吡啶二羧酸修饰的稀土嵌入碲钨酸盐的合成及电化学生物识别性质
蒋 君,宫田田,张成鹏,刘晓倩,赵俊伟
(河南大学化学化工学院,河南省多酸化学重点实验室,开封 475004)
1 Introduction
Polyoxometalates(POMs)are a remarkable class of distinctive structure-featuring nanosized anionic clusters constructed from early transition-metal-oxo{MOm}(M=VV,NbV,TaV,MoVI,WVI;m=3—6)polyhedra aggregated together by sharing corners,edges,or faces,which have attracted a great deal of attention because of their structural versatility,specific electronic properties as well as potential applications in catalysis,magnetism,electrochemistry,and medicine,etc.[1—5].As an important research branch of POM chemistry,heteropolyoxotungstates(HPOTs)have displayed definite structural stability and wide-ranging application potentials,and sufficient availability in the construction of diversiform lacunary building blocks[6].These highly lacunary HPOM building blocks with more reactive sites as multidentate inorganic ligands can further connect with transition-metal(TM)or rare-earth(RE)ions to prepare complicated and captivating TM-or RE-incorporated HPOTs[7—10].
Tellurotungstates(TTs)as a representative category of HPOTs are an emerging branch of POMs and have been greatly developed in the past decade.Unlike other HPOTs,the characteristics of TTs can be summarized as follows[11—30].Two types of valence states of Te(TeIVand TeVI)atoms can work as heteroatoms to construct TTs.TeVIO66-acts as an octahedral heteroanion template on account of TeVIwithout lone-pair electron,and the formed TTs are mainly based on Dawson-like or Anderson-type building blocks,most of which are constituted by saturated TT fragments[11,12].While TeIVO23-serves as a trigonal pyramidal heteroanion template,and the lone-pair electron effect on TeIVefficaciously prevents the formation of plenary Keggin units and guides to generate a diverse range of multilacunary TT building blocks.As a result,TT building blocks can manifest a great structural diversity in the reaction.On one hand,although Keggin-type fragments in TTs generally are trivacant,other higher vacant segments can also be encountered,such as tetravacant[B-α-TeW8O31]10-and pentavacant[B-α-TeW7O28]12-.On the other hand,TT building blocks with the high Te/W ratio and TTs containing mixed building blocks(e.g.Keggin and Lindqvist)were also reported,which illustrate flexible combination modes among Te,O and W[13—30].These phenomena are rather rare in other HPOTs and also show that multifarious TeIV-templating TT building blocks can offer a great possibility for capturing much more TM or RE electrophiles into TT frameworks to synthesize TM-or RE-incorporated TTs with fabulous structures.The early researches are mostly focused on“pure”TTs and TM-incorporated TTs(TMITTs)[13—23].However,although many TMITTs have been synthesized,their structural types are very few and are focused on classical Krebs-type derivatives[16—23].Compared with TM ions,RE ions display obvious advantages.For one thing,RE ions possess higher coordination number and abundant coordination modes,which can be combined with TT building blocks to give rise to complex and diversiform structures.For the other thing,special electronic structures of RE ions make them own peculiar fluorescent properties,which play an indispensable role in the optical field.Therefore,preparing intriguing RE-incorporated TTs(REITTs)and exploring their interesting properties have become a new research direction[24—26].For instance,Su’s group[24]reported the first REITT K32Na16[{(TeO3)W10O34}8{Ce8(H2O)20}(WO2)4(W4O12)]·114H2O in 2013,which is an octameric polyanion and can self-assembly into inorganic hollow spheres in dilute solution.Until now,only less REITTs have been reported probably because the rapidly reaction of oxophilicity of RE ions with TT segments form amorphous precipitates,which makes it difficult to determine their structures[27—29].Furthermore,it also remains a great challenge for synthesizing organic-inorganic hybrid REITTs.
Enlightened by previous results,we performed systematic explorations to discover original organicinorganic hybrid REITTs.Firstly,rigid dicarboxylic pyridine ligands come into our view since coordination ability possibly increases with augment of coordination sites,which could more easily react with metal ions in the process of fabricating REITTs.Secondly,the introduction of larger-sized organic amines under acidic conditions is helpful to the formation of target products.Organic amines can not only serve as the solubilizers of RE ions but also function as counter cations to balance charges of polyanions.Thirdly,microwave heating can facilitate to the reaction owing to the heating mechanism of molecular collision.On the basis of above thoughts,a series of 2,6-pyridine dicarboxylic acid decorated REITTs,(PTEA)7H3K[RE2(B-α-TeW9O33)3W3O6(H2O)2(HDPA)]·22H2O[RE=Ce3+(1),Pr3+(2),Nd3+(3),Sm3+(4);H2DPA=2,6-pyridinedicarboxylic acid,PTEA=protonated triethanolamine],was synthesizedviaone-step self-assembly synthetic strategy by microwave heating method in aqueous solution.Their polyanions can be identified as a{RE2W3O5(H2O)3·(HDPA)}13+heterometal cluster surrounded by three[B-α-TeW9O33]8-building blocks.In the central heterometal cluster,HDPA acted as a quadridentate ligand coordinates with two RE ions(RE1 and RE2)to form a planar“anthracene-like”tri-heterocycle structure,which is perpendicular to the isosceles triangle constructed by W2,W2A and W16 centers and passes through the bisector of the top angle.So far,with the development of POM-based materials,water-soluble POMs can often be composited with other materials to prepare waterinsoluble materials and explore their excellent properties[30—32].Consequently,compound 1 was loaded on carboxylic multi-walled carbon nanotube(CMWCNT)to produce the 1-CMWCNT composite,which then served as the electrode material to fabricate electrochemical biosensor(ECBS).With the help of capture probe DNA,together with Au nanoparticles(Au NPs)as signal amplifiers and methylene blue(MB)as an indicator,this ECBS successfully realized to detect sequence-specific DNA(target DNA).
2 Experimental
2.1 Syntheses of Compounds 1—4
2.1.1 Synthesis of(PTEA)7H3K[Ce2(B-α-TeW9O33)3W3O5(H2O)3(HDPA)]·22H2O(1)K2TeO3(0.201 g,0.792 mmol),Na2WO4·2H2O(1.502 g,4.554 mmol),H2DPA(0.100 g,0.598 mmol)and NaAc·3H2O(1.00 g,7.349 mmol)were dissolved in 15 mL of distilled water under stirring and 2 mL of triethylamine(TEA)was dropwise added into the solution.Then the pH value of the solution was adjusted to 3.5 with 6 mol/L HCl aqueous solution followed by the addition of Ce(NO3)3·6H2O(0.300 g,0.691 mmol)in the system.Then the pH value was adjusted to 3.5 again by 4 mol/Land 0.5 mol/L NaOH.The obtained turbid solution was directly heated until the system was clear(ca.10 min)and kept heating for another 5 min accompanied with stirring.The clear solution was immediately put into a 70°C microwave oven for 40 min and then cooled to room temperature and filtrated,slow evaporation of the filtrate at room temperature led to orange rodlike crystals of compound 1 in about one month.Yield:22.76%(0.33 g)based on Na2WO4·2H2O.Elemental analysis(%)calcd.:C 6.16,H 1.78,N 1.17,W 57.74,Te 4.01,Ce 2.93,K 0.41;found:C 6.46,H 1.62,N 1.13,W 57.96,Te 3.77,Ce 3.15,K 0.55.
2.1.2 Synthesis of(PTEA)7H3K[Pr2(B-α-TeW9O33)3W3O5(H2O)3(HDPA)]·22H2O(2) The synthetic process of compound 2 imitated compound 1 except that Pr(NO3)3·6H2O(0.301 g,0.691 mmol)took the place of Ce(NO3)3·6H2O(0.300 g,0.691 mmol).Green rodlike crystals of compound 2 were obtained.Yield:22.07%(0.32 g)based on Na2WO4·2H2O.Elemental analysis(%)calcd.:C 6.16,H 1.78,N 1.17,W 57.73,Te 4.01,Pr 2.95,K 0.41;found:C 6.45,H 1.52,N 1.12,W 58.05,Te 3.88,Pr 3.27,K 0.51.
2.1.3 Synthesis of(PTEA)7H3K[Nd2(B-α-TeW9O33)3W3O5(H2O)3(HDPA)]·22H2O(3) The synthetic procedure of compound 3 is similar to compound 1 except that Ce(NO3)3·6H2O(0.300 g,0.691 mmol)was replaced by Nd(NO3)3·6H2O(0.300 g,0.691 mmol).Green rodlike crystals of compound 3 were obtained.Yield:25.50%(0.37 g)based on Na2WO4·2H2O.Elemental analysis(%)calcd.:C 6.16,H 1.78,N 1.17,W 57.69,Te 4.00,Nd 3.02,K 0.41;found:C 6.43,H 1.60,N 1.11,W 57.83,Te 4.16,Nd 3.18,K 0.57.
2.1.4 Synthesis of(PTEA)7H3K[Sm2(B-α-TeW9O33)3W3O5(H2O)3(HDPA)]·22H2O(4) The synthetic course of compound 4 is analogous to compound 1 except that Sm(NO3)3·6H2O(0.302 g,0.691 mmol)replaced Ce(NO3)3·6H2O(0.300 g,0.691 mmol).Green rodlike crystals of compound 4 were obtained.Yield:20.69%(0.30 g)based on Na2WO4·2H2O.Elemental analysis(%)calcd.:C 6.15,H 1.78,N 1.17,W 57.62,Te 4.00,Sm 3.14,K 0.41;found:C 6.39,H 1.53,N 1.13,W 57.89,Te 3.74,Sm 3.28,K 0.49.
2.2 Preparation of 1-CMWCNT Composite
1-CMWCNT composite was prepared as follows.After 30 mg of compound 1 was dissolved in 1 mL of ultrapure water,10 mg of CMWCNT was added to the solution.A homogeneous and stable suspension was obtained by dispersing ultrasonically for 6 h,which was then centrifuged at a rate of 12000 r/min for 20 min.The gathered precipitates were washed by ultrapure water for three times and then were dried at 60℃for 3 d.The 1-CMWCNT composite was obtained and used standby.
3 Results and Discussion
3.1 Structural Description
Compounds 1—4 are isomorphic discr ete structures and belong to the orthorhombic space groupPnma(Table S1,see the Supporting Information of this paper),all of them contain an unusual trimeric polyanion[RE2(B-α-TeW9O33)3W3O5(H2O)3(HDPA)]11-.As far as we are aware,compounds 1—4 are on behalf of the first class of H2DPA-substituted LITTs,and only compound 4 was selected as a representative to describe their structural feature.The holistic polyanion framework of compound 4[Fig.1(A)]could be viewed as three trivacant Keggin-type[B-α-TeW9O33]8-{TeW9}building blocks linked by a central heterometallic{RE2W3O5(H2O)3(HDPA)}13+cluster.The three{TeW9}units[Fig.1(B)]can be regarded as three vertexes of an isosceles triangle[Fig.1(C)].The distances of the three edges(Te…Te distances)equal to 0.7324 nm for the“waist”edges and 0.7047 nm for the bottom edge.Interestingly,the linking groups between each two{TeW9}units are just three{WOn}(n=5 or 6)groups in the central heterometallic{RE2W3O5(H2O)3(HDPA)}13+cluster[Fig.1(D)],which occupy the vertexes of another isosceles triangle[Fig.1(E)]with the{W16O5}group sitting on the vertex angle and{W2O6}and{W2AO6}groups residing in two bottom angles.The distance between W16 and W2 centers is 0.6265 nm and the distance between W2 and W2A centers is 0.4583 nm.Two above-mentioned isosceles triangles are reversely arrayed[Fig.1(F)].Upon further observing the linking modes of three{WOn}(n=5 or 6)groups,we can find that three{WOn}(n=5 or 6)groups are connected by two{SmOn}(n=8 or 9)groups by sharingμ2-O atoms(O4 and O4A atoms between W2,W2A and Sm1 centers,O33 and O33A atoms between W2,W2A and Sm2 centers as well as the O21 atom between W16 and Sm2 centers).More intriguingly,Sm13+and Sm23+ions are not mutually independent,which are bridged by an HDPA ligand.The HDPA ligand ion not only coordinates with the Sm23+ion through the two carboxylic O atoms(O19,O52)in the four-numbered ring motif[Fig.1(G)],but also is combined with the Sm13+ion through the N atom and two O atoms from two different carboxylic groups in two edge-sharing five-numbered ring mode[Fig.1(G)].The similar four-or five-numbered ring modes(M—O—O—C or M—N—C—C—O,M=metal ions)have been previously reported and the double edge-sharing five-numbered ring mode has also been discovered(Fig.S3,see the Supporting Information of this paper)[33—36],but such“anthracene”-like ring structure[Fig.1(G)]composed of two edge-sharing five-numbered rings and one four-numbered ring by sharing two edges(N1—Sm1 and C1—O19)is extremely infrequent in POM field.The scarce coordination mode results from the congenitally five adjoining coordination atoms of the HDPA ligand,which is convenient to chelate more than one metal centers to form stable multi-ring structure.Moreover,the“anthracene”-like ring structure(Sm1—O37—C7—C6—N1,Sm1—N1—C2—C1—O19 and O19—C1—O52—Sm2)is located at one plane which also passes through every atom of HDPA ligand[Fig.1(H)].This plane is perpendicular to the isosceles triangle defined by three W atoms(W16,W2 and W2A)in the central cluster[Fig.1(H)]and acts as the symmetry plane of the whole polyanion[Fig.1(D)].The crystallographically independent Sm13+and Sm23+ions adopt the nona-coordinate and octa-coordinate modes,respectively.The Sm13+ion exhibits a distorted mono-capped square antiprism geometry[Fig.1(I)]that is established by one N atom(N1)and two carboxylic O atoms(O19,O37)from the HDPA ligand,two O atoms(O24,O24A)from a{TeW9}unit,two O atoms(O4,O4A)of two{WO6}groups in the central cluster and another two O atoms(O1W,O1WA)of water ligands.In the mono-capped square antiprism of the Sm13+ion,two bottom planes are formed by O19,O1W,O37,O1WA group and O24A,O4A,O4,O243 group,respectively,while the cap position is occupied by N1 atom.The Sm1—L(L=N or O)bond lengths are in the range of 0.2429(10)—0.2523(16)nm and the L—Sm1—L bond angles are in the range of 62.7(7)°—143.8(5)°.Moreover,the Sm23+ion is coordinated by two carboxylic O atoms(O19,O52)from the HPDA ligand,two O atoms(O53,O53A)of two{TeW9}units,three O atoms(O33,O33A,O21)from two{WO6}groups({W2O6},{W2AO6})and the disordered{W16O5}group in the central cluster severally and one O atom(O2W)of water molecule and displays a bicapped triangular prism configuration[Fig.1(J)],in which two bottom planes are respectively occupied by O53A,O52,O19 group and O21,O57,O35 group while the two cap positions are situated by O2W and O33A,respectively.The Sm2—O distances range from 0.2347(15)to 0.2615(18)nm and the O—Sm—O bond angles range from 49.4(6)°to 155.5(5)°.Besides,a K+ion is incorporated in the cavity formed by three{TeW9}units,which is beneficial to stabilize the whole skeleton of the polyanion(Fig.S4,see the Supporting Information of this paper).
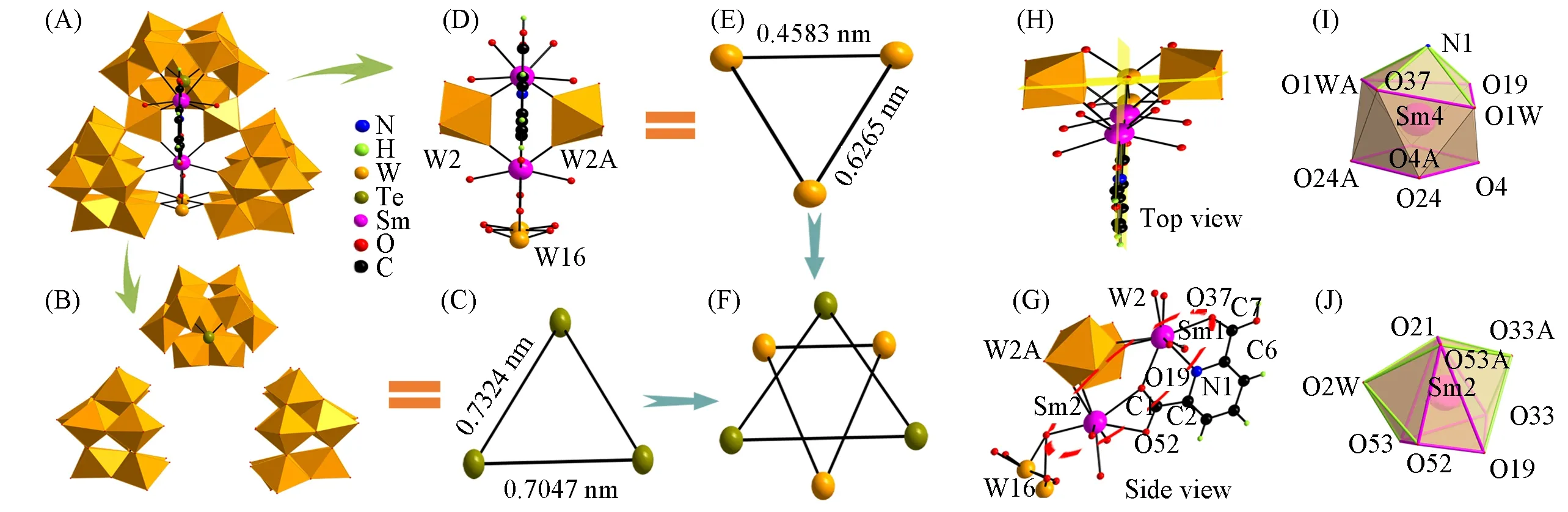
Fig.1 Trimeric[Sm2(B-α-TeW9O33)3W3O5(H 2O)3(HDPA)]11-polyanion(A),three{TeW9}building blocks(B),isosceles triangle formed by three{TeW9}units(C),top view of the{Sm2W3O5(H 2O)3(HDPA)}13+central cluster(D),isosceles triangle constructed by three{WOn}atoms in the central cluster(E),reversely arrayed fashion of two isosceles triangles(F),side view of the{Sm2W3O5(H 2O)3(HDPA)}13+central cluster(G),highlight of the“anthracene”-like ring plane being perpendicular to the isosceles triangle defined by three W atoms(W16,W2 and W2A)in the central cluster(H),coordination geometry of the Sm13+ion(I),coordination geometry of the Sm23+ion(J)
Additionally,the discrete[Sm2(B-α-TeW9O33)3W3O5(H2O)3(HDPA)]11-polyanions are regularly arranged to establish the 3D packing structure(Fig.2)through H-bonding interactions(N—H…O:0.281—0.316 nm or O—H…O:0.249—0.326 nm)between PTEA+cations and the surface oxygen atoms of polyanions or water molecules,electrostatic interactions between polyanions and counter cations as well as van der Waals interactions among molecules and ions.The packing of the polyanions of 4 trimly manifests the—ABAB—fashion in theabplane[Fig.2(A)].While it is obvious that the polyanions of 4 are not arranged in the same spatial orientation and their spatial orientations can be divided into four kinds(A,B,A',B')[Fig.2(B)].So,in the layer A and layer B,the polyanions of 4 are also stacked in a staggered array with—ABAB—and—A'B'A'B'—fashions[Fig.2(C)—(F)].Line A can be regarded as that Line B counterclockwise rotates 180°along thebaxis and the relation between Line A'and Line B'is the same as the former[Fig.2(B)].Although the polyanions of 4 exhibit four orientations,in every line they are regularly arranged as—AAA—fashion respectively.Adjacent lines could be stacked in a staggered array,which is the result of minimizing the steric hindrance.Then,the layers of polyanions of 4 are further stacked together for minimizing the steric hindrance[Fig.2(G)and(H)].
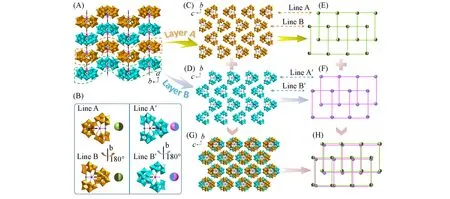
Fig.2 Packing architecture of the polyanions of 4 with a regular—ABAB—fashion in ab plane(A),four spatial orientations of the polyanions of 4(B),layer A in the bc plane(C),layer B in the bc plane(D),simplified layer A(E),simplified layer B(F),3D stacking of the polyanions of 4 in the bc plane(G),simplified 3D stacking of the polyanions of 4 in the bc plane(H)
3.2 Characterization of 1-CMWCNT and Au-1-CMWCNT Composites
Scanning electron microscopy(SEM)images of CMWCNT and the 1-CMWCNT composite reveal that the 1-CMWCNT composite exhibits a smooth and tubulose morphology,which is similar to CMWCNT[Fig.3(A)—(D)].After electrodepositing Au NPs on the 1-CMWCNT composite,SEM images of the Au-1-CMWCNT composite show that Au NPs evenly adhere to the surface of the 1-CMWCNT composite[Fig.3(E)and(F)],and the diameters of Au NPs are 10—20 nm.Compared with the CMWCNT composite[Fig.3(G)],the signals of W,Ce,Te and K elements are observed in energy dispersive spectroscopy(EDS)of the 1-CMWCNT composite[Fig.3(H)],illustrating that compound 1 has been loaded on CMWCNT.In EDS profile of the Au-1-CMWCNT composite,the signal of the Au element is observed[Fig.3(I)],which also exhibits that Au NPs have existed in the Au-1-CMWCNT composite.
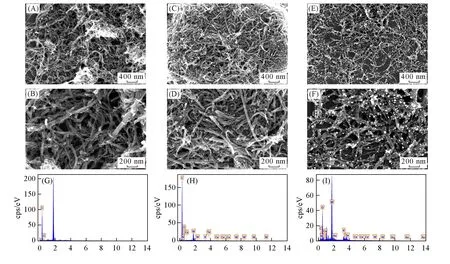
Fig.3 SEM images of CMWCNT(A,B),1-CMWCNT composite(C,D),Au-1-CMWCNT composite(E,F)and EDS of CMWCNT(G),1-CMWCNT composite(H)and Au-1-CMWCNT composite(I)
3.3 Sensing Principle
Recently,electrochemical DNA biosensors are flourishing and have aroused considerable concern owing to their intrinsic advantages,such as low cost,high stability,fast signal response,easy operation and high sensitivity[37—43].Until now,POM-based materials as electrode materials for establishing ECBSs are still extremely rare.In this article,the 1-CMWCNT composite was served as the electrode material to manufacture DNA ECBS to detect sequence-specific DNA.The sensing principle is listed in Fig.4[37].The 1-CMWCNT composite dropped on the electrode can not only promote electron transfer and increase the current signal,but also provide the substrate for electrodepositing Au NPs[Fig.4(A)and(B)].After electrodepositing Au NPs on the electrode surface[Fig.4(C)],capture probe(CP)is combined with Au NPs by Au—S bond interactions to form the CP-Au-1-CMWCNT GCE[Fig.4(D)].In addition,unreacted Au NPs with CP can influence experimental results,which should be blocked by 6-mercapto-1-hexanol(MCH)[Fig.4(E)].Then target DNA(TD)could be combined with CPviaspecific base pairing interactions to be immobilized on the electrode[Fig.4(F)].At last,MB interacts with guanine groups on CP and TD[Fig.4(G)],and so the TD concentration can be detected by monitoring the intensity of MB signal[Fig.4(H)].

Fig.4 Preparation of the 1-CMWCNT composite(A),the 1-CMWCNT composite was dropped on GCE(B),schematic of Au-1-CMWCNT GCE(C),schematic of CP-Au-1-CMWCNT GCE(D),MCH blocking nonspecific binding sites on CP-Au-1-CMWCNT GCE(E),schematic of TD-CP-Au-1-CMWCNT GCE(F),schematic of MB-TD-CP-Au-1-CMWCNT GCE(G)and detection curves of MB-TD-CP-Au-1-CMWCNT GCE(H)
3.4 Electrochemical Characterization
Cyclic voltammetry(CV)is an effective method for exploring electrochemical features of electrodes.In 5.0 mmol/L[Fe(CN)6]4-/[Fe(CN)6]3-solution,the higher peak current can illustrate the better electroconductibility of the modified electrode.As shown in Fig.5(A),the bare GCE exhibits a pair of well-defined redox peak of[Fe(CN)6]4-/[Fe(CN)6]3-with the peak separation(ΔEP)of 0.098 V(purple line),and the CMWCNT GCE shows higher peak current(orange line).Compared with CMWCNT GCE,the peak current of 1-CMWCNT GCE becomes stronger(green line),implying better electroconductibility of 1-CMWCNT because of the favorable redox activity of compound 1 as well as the fact that compound 1 promotes the dispersion of CMWCNT.Then,after electrodepositing Au NPs on 1-CMWCNT GCE,the peak current further increase(red line),indicating that Au NPs facilitate electron transfer.Next,after fixing CP on Au-1-CMWCNT GCE,the peak current of CP-Au-1-CMWCNT GCE decreases distinctly on account of combination of CP with Au NPs hindering electron transfer between solution and electrode(cyan line).With the addition of MCH and TD,the peak current of TD-CP-Au-1-CMWCNT GCE ulteriorly becomes weak(yellow line).
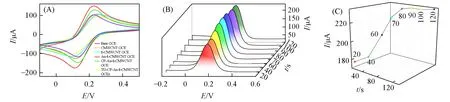
Fig.5 CV curves of different electrodes(A),DPV curves of Au-1-CMWCNT GCEs in different electrodeposition times(t=20,40,60,80,100,120 s)(B)and the plot of DPV peak current of Au-1-CMWCNT GCEs versus electrodeposition time(t=20,40,60,80,100,120 s)(C)
3.5 Optimization of Assay Conditions
For achieving the best detection effect,the optimization of assay conditions is indispensable.Firstly,Au NPs deposited on the electrode not only can act as signal amplifiers to facilitate electron transfer,but also can function as linkers to immobilize CP onto the electrode surface through strong Au—S bond interactions.The electroconductibility of Au-1-CMWCNT GCEs can be judged by DPV signal of Fe2+/Fe3+in[Fe(CN)6]4-/[Fe(CN)6]3-solution.As illustrated in Fig.5(B)and(C),the DPV peak current rises with varying electrodeposition time from 20 s to 80 s probably due to the increase of Au NPs amount.Continuing to prolong electrodeposition time,the DPV peak current almost remains unchanged after 80 s,which illustrates that the number of Au NPs reaches the maximum.So,the best electrodeposition time of 80 s is employed in the subsequent experiments.
Then,the reaction time of CP immobilized on Au-1-CMWCNT GCEs by Au—S bonds was explored.As illustrated in Fig.6(A)and(B),the DPV peak current decreases when increases reaction time from 0.5 to 2 h,because CP immobilized on the electrode could hinder electron transfer.The longer the reaction time goes on,the more CP are immobilized on the electrode,leading to the decline of the DPV peak current.When the reaction time is longer than 2 h,the DPV peak current no longer diminishes,which indicates the reaction between CP and Au NPs arrives at a plateau.Therefore,2 h is the suitable reaction time for constructing CP-Au-1-CMWCNT GCEs.
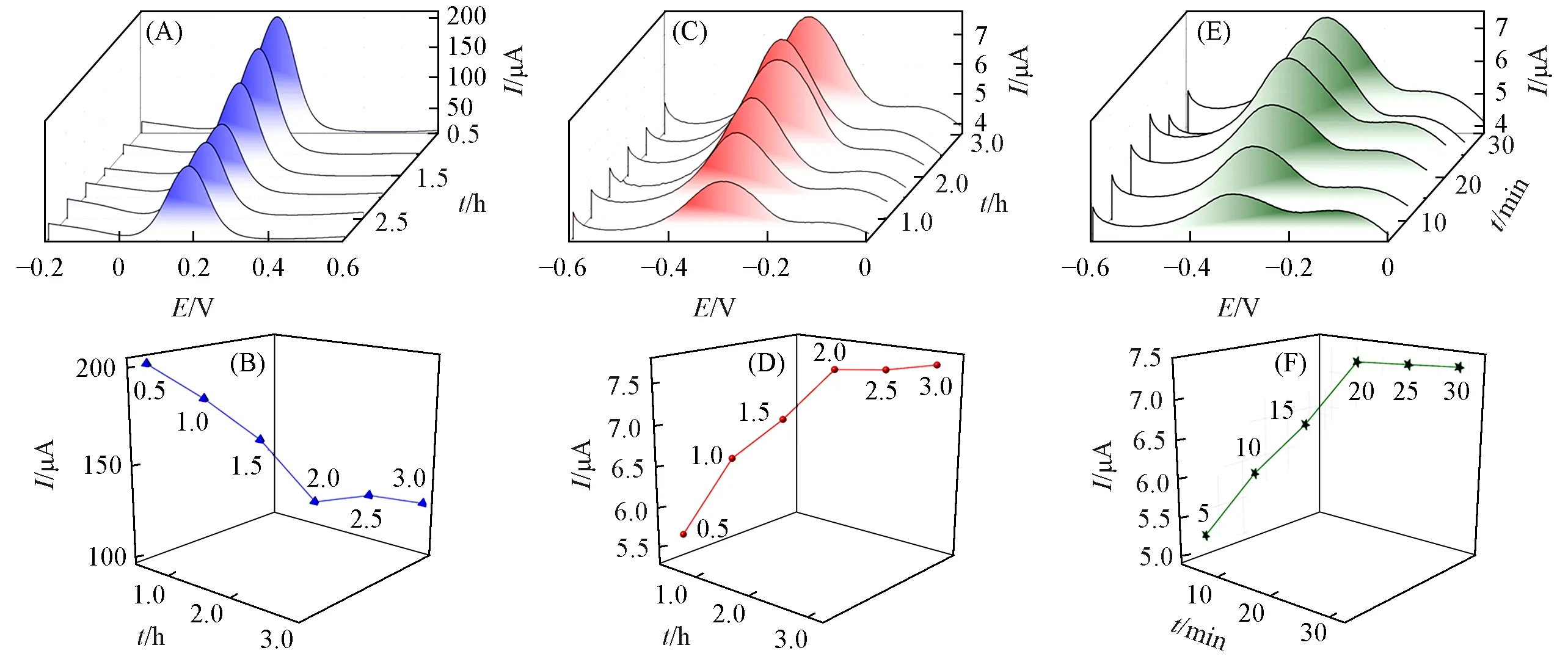
Fig.6 DPV curves of CP-Au-1-CMWCNT GCEs with different reaction times between CP and Au NPs(t=0.5,1,1.5,2,2.5,3 h)(A),plot of DPV peak current of CP-Au-1-CMWCNT GCEs versus reaction time between CP and Au NPs(t=0.5,1,1.5,2,2.5,3 h)(B),DPV curves of MB-TD-CP-Au-1-CMWCNT GCEs with different hybridization times between CP and TD(t=0.5,1,1.5,2,2.5,3 h)(C),plot of DPV peak current of MB-TD-CP-Au-1-CMWCNT GCEs versus hybridization time between CP and TD(t=0.5,1,1.5,2,2.5,3 h)(D),DPV curves of MB-TD-CP-Au-1-CMWCNT GCEs with different incubation times in MB solution(t=5,10,15,20,25,30 min)(E),and plot of DPV peak current of MB-TD-CP-Au-1-CMWCNT GCEs versus incubation time in MB solution(t=5,10,15,20,25,30 min)(F)
The electroconductibility of CP-Au-1-CMWCNT GCEs is so poor that the signal of[Fe(CN)6]4-/Fe(CN)6]3-could not be used to optimize hybridization time between CP and TD.While MB has been proved to be as an electrochemical indicator in DNA hybridization detection[42,43],and DPV peak current signal of MB on GCEs can be used to optimize hybridization time and detect TD.As shown in Fig.6(C)and(D),with prolongation of hybridization time from 0.5 h to 2 h,the DPV peak current of MB-TD-CP-Au-1-CMWCNT GCEs gradually rises.When the hybridization time is longer than 2 h,the DPV peak current no longer increases,demonstrating that the amount of TD arrives at the saturation by complementary base pairing with CP.Thus,hybridization time of 2 h is the optimum time for the establishment of MB-TD-CP-Au-1-CMWCNT GCEs.
Finally,the incubation time of MB was also investigated.The result was provided in Fig.6(E)and(F).With the incubation time of MB increasing from 5 min to 20 min,the DPV peak current of MB-TD-CP-Au-1-CMWCNT GCE gradually increases.Then the peak current reaches a platform after 20 min,which indicates the majority of MB has been immobilized on the electrode.So,the incubation time of MB is set as 20 min in the following experiments.
3.6 Detection of TD
Under the optimum conditions,the electrochemical performances of MB-TD-CP-Au-1-CMWCNT GCEs for detecting TD were evaluated.As shown in Fig.7,in the TD concentration range of 10-14—10-7mol/L,DPV peak current gradually increases as increasing the TD concentration and DPV peak current is remarkably linearly correlated with the TD concentration,which can be fitted byI=0.2461lgc+9.962 with a regression coefficientR2=0.988.The limit of detection(LOD)of 8.85×10-15can be calculated by triple standard deviation of the blank sample divided by the slope(LOD=3s/k).

Fig.7 DPV curves of MB-TD-CP-Au-1-CMWCNT GCEs with TD concentrations of 10-14—10-7 mol/L(A)and plot of the DPV peak current of MB-TD-CP-Au-1-CMWCNT GCEs versus the logarithm of the TD concentration in the range of 10-14—10-7 mol/L(B)
3.7 Selectivity,Reproducibility and Stability
The selectivity of MB-TD-CP-Au-1-CMWCNT GCE was also assessed.1MT,3MT and NC were selected as referencing ssDNAs.As shown in Fig.8(A),the DPV peak currents of MB-1MT-CP-Au-1-CMWCNT GCE,MB-3MT-CP-Au-1-CMWCNT GCE and MB-NC-CP-Au-1-CMWCNT GCE are significantly lower than that of MB-TD-CP-Au-1-CMWCNT GCE,because these referencing ssDNA chains can’t hybridize with CP,leading to the very weak DPV signal response.Thus,MB-TD-CP-Au-1-CMWCNT GCE exhibits an outstanding selectivity for sensing TD.
Reproducibility is another important index.To justify the good reproducibility,six independent MB-TDCP-Au-1-CMWCNT GCEs were established to detect TD.It can be seen from Fig.8(B)that their DPV peak currents are almost the same,indicating that MB-TD-CP-Au-1-CMWCNT GCEs have a good reproducibility.

Fig.8 Comparison of DPV peak currents of MB-TD-CP-Au-1-CMWCNT GCE(a),MB-1MT-CP-Au-1-CMWCNT GCE(b),MB-3MT-CP-Au-1-CMWCNT GCE(c)and MB-NC-CP-Au-1-CMWCNT GCE(d)(A),comparison of DPV peak currents of six different MB-TD-CP-Au-1-CMWCNT GCEs for detecting 10-8 mol/L TD(B)and comparison of DPV peak currents of MB-TD-CP-Au-1-CMWCNT GCEs established immediately(a)and after 7 d(b—f)(C)
To evaluate the stability,five 1-CMWCNT GCEs were placed at room temperature for 7 d.Seven days later,these 1-CMWCNT GCEs were utilized for manufacturing MB-TD-CP-Au-1-CMWCNT GCEs to detect TD[Fig.8(C)].All DPV peak currents show a slight change in comparison with that established immediately,which reveal that the 1-CMWCNT GCEs possess an acceptable stability.
4 Conclusions
A series of 2,6-pyridinedicarboxylic acid functionalized REITTs(1—4)was obtainedviathe one-step self-assembly synthetic strategy by microwave heating method.Their polyanions consist of three trivacant{TeW9}building blocks together with a specific{RE2W3O5(H2O)3(HDPA)}13+heterometal cluster.In this heterometal cluster,one HDPA coordinates with two RE ions to generate the tri-heterocycle structure,which is infrequent in POM chemistry.Moreover,compound 1 loaded on CMWCNT can generate the 1-CMWCNT composite,which was then used as electrode material to establish electrochemical biosensor for detecting sequence-specific DNA.This work not only enriches organic-inorganic hybrid REITTs,but also expands the application of POM-based materials in electrochemical sensing field for detecting ssDNA.
The supporting information of this paper see http://www.cjcu.jlu.edu.cn/CN/10.7503/cjcu20210561.

