一种多孔配位聚合物的氢键协同客体响应
莫宗文,张学文,周浩龙,周东东,张杰鹏
(1.中山大学化学学院,生物无机与合成化学教育部重点实验室,广州 510275;2.五邑大学生物科技与大健康学院,江门 529000)
1 Introduction
Porous coordination polymers(PCPs),or metal-organic frameworks(MOFs),have attracted great interest for their large structural diversity and many potential applications[1—3].Benefiting from their long-range ordered structures,the locations and interactions of guest molecules can be straightforwardly visualized at the atomic level by diffraction techniques especially using single-crystalline samples,which is critical for understanding the host-guest chemistry[4—6].Nevertheless,retention of single-crystallinity after guest adsorption/desorption is usually difficult,particularly for those involving large structural transformations[7].
Consisting of many reversible and movable components,PCPs can undergo various types of structural transformations,such as shrinkage,expansion,distortion,sliding,ligand exchange,catenation rearrangement,etc[8—11].While many chemical and physical stimuli can induce the structural transformations of PCPs,most examples arise from adsorption/desorption of guest[12,13].Therefore,the interaction between the guest molecule and the host framework plays an important role.As compared with the structure of the host framework,the position and orientation of guests,especially gas molecules,are much more difficult to determine[4,14,15].Counter ions can also be considered as a special type of guests,since they can be exchanged rather than adsorbed/desorbed[16—19].Nevertheless,the role of counter ions on host-guest interactions and/or structural transformations have been rarely studied[20,21].
In this work,we synthesized a new flexible PCP consisting of synergetic hydrogen-bonding networks among the anionic pillared-ribbon 3-dimensional(3D)host framework,Me2NH2+counter cations,and neutral guest molecules.This PCP exhibited significant structural responses to the desorption/adsorption of neutral guest molecules in terms of the unit-cell volume,porosity and symmetry.Single-crystal X-ray diffraction analyses showed the vital roles of the reconstitution of hydrogen-bonding network and the movement/rotation of Me2NH2+counter cations.
2 Experimental
2.1 Reagents and Instruments
All reagents and solvents were commercially purchased and used without further purification unless otherwise noted.Thermogravimetry analysis was carried out on a TA-Q50 system under N2flow with a heating rate of 10℃/min.Power X-ray diffraction(PXRD)data were collected using a Bruker D8 advance X-ray powder diffractometer(CuKα)at room temperature with a scanning speed of 0.02°/step and 0.2 s/step.Gas sorption isotherms were measured with a Micromeritics ASAP 2020M instrument.Before the sorption experiment,the as-synthesized sample was activated under high vacuum at 170℃for 6 h.Infrared(IR)spectrum was obtained from KBr pellet using a Bruker Tensor 27 FTIR spectrometer in the 400—4000 cm-1region.
2.2 Synthesis of(Me2NH 2)[Zn2(pzdc)(abdc)]·DMF·H 2O(1·g)
A mixture of Zn(NO3)2·6H2O(1 mmol,0.298 g),H3pzdc(1 mmol,0.120 g),H2abdc(0.5 mmol,0.208 g)andN,N-dimethylformamide(DMF,50 mL)was stirred for 30 min,and then sealed in a 100 mL Teflon-lined reactor and heated at 140℃for 3 d.After cooling to room temperature at a rate of 10℃/h,yellow block crystals were collected,washed with DMF,and dried in air.Yield based on Zn(NO3)2·6H2O:90%.
Diffraction data were collected on a Rigaku XtaLAB P300DS single-crystal diffractometer by using CuKα radiation.The structures were solved by the direct method and re f ined with the full-matrix least-squares method onF2by the SHELXTL package.Anisotropic thermal parameters were used to refine all non-hydrogen atoms.Hydrogen atoms were generated geometrically.The crystal data and structure refinement results are listed in Table S1(see the Supporting Information of this paper).CCDC:2098954 contain the supplementary crystallographic data.
3 Results and Discussion
Solvothermal reaction of Zn(NO3)2·6H2O,H3pzdc and H2abdc in DMF yielded crystals of(Me2NH2)·[Zn2(pzdc)(abdc)]·H2O·DMF(1·g).Single-crystal X-ray diffraction revealed that compound 1·g crystallizes in the orthorhombic space groupPnma.Each asymmetry unit contains two Zn2+ions,one pzdc3-ligand,and one Me2NH2+counter cation(all locating at the mirror plane with half-occupancy),and an abdc2-ligand,as well as one H2O and one DMF guest molecules(Fig.S1 and Table S1,see the Supporting Information of this paper).The presence of counter cation Me2NH2+in the crystal structure was consistent with the common observations of hydrolysis of the DMF,and further supported by infrared spectrum,in which the absorption peaks at 1358 cm-1and 2500—3000 cm-1can be assigned to the deformation and stretching vibrations of—CH3group,respectively(Fig.S2,see the Supporting Information of this paper)[16,22].Each pzdc3-ligand coordinates with four Zn2+ions on its molecular plane parallel with theac-plane,which extends to give a flat{Zn2(pzdc)}+ribbon[Fig.1(A)].In the ribbon,each Zn2+ion is T-shaped coordinated by one nitrogen and two oxygen atoms from two pzdc3-ligands.The trigonal bipyramidal coordination geometry of Zn2+is furnished by two oxygen atoms from two abdc2-ligands.Each abdc2-coordinates with four Zn2+ions from two adjacent{Zn2(pzdc)}+ribbons,using the four carboxylate oxygen atoms in the common coordination mode.Consequently,the parallel and coplanar ribbons are connected by the abdc2-linkers to form a 3D pillared-ribbon coordination network[Fig.1(B)and Fig.S3,see the Supporting Information of this paper],defining a 3D intersecting channel with a large void ratio of 61%(Me2NH2+omitted)or 47%(Me2NH2+retained).The Me2NH2+counter cations and H2O guest molecules locate in the empty space between two adjacent coplanar{Zn2(pzdc)}+ribbons,forming typical N/O—H···O hydrogen bonds among the host and guests[Fig.1(A)].Note that the—NH2and—NMe2moieties of Me2NH2+are parallel and perpendicular with the ribbon plane,respectively.
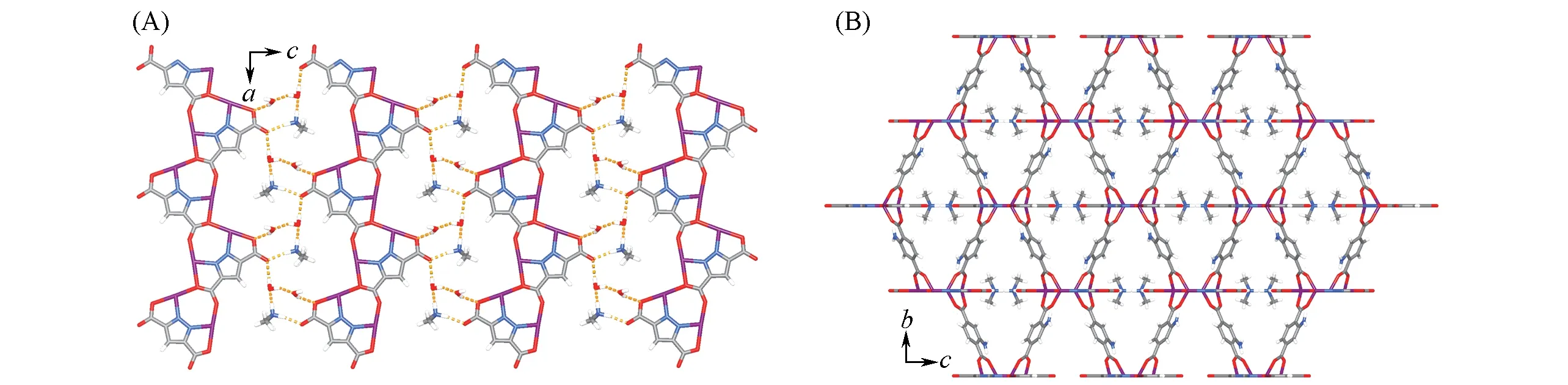
Fig.1 Coplanar{Zn2(pzdc)}+ribbons with hydrogen⁃bonded Me2NH 2+and H 2O guests(A)and the 3D pillared⁃ribbon framework(B)of 1·g
Thermogravimetry analysis of compound 1·g showed no obvious plateau,which can be attributed to the gradual removal of the high boiling-point DMF molecules(Fig.S4,see the Supporting Information of this paper).Variable-temperature PXRD showed that,after guest removal above 150℃,the guest-free framework 1'adopted a shrunk structure,which can be stable up to 200℃(Fig.S5,see the Supporting Information of this paper).When compound 1'was exposed to the saturated vapor of DMF or immersed directly in DMF,compound 1·g was recovered(Fig.S6,see the Supporting Information of this paper),meaning that the structural transformation is reversible and guest-induced.
Although the crystallinity significantly decreased after guest removal,we successfully determined the single-crystal structure of compound 1'(CCDC:2098955)by slowly increasing the temperature,after multiple attempts.The guest-free framework adopts a chiral space groupP212121and an obviously shrunk unit-cell volume(-11%)(Table S1 and Fig.S1),as well as a smaller void ratio of 56%(Me2NH2+omitted)or 38%(Me2NH2+retained)(Fig.2 and Fig.S3).From compound 1·g to compound 1',adjacent{Zn2(pzdc)}+ribbons approach each other,as illustrated by the decrease of periodicity from 1.44 nm to 1.28 nm and distortion/rotation of the ribbons.While the{Zn2(pzdc)}+ribbons in compound 1·g are planar and parallel to theab-plane,they exhibit two tilting directions(about±23°)to theab-plane in compound 1'.Consequently,Me2NH2+cations shift from the ribbon plane in compound 1·g to the upper/lower sides in compound 1',corresponding to the symmetry lowering of the crystal.While each Me2NH2+forms only one hydrogen bond with one of the two neighboring ribbons[N…O 0.272(3)nm,∠N—H…O 163.4(2)°]in compound 1·g,it forms two hydrogen bonds[N…O 0.270(7)and 0.279(7)nm,∠N—H…O 154.5(6)°and 172.9(5)°]with two ribbons in compound 1',highlighting the change of the ribbon alignments.In this context,the Me2NH2+ions provide the attraction forces to shrink the host framework,while the neutral DMF/H2O guest provide the repulsive forces to expand the host framework.The relatively high and low mobilities of the Me2NH2+ions in compound 1·g and compound 1'arising from their different hydrogen-bonding environments can also be visualized from their theral ellipsoids(Fig.S1).
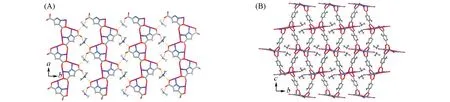
Fig.2 Tilted{Zn2(pzdc)}+ribbons with hydrogen⁃bonded Me2NH 2+ions(A)and 3D coordination network(B)of compound 1'
The porosity of compound 1'was studied by N2and CO2sorption isotherms at 77 K and 195 K,respectively(Fig.3).The N2adsorption-desorption isotherms showed two steps with saturated uptakes of 6.8 and 7.3 mmol/g,corresponding to pore volumes of 0.24 and 0.25 cm3/g,respectively.An obvious hysteresis was observed between the two steps.The CO2adsorption-desorption isotherms exhibited the type-I character with negligible hysteresis.At high pressures,the CO2uptake gradually increased from 4.8 mmol/g atp/p0=0.08 to 6.1 mmol/g atp/p0=0.95,corresponding to the pore volume increase from 0.19 to 0.24 cm3/g.These data indicate that the flexible host framework of compound 1'response differently to N2and CO2.Unfortunately,so far we cannot obtain the gas-loaded single-crystal structures,because gas adsorption further degrades the sample crystallinity.

Fig.3 N2(77 K)and CO2(195 K)adsorption(solid sym⁃bols)and desorption(open symbols)isotherms of compound 1'
4 Conclusions
We synthesized a pillared-ribbon PCP showing competition and cooperation of hydrogen bonds among the host framework,counter cations,and neutral guest molecules.The reversible guest-responsive structural transformations involve the movement and rotation of the counter cation,giving rise to the distortion and rotation of the ribbons and significant changes of the volume and symmetry of the whole host framework.These results enrich our understanding of the role of host-guest interactions for the structural transformations of flexible PCPs.
The Supporting Information of this paper see http://10.7503/cjcu20210576.

