reaction is biased toward the raw material. Therefore, the application of a membrane reactor that extracts hydrogen gas as a product from the reaction field by means of a hydrogen separation membrane is being studied to improve this reaction. The Bunsen reaction (5) is operated at below 100 °C where ΔG is negative; a large quantity of exothermic heat is generated.
As described above, thermochemical cycles can be driven by thermal energy alone by operating the chemical reactions in temperature ranges in which the free energy change becomes negative.Thus, the process works as a heat engine that absorbs high-temperature heat and exhausts low-temperature heat to produce the work required for water splitting.
The hybrid sulfur process (also known as the Westinghouse process) is a method in which reactions (4) and (5) are replaced with the following reaction (6).
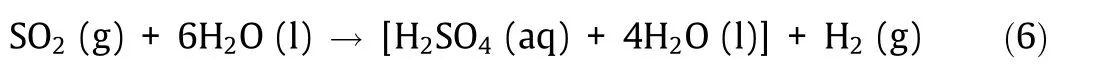
Reaction(6)is a liquid-phase electrochemical reaction to obtain hydrogen and H2SO4by means of the electrolytic oxidation of sulfurous acid.This use of electrical energy in part simplifies the num-ber of chemical reactions to two.The reaction is operated at 140°C[6] or lower, and the required voltage is about 0.37 V (25 °C), as shown in Fig. 4(a), which has the advantage of being smaller than the 1.48 V required for water electrolysis.
Since the IS process and the hybrid sulfur process require a high-temperature reaction field of above 600 °C to drive the H2SO4decomposition reaction (in practice, it should be around 850 °C to obtain a high conversion ratio), the high-temperature gas-cooled reactor is suitable for use as the heat source.
Fig. 4(b) shows an energy conversion diagram that converts nuclear thermal energy into hydrogen energy using thermochemical cycles. In principle, 1 mol of hydrogen can be obtained from 447 kJ of heat (900 °C). By exhausting nearly half of the nuclear thermal energy with an exergy ratio of about 0.5 (900 °C), the nuclear thermal energy can be converted into hydrogen energy with a high exergy ratio of about 0.8.
5. Hydrogen production from methane
Methane is an abundant fossil resource, with 50 years(2 × 1014m3) of proved reserves and 200 years (8 × 1014m3) of possible reserves [7]. The steam reforming method for methane is a process that produces hydrogen (and CO2) by reacting hydrocarbon fuels such as natural gas(i.e.,methane)with steam at high temperatures(800°C).This method is an industrially mature technology, with natural gas (methane) reforming accounting for 48%of global total hydrogen production and naphtha steam reforming accounting for 30% of the total.
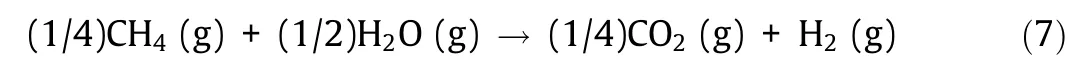
The reaction formula of the steam reforming method is shown below.Methane is one of the most stable organic molecules due to its strong C–H bonds.Research and development of the direct pyrolysis of methane is under way as a technology with the potential to produce hydrogen by forming solid carbon that does not diffuse into the atmosphere. The reaction formula for the pyrolysis of methane is shown below.
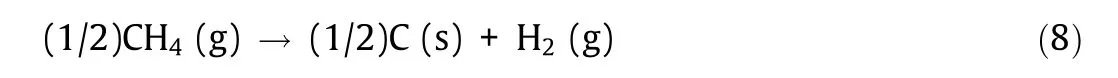
where ‘‘s” denotes the solid phase. Instead of consuming fossil fuels to obtain high temperature and reaction heat,methane usage can be reduced by supplementing the heat with nuclear energy.
Fig.5(a)shows a ΔH–T and ΔG–T diagram of the chemical reactions of steam reforming and the pyrolysis of methane (thermochemical data of solids and gases [2]). Both reactions are endothermic, and ΔG becomes negative above 600 °C, which is advantageous for the progress of the reaction.Thus,the high-temperature gas-cooled reactor is suitable as a heat source.
Fig. 5(b) shows an energy conversion diagram that converts methane into hydrogen using nuclear thermal energy. The exergy ratio of methane is about 0.9. By adding nuclear thermal energy with the exergy ratio of about 0.5 to methane, hydrogen with an enthalpy of 286 kJ and an exergy of 237 kJ can be obtained.
Thus, the process of converting methane into hydrogen using nuclear thermal energy can be understood as follows: If nuclear energy does not contribute,1.28 times(including the fuel that supplies the reaction heat)the amount of methane will be required;in principle,the use of nuclear energy can economizes this amount of methane. By adding nuclear thermal energy with the exergy ratio of about 0.5 (900 °C) to the endothermic reaction using methane,low-quality thermal energy is pumped up to hydrogen levels with a high exergy ratio like a heat pump,thereby improving the quality of that energy. In this conversion, since exhaust heat is not generated in principle, nuclear thermal energy can be effectively utilized.
6. Advantages of hydrogen production using nuclear energy
Hydrogen has the value of being applicable to industrial applications (as a fuel, a chemical raw material, a reducing agent, etc.)that cannot be covered by electrification.This paper makes the following statements based on energy-form conversion: Nuclear energy can supply the primary energy of heat and/or electricity required for hydrogen production; it can provide the temperature levels required for the chemical reactions used in hydrogen production. Hydrogen production methods that can harness nuclear energy include electrolysis,thermochemical cycles,and production methods from hydrocarbons. The temperature level that can be supplied depends on the reactor type,so each type of reactor must be combined with the appropriate hydrogen production method.The light-water reactor,fast breeder reactor,and high-temperature gas-cooled reactor can all supply energy for electrolysis.Due to the limited temperature range at which the relevant chemical reactions proceed, the high-temperature gas-cooled reactor is suitableas a heat source for thermochemical cycles (the sulfur family)and for methane reforming and pyrolysis. Furthermore, the heat quality of nuclear power can be increased to the hydrogen level,as hydrogen has a high exergy ratio. In this way, nuclear energy can contribute to the substitution of fossil resources by playing a role in hydrogen production processes.
- Engineering的其它文章
- Editorial for the Special Issue on High-End Measuring Instruments
- First Pig-to-Live Human Xenotransplant Produces Mixed Results
- Climate Change Action Alights on Satellite Detection of Methane
- Water Security: Why We Need Global Solutions
- The First Stage of the Middle-Line South-to-North Water-Transfer Project
- Fluorescence Nanoscopy in Neuroscience

