DNA Barcoding and Systematic Review of Minervaryan Frogs (Dicroglossidae:Minervarya) of Peninsular India:Resolution of a Taxonomic Conundrum with Description of a New Species
Sonali GARG and S.D.BIJU
Systematics Lab,Department of Environmental Studies,University of Delhi,Delhi 110007,India
Abstract The genus Minervarya is among the most widely distributed,commonly occurring,and taxonomically confusing groups of dicroglossid frogs in India.Recent studies have provided evidence that this genus contains complexes of morphologically conserved but genetically divergent taxa—some widely distributed across South and Southeast Asia,and many particularly restricted to the Western Ghats region of the Indian Peninsula—posing several challenges in resolving long-standing taxonomic confusions.Here,we present a systematic review of minervaryan species found in Peninsular India,based on extensive DNA barcoding with nearly 400 samples from the entire range of the genus,including 277 new samples and topotypic material for most available names from the study area,combined with detailed morphological studies.As a result,we recognise 18 species in Peninsular India,including a new species described herein as Minervarya pentali sp.nov.Due to the comprehensive nature of the study,including comparisons with all available types,certain long-standing taxonomic uncertainties on the status of ten previously known taxa are resolved. Rana (Tomopterna) parambikulamana Rao,1937 (=Minervarya parambikulamana),Rana (Hylorana)sauriceps Rao,1937 (=Minervarya sauriceps), and Fejervarya kudremukhensis Kuramoto,Joshy,Kurabayashi,and Sumida,2008“2007”(=Minervarya kudremukhensis),are considered as junior subjective synonyms of Rana (Rana) limnocharis mysorensis Rao,1922 (=Minervarya mysorensis);Nyctibatrachus sanctipalustris var.modestus Rao,1920 (=Minervarya modesta)is proposed to be a synonym of Rana limnocharis syhadrensis Annandale,1919 (=Minervarya syhadrensis);while Rana murthii Pillai,1979 (=Minervarya murthii) and Fejervarya mudduraja Kuramoto,Joshy,Kurabayashi,and Sumida,2008 “2007”(=Minervarya mudduraja) are considered as junior subjective synonyms of Rana nilagirica Jerdon,1853 (=Minervarya nilagirica).At the same time,Rana brevipalmata Peters,1871 (=Minervarya brevipalmata),previously known only from its original description and the type specimen,is recognised as a distinct species referable to live populations in the Western Ghats.The study results in taxonomic stability of all the currently recognised members of the genus in Peninsular India.Significant geographical range extensions of species previously known from single localities are also provided based on morphologically and genetically confirmed records.Additionally,we classify all the recognised species into eight species-groups,with the aim of facilitating a better working taxonomy and future systematic studies on minervaryan frogs across their entire known range in Asia.
Keywords amphibia,distribution,Fejervarya,integrative taxonomy,Minervarya pentali sp.nov.,morphology,species groups,Western Ghats Biodiversity Hotspot
1.Introduction
The systematics of the genusMinervarya
Dubois,Ohler,and Biju,2001 (sensu lato) has been a subject of considerable discussion in the past few years,primarily owing to the conserved and confusing morphology of its members,as well as the largely unresolved genus-level relationships with the closely relatedFejervarya
Bolkay,1915 andSphaerotheca
Günther,1859 (e.g.,Dineshet al
.,2015;Garg and Biju,2017;Sanchezet al
.,2018).As currently recognised (sensu Sanchezet al
.,2018),the genus constitutes a relatively large radiation of small to largesized species (male SVL 16–50 mm;female SVL 19–65 mm),characterised by the presence of vomerine ridge;presence of pineal eye;presence of rictal glands,weakly to well developed(especially in males),and a light greyish-brown glandular stripe extending from the lower half of the tympanum up to the level of the shoulders on either side;presence of fejervaryan lines on either side of the belly;absence of webbing between fingers;presence of webbing between toes,ranging from reduced to extensive but never complete;absence of finger and toe discs;presence of skin folds,either continuous or discontinuous,with glandular warts on the dorsum;and smooth ventral skin(Duboiset al
.,2001;Garg and Biju,2017).The recognised thirty-five species of the genus are predominantly distributed in South Asia (India including the Andaman and Nicobar Archipelago,Sri Lanka,Pakistan,Nepal,Bhutan,and Bangladesh),with only two members found in the Southeast Asian countries of Myanmar andThailand,and neighbouring regions of southern China (Frost,2021;Khatiwadaet al
.,2021).Most of these (31 species) are known to occur in India,with the highest diversity (23 species)and endemism (21 species) in the Western Ghats Biodiversity Hotspot,which encompasses the southwest coast of the Indian Peninsula (Dineshet al
.,2015;Frost,2021;Garg and Biju,2017).However,severalspecies of the region,such as
Minervarya brevipalmata
,M.modesta
,M.murthii
,M.mysorensis
,M.nilagirica
,M.parambikulamana
,andM.sauriceps
,are known only from their original descriptions or type specimens,in the absence of any new collections or reliable reports ever since their descriptions (Garg and Biju,2017).Although new species of this genus have been described in the recent years,of which 12 species alone are from regions in Peninsular India (e.g.,Kuramotoet al
.,2008“2007”;Dineshet al
.,2015;Garg and Biju,2017;Rajet al
.,2018;Phugeet al
.,2019),attempts to either address or resolve the taxonomic status of the poorly known taxa have been limited.More recently,Garg and Biju (2017) assigned all the Western Ghats species into four morphologically diagnosed species groups.Phugeet al
.(2020) clarified the taxonomic status ofM.syhadrensis
(Annandale,1919),also leading to the synonymy ofM.caperata
Kuramoto,Joshy,Kurabayashi,and Sumida,2008.Khatiwadaet al
.(2021) recently clarified also the taxonomic status and generic placement ofMinervarya
species found in Nepal based new collections along with molecular data,with implications for species found in India.However,the taxonomic status of a large number of named taxa still remains doubtful,thereby deterring proper identification and range delineation of minervaryan frogs,particularly within the Western Ghats.At the same time,integrated morphological and molecular studies based on comprehensive as well as wide taxon sampling for all known species of the genus,such as those undertaken on other groups of frogs in the region (e.g.,Biju and Bossuyt,2009;Bijuet al
.,2011,2014a,2014b;Dahanukaret al
.,2016,2017;Garg and Biju,2016;Garget al
.,2018,2019,2021;Vijayakumaret al
.,2014),remain unattempted on minervaryan frogs.We undertook a study on minervaryan frogs,based on sampling of all known species of the genus in Peninsular India over a period of nearly two decades.Here,for the first time,we report specimens for all the recognised species of the region identified based on detailed morphological studies,along with molecular data from 277 newly sampled individuals combined into a DNA barcoding study using mitochondrial 16S rRNA gene sequences.The primary aim of our study was to resolve the taxonomic identities of species,particularly of members known only from their original descriptions and possibly confused with other widespread species.In turn,our wide sampling along with new topotypic material also enabled us to investigate the distribution ranges of species,the presence of potentially undescribed diversity,as well as understand systematic affinities for defining species-groups in this large and taxonomically challenging group of frogs.
2.Materials and Methods

2.2.Molecular study
Genomic DNA was extracted from 277 new samples using Qiagen DNeasy blood and tissue kit (Qiagen,Valencia,CA,USA),following manufacturer’s protocol.A fragment of the mitochondrial 16S rRNA gene was PCR-amplified using previously published primer set 16Sar and 16Sbr (Simonet al
.,1994) and sequenced on both forward and reverse strands with BigDye Terminator v3.1 Cycle Sequencing kit using an ABI 3730 automated DNA sequencer (Applied Biosystems).The raw sequence data was assembled and checked in ChromasPro v1.4 (Technelysium Pty Ltd.),and deposited in the GenBank under accession numbers MZ156076–MZ156352.In addition to our newly sequenced data,we retrieved 120 homologous sequences for all knownMinervarya
species from the GenBank.Limnonectes khasianus
was used as an outgroup taxon for the phylogenetic analyses.A dataset of total 398 sequences was assembled in MEGA 7.0 (Kumaret al
.,2016).Sequences were aligned using the MUSCLE tool (Edgar,2004)in MEGA 7.0,and the alignment was compared using MAFFT(Madeiraet al
.,2019).The resultant dataset of 544 characters was analysed using the Maximum Likelihood (ML) and Bayesian approaches.The appropriate model of sequence evolution was determined by implementing Akaike Information Criteria in ModelTest 3.4 (Posada and Crandall,1998).Bayesian analyses were executed in MrBayes (Ronquist and Huelsenbeck,2003) for five million generations using uniform priors,four Metropolis-Coupled Markov Chain Monte Carlo (MCMCMC)chains,and the best-fit General Time Reversible (GTR) model with a proportion of invariant sites (+I) and gamma-distributed rate variation among sites (+G).Trees were sampled at every 1000th generation and the Bayesian posterior probabilities(BPP) for clades were summarised after discarding the first 25 percent trees as burn-in (Huelsenbecket al
.,2001).Clade support was also assessed in the ML framework through 10,000 ultrafast bootstrap replicates (UBS) executed using the GTR+I+G model in IQ-TREE (Minhet al
.,2013),as implemented on the IQ-TREE webserver (Trifinopouloset al
.,2016).Intra-and interspecific uncorrected pairwise genetic distances among species were computed using MEGA 7.0 (Kumaret al
.,2016).2.3.Morphological study
Morphological comparisons were made with the available type specimens,original descriptions,and new topotypic specimens for all the currently recognisedMinervarya
species known to occur in Peninsular India.The types of following species were examined:M.agricola
andM.murthii
(ZSI/SRS—Zoological Survey of India,South Regional Station,Chennai);M.andamanensis
,M.modesta
,andM.syhadrensis
(ZSIC—Zoological Survey of India,Kolkata);M.brevipalmata
(ZMB—Zoological Museum,Berlin);M.cepfi
,M.gomantaki
,M.kadar
,M.manoharani
,andM.neilcoxi
(ZSI/WGRC—Zoological Survey of India,Western Ghats Regional Centre,Kozhikode);M.goemchi
,M.kalinga
,M.krishnan
,andM.marathi
(ZSI/WRC—Zoological Survey of India,Western Regional Centre,Pune);M.keralensis
andM.mysorensis
(NHM—Natural History Museum,London);M.kudremukhensis
andM.mudduraja
(BNHS—Bombay Natural History Society,Mumbai);M.nilagirica
,M.rufescens
,andM.sahyadris
(MNHNP—Muséum National d’Histoire Naturelle,Paris).For two speciesM.parambikulamana
andM.sauriceps
,for which type specimens are lost,comparisons were made with the original descriptions and new topotypic specimens.Morphological characters for other congeners with their geographical ranges restricted outside the study region were largely derived from their original descriptions or subsequent literature,available types or other museum specimens,published photographs,or new collections.Shared morphological characters were also identified for grouping of species.We used only adult (mature) individuals for the morphometric studies.Sex and maturity were determined by the presence of secondary sexual characters (such as nuptial pads and vocal sacs in males) or examination of gonads.Measurements and associated terminologies follow Biju and Bossuyt (2009).The following measurements were taken to the nearest 0.1 mm using digital slide-calipers or a binocular microscope with a micrometer ocular:snout-vent length (SVL),head width (HW,at the angle of the jaws),head length (HL,from rear of mandible to tip of snout),MN (distance from the rear of the mandible to the nostril),MFE (distance from the rear of the mandible to the anterior orbital border),MBE(distance from the rear of the mandible to the posterior orbital border),snout length (SL,from tip of snout to anterior orbital border),eye length (EL,horizontal distance between bony orbital borders),inter upper eyelid width (IUE,the shortest distance between the upper eyelids),maximum upper eyelid width (UEW),internarial distance (IN),internal front of the eyes (IFE,shortest distance between the anterior orbital borders),internal back of the eyes (IBE,shortest distance between the posterior orbital borders),NS (distance from the nostril to the tip of the snout),EN (distance from the front of the eye to the nostril),TYD (greatest tympanum diameter),TYE (distance from the tympanum to the back of the eye),forearm length(FAL,from flexed elbow to base of outer palmar tubercle),hand length (HAL,from base of outer palmar tubercle to tip of third finger),FL(finger length),thigh length (TL,from vent to knee),shank length (SHL,from knee to heel),foot length(FOL,from base of inner metatarsal tubercle to tip of fourth toe),total foot length (TFOL,from heel to tip of fourth toe),FD(maximum disc width of finger),width of finger (FW,measured at the base of the disc),TD (maximum disc width of toe),width of toe (TW,measured at the base of the disc).Digit number is represented by roman numerals I–V in subscript.Measurements and photographs were largely taken of the right side of the specimen,and sometimes on the left side when a character was damaged.All measurements provided in the taxonomy section are in millimetres.For the convenience of discussion,species of the genusMinervarya
are categorised based on their body size,as modified from Garg and Biju (2017):small (male SVL 16.0–25.0 mm),medium (male SVL 25.1–45.0 mm) and large (male SVL 45.1–65.0 mm).2.4.ZooBank registration
This published work and the nomenclatural acts it contains have been registered in ZooBank,the online registration system for the International Commission on Zoological Nomenclature (ICZN).The ZooBank LSID(Life Science Identifier) for this publication is urn:lsid:zoobank.org:pub:3D20A723-AA21-456E-9C95-F3DF0F56A2FC and for the new species described herein LSID is urn:lsid:zoobank.org:act:1CBAF3D4-17A1-4A1F-B1A1-8E58A17C8FF9.The LSIDs and associated information can be resolved through any standard web browser by appending the LSID to the prefix http://zoobank.org.3.Results
3.1.DNA barcoding and phylogenetic relationships
The results of our BI and ML phylogenetic analyses were highly congruent with respect to the monophyly of the major wellsupported clades.We recovered 26 species-level lineages(representing recognised or potential species) within the genusMinervarya
(Figure 1).Our 277 new samples nested with 12 previously known species identified from topotypic material.Of the total 24 previously recognised species in the genus represented on the tree,16 were found in the Western Ghats or the Indian Peninsula.Of these,13 species were restricted to the region,while three species had their distributions extending towards central,eastern,and northern Indian regions (Table S1).In addition,our analyses recovered two hitherto unrecognised lineages—one identified as belonging toMinervarya brevipalmata
(see section ‘3.2 Taxonomic status of some nominal taxa’),and the other representing a new species (formally described asMinervarya pentali
sp.nov.
under the section ‘3.3 Description of a new species’).The species-level relationships were largely in agreement with previous studies (Dineshet al
.,2015;Garg and Biju,2017;Köhleret al
.,2019;Phugeet al
.,2019;Rajet al
.,2018;Sanchezet al
.,2018),and differed primarily with respect to either the level of support received for various clades or their taxonomic identities (as clarified in the subsequent section ‘3.2 Taxonomic status of some nominal taxa’).Twenty species recovered as highly supported (BPP ≥ 95,UBS ≥ 90)monophyletic clusters both in the BI and ML analyses (IQTREE) includedM.sahyadris
,M.krishnan
,M.agricola
,M.asmati
,M.teraiensis
(based on conspecific sequence identity with the typical sequences ofM.dhaka
,as per sequences available from Howladeret al
.,2016;Khatiwadaet al
.,2021),M.chiangmaiensis
,M.syhadrensis
,M.pentali
sp.nov.,
M.brevipalmata
,M.goemchi
,M.mysorensis
,M.kirtisinghei
,M.manoharani
,M.cepfi
,M.neilcoxi
,M.marathi
,M.muangkanensis
,M.keralensis
,M.kalinga
,andM.nilagirica
.The only exceptions were:(1)M.gomantaki
,for which the typical populations from Goa were recovered as a wellsupported cluster (BPP 100,UBS 99),however,the relationship of other genetically and geographically close populations from Maharashtra,which were also morphologically referable to this species,remained unresolved in both the analyses;and (2)M.rufescens
,for which the monophyly of all the assigned populations was highly supported in our BI (BPP 100) but moderately supported in the ML analysis (UBS 80).Furthermore,the clade support could not be assessed for four species currently represented by a single GenBank sequence each (M.nepalensis
,M.greenii
,M.kadar
,andM.andamanensis
).All theMinervarya
species included in our phylogenetic analyses were recovered in eight distinct,largely well supported,and some moderately supported (BPP ≥ 90,UBS ≥ 70) clades representing species-groups—(1)Minervarya sahyadris
group:M.gomantaki
,M.krishnan
,andM.sahyadris
;(2)Minervarya syhadrensis
group:M.syhadrensis
,M.nepalensis
(sensu Khatiwadaet al
.,2021),andM.pentali
sp.nov.;
(3)Minervarya agricola
group:M.agricola
,M.asmati
,M.chiangmaiensis
,andM.teraiensis
;(4)Minervarya mysorensis
group:M.brevipalmata
,M.goemchi
,andM.mysorensis
;(5)Minervarya rufescens
group:M.cepfi
,M.kadar
,M.manoharani
,M.neilcoxi
,andM.rufescens
;(6)Minervarya andamanensis
group:M.andamanensis
andM.muangkanensis
;(7)Minervarya greenii
group:M.greenii
andM.kirtisinghei
;(8)Minervarya nilagirica
group:M.kalinga
,M.keralensis
,andM.nilagirica
.Based on further morphological studies,these clades are herein defined as species-groups,primarily adapted and expanded from our previous work (Garg and Biju,2017).A single species,M.marathi
was provisionally assigned to theMinervarya andamanensis
group based on morphology,although it showed a deeply divergent yet highly to moderately supported sister-group relationship with members of the morphologically disparateMinervarya rufescens
group.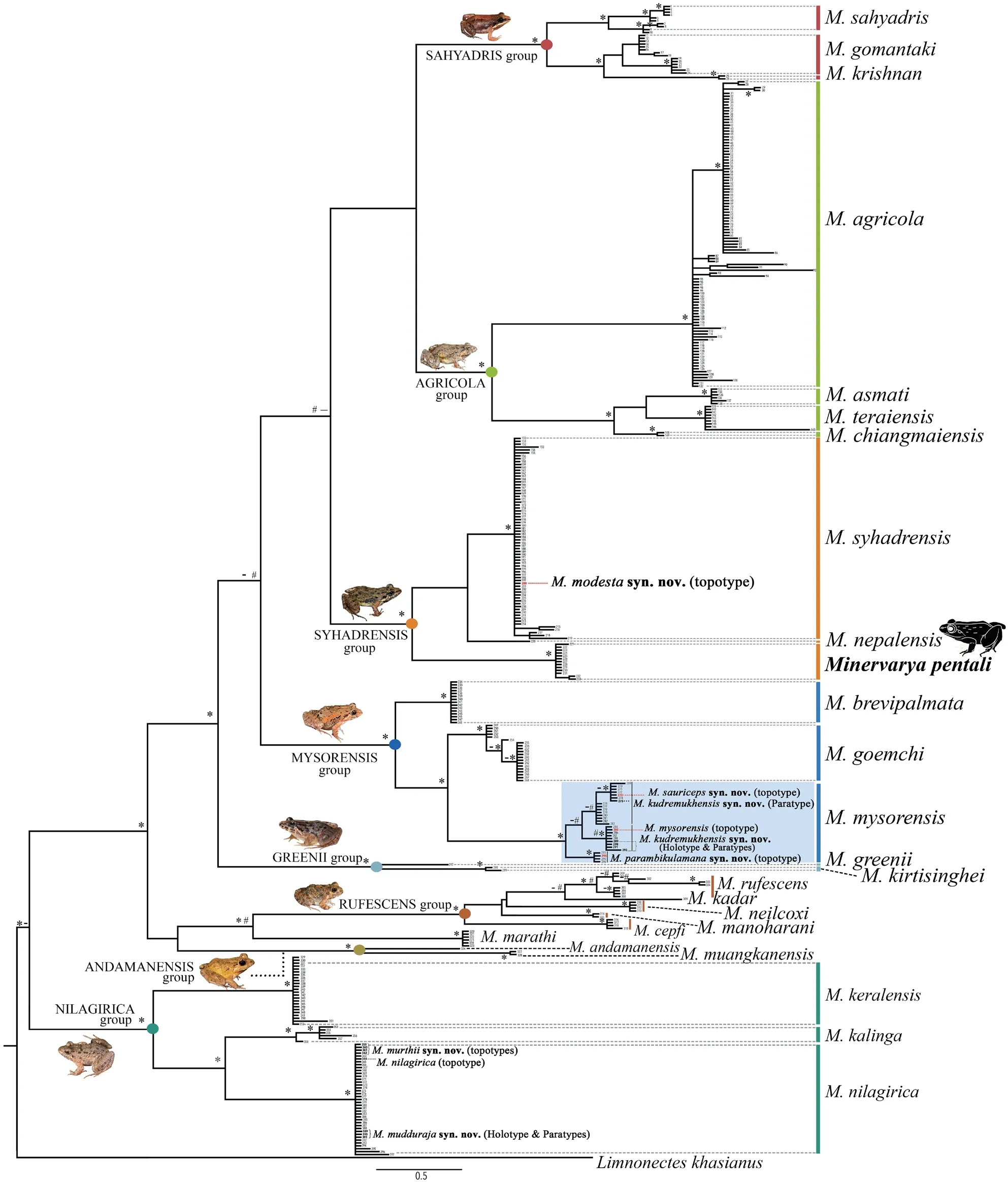
Figure 1 Bayesian consensus phylogram depicting phylogenetic relationships among 397 Minervarya samples from regions across the known range of the genus in Asia based on mitochondrial 16S rRNA sequences.The focal genus Minervarya is represented by 25 previously known and one new species,comprising eight species-groups discussed in the study.Coloured vertical bars alongside the terminal nodes indicate species;bold letters indicate the new species;and coloured circles at the internal nodes indicate species-groups.Asterisk symbol (*) above the branch indicates Bayesian Posterior Probabilities (BPP) ≥ 95% and Ultrafast Bootstrap Support (UBS) values ≥ 90%;hash symbol (#) indicates BPP ≥ 90% and UBS values ≥ 70%;dash (-) indicates branches with BPP <90%or UBS <70% in cases where BPP ≥ 90% or UBS ≥ 70% in one of the analyses.The tree is rooted with Limnonectes khasianus as the outgroup taxon.
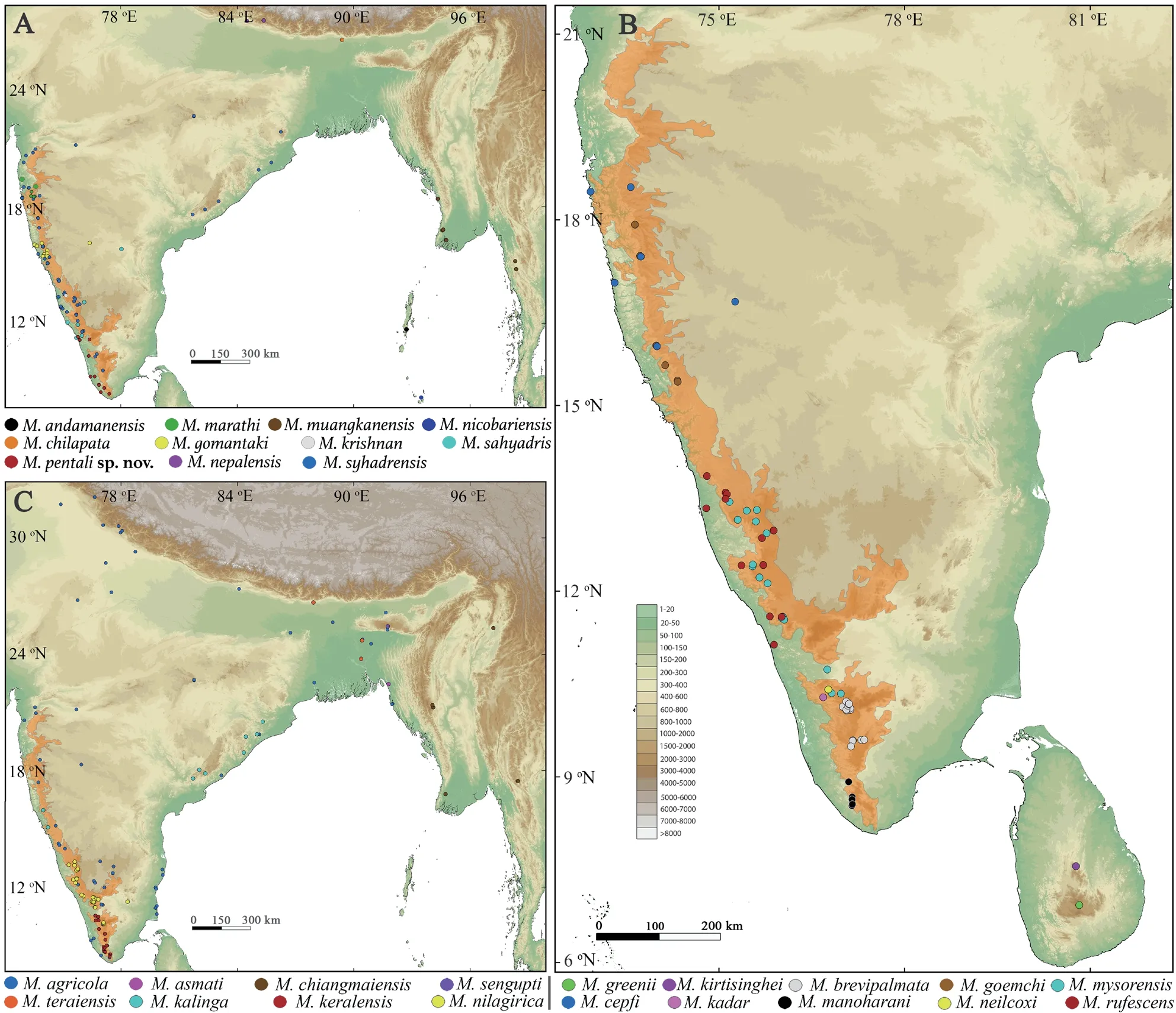
Figure 2 Geographical distribution of twenty-nine species in the genus Minervarya based on genetic samples used in the study and the known type localities.(A) Eleven species of the Minervarya andamanensis group (M.andamanensis,M.marathi,M.muangkanensis,and M.nicobariensis),Minervarya sahyadris group (M.chilapata,M.gomantaki,M.krishnan,and M.sahyadris),and Minervarya syhadrensis groups (M.pentali sp.nov.,M.nepalensis,and M.syhadrensis);(B) Eight species of the Minervarya agricola group (M.agricola,M.asmati,M.chiangmaiensis,M.sengupti,and M.teraiensis) and Minervarya nilagirica group (M.kalinga,M.keralensis,and M.nilagirica);(C) Ten species of the Minervarya greenii group (M.greenii and M.kirtisinghei),Minervarya mysorensis group (M.brevipalmata,M.goemchi,and M.mysorensis),and Minervarya rufescens group (M.cepfi,M.kadar,M.manoharani,M.neilcoxi,and M.rufescens).
Among the recognised species-groups,several notable intra-and inter-group relationships were observed.Within theMinervarya sahyadris
group,M.sahyadris
comprised of two well-supported divergent sub-lineages (uncorrectedp
-distances ranging between 1.3%–1.8%) from localities in Karnataka and Kerala,respectively (Figures 1 and 2).This species showed a well-supported sister-group relationship with the clade containing two weakly resolved but divergent sub-populations ofM.gomantaki
from Goa and Maharashtra (uncorrectedp
-distance ranging between 1.1%–1.7%) andM.krishnan
.A sister-group relationship betweenMinervarya sahyadris
group and theMinervarya agricola
group was recovered in the BI analysis with low support (BPP 82),whereas the ML analysis suggested a possible sister relationship between theMinervarya sahyadris
group andMinervarya syhadrensis
group,albeit with much lower confidence (UBS 54,not shown).However,the close relationship of these three groups received moderate to weak support in our analyses (BPP 93,UBS 56).Within theMinervarya agricola
group,a sister-group relationship betweenM.asmati
andM.teraiensis
was recovered in BI with low support (BPP 61),but together the two species showed a wellsupported sister-group relationship withM.chiangmaiensis
.These three species were more distantly related toM.agricola
,which also showed high levels of genetic differentiation(uncorrectedp
-distances ranging between 0.6%–4.0%) among various poorly resolved population sub-clusters,largely with either south-ranging (Tamil Nadu,Kerala,and Karnataka)or north-ranging (Maharashtra,Andhra Pradesh,Gujarat,Rajasthan,Punjab,Himachal Pradesh,Haryana,Delhi,Uttar Pradesh,Uttarakhand,Madhya Pradesh,Chhattisgarh,Odisha,Bihar,up to West Bengal) distribution pattern.The findings suggest thatM.agricola
could possibly also represent a complex of multiple species requiring further detailed population studies.Within theMinervarya syhadrensis
group,the relationships between three recognised species received low support,but were suggestive of a sister relationship betweenM.syhadrensis
andM.nepalensis
(BPP 74,UBS <50),which were together likely to form the sister-group to the new speciesM.pentali
sp.nov.At the same time,intraspecific divergence among the various widely distributedM.syhadrensis
populations (from Kerala,Karnataka,Goa,Maharashtra,Gujarat,Andhra Pradesh,Madhya Pradesh,up to Odisha) reached up to 1.2%.The otherwise well-supported and distinctMinervarya brevipalmata
group was recovered as having a weakly supported and unresolved relationship with three groups (Minervarya sahyadris
group,Minervarya syhadrensis
group,andMinervarya agricola
group).Within theMinervarya brevipalmata
group,M.mysorensis
andM.goemchi
showed a well-supported sister relationship,and together the two species formed a sister-group relationship withM.brevipalmata
with high support (the taxonomic identities of these taxa are discussed in the section ‘3.2 Taxonomic status of some nominal taxa’).The intraspecific divergence among the various populations ofM.mysorensis
ranged up to 1.1%between three major population sub-clusters:(1) Anaimalais up to Nelliyampathy hills encompassing regions south of Palghat gap in Kerala and Tamil Nadu;(2) Wayanad plateau in northern Kerala up to Coorg plateau in southern Karnataka;and (3) Chikamagalur hills up to Sharavathi basin.ForM.goemchi
,the observed intraspecific divergences were up to 0.4%among populations distributed from northern Karnataka up to Maharashtra.On the other hand,the distribution ofM.brevipalmata
was restricted to regions south of the Palghat gap,from Anamalai and Cardamom hills down to Meghamalais.TheMinervarya greenii
group,comprising two species endemic to Sri Lanka,was recovered as having a sister relationship with the clade containing four groups (Minervarya sahyadris
group,Minervarya syhadrensis
group,Minervarya agricola
group,andMinervarya brevipalmata
group).TheMinervarya rufescens
group showed a well-supported sister-group relationship withM.marathi
.Together,these members were recovered as having a close relationship with theMinervarya andamanensis
group,albeit with weak support.TheMinervarya nilagirica
group formed the most distinct clade in the genus with a basal relationship to all the remaining knownMinervarya
species and species groups.Within theMinervarya nilagirica
group,the sister relationship betweenM.nilagirica
(predominantly restricted to the Western Ghats regions of Tamil Nadu,Kerala,and Karnataka) andM.kalinga
(currently known from the northern Western Ghats states of Goa and Maharashtra,and also from the Eastern Ghats regions in Andhra Pradesh and Odisha,with intraspecific divergences of up to 1.1%) was well supported.The third member of the group,M.keralensis
(restricted to south of the Palghat gap in Kerala and Tamil Nadu),formed a sistergroup toM.nilagirica
andM.kalinga
with high support (Figures 1 and 2).Although no other previous comparable study on minervaryan frogs of Peninsular India is available in terms of taxon sampling,the relationships recovered in our phylogenetic analyses among the major clades were largely congruent with the recent multi-gene and mitochondrial 16S rRNA phylogenies(e.g.,Dineshet al
.,2015;Garg and Biju,2017;Köhleret al
.,2019;Phugeet al
.,2019;Rajet al
.,2018;Sanchezet al
.,2018).3.2.Taxonomic status of ten nominal taxa
(Peters,1871):taxonomic identity and nomenclatural stability.
While describing this species asRana brevipalmata
,Peters (1871) provided a detailed description of the holotype (Figure 3),which he stated to have been purchased from Pegü (Burma,Myanmar).Boulenger (1905“1904”) doubted the type locality of this taxon,apparently due to the availability of several comparable specimens“from the Nilgherry and Travancore hills”in southern India,which he identified as belonging toRana brevipalmata
Peters,1871.Subsequently,this taxon was subjected to frequent taxonomic changes.Boulenger(1890) synonymised it underRana limnocharis
Gravenhorst,1829 and referred to it as“var.brevipalmata
”based on examination of some specimens from“Pegu and S.India”.Subsequent authors(e.g.,Annandale,1917;Boulenger,1920;Roux,1928;Bourret,1942;Gorham,1974;Daniel,1975) followed this by treating it either as a subspecies or a junior subjective synonym ofRana limnocharis
.Pillai (1980) specifically addressed the taxonomic status of this taxon.Based on multiple new collections from Kerala and Tamil Nadu,he discussed morphological characters as well as behavioural patterns distinguishingRana brevipalmata
as a distinct species fromRana limnocharis nilagirica
,with which he stated it to have been previously confused (Boulenger,1920).However,Dubois (1984) consideredRana brevipalmata
to be a junior subjective synonym ofRana nilagirica
Jerdon,1853,but subsequently treated it asincertae sedis
in the genusFejervarya
(Dubois,1987“1986”).Dubois and Ohler (2000) later concluded that this name does not refer to any biological species of frog.Yet,Rana brevipalmata
Peters,1871 continued to be treated as a valid species,in various genera such asLimnonectes
(Dubois,1987;Dutta and Singh,1996),Fejervarya
(Iskandar,1998;Feiet al
.,2002;Dineshet al
.,2015),Zakerana
(Howlader,2011),and the most recent transfer toMinervarya
based on the separation of this predominantly South Asian genus from the largely Southeast AsianFejervarya
(Sanchezet al
.,2018).Most available literature includes this species in the list of Western Ghats frogs,where it is also believed to be restricted (e.g.,Daniels,2005;Dineshet al
.,2009;Dutta,1997;Frost,2021).However,none of the recent studies have revisited the identity of this taxon and provided new collections or insights on its taxonomic status.Also surprisingly,even though the type ofRana brevipalmata
was originally stated to have originated from Myanmar,it appears to have been included in the region’s amphibian inventories only rarely (Frost,2021),including its absence from a recent comprehensive study onFejervarya
andMinervarya
frogs of that country (Köhleret al
.,2019).
Figure 3 Type specimens of eight Minervarya taxa described from Peninsular India showing (left to right) dorsal view,ventral view of hand,and ventral view of foot.(A–C) holotype (ZMB 6130) of Rana brevipalmata;(D–F) holotype (BNHS 4653) of Fejervarya kudremukhensis;(G–I) holotype (BMNH 1947.2.1.80) of Rana (Rana) limnocharis mysorensis;(J–L) neotype (BNHS 6113) of Rana(Tomopterna) parambikulamana;(M–O) neotype (BNHS 6114) of Rana (Hylorana) sauriceps;(P–R) neotype (MNHNP 1984.2340) of Rana nilagirica;(S–U) holotype (BNHS 4645) of Fejervarya mudduraja;(V–Y) holotype (ZSI-SRS VA/773) of Rana murthii,(W) ventral view of throat and chest showing the absence of“pearl-like papillae”.Bottom row:Topotypes of four synonymised nomen in life.
We studied the type specimen ofRana brevipalmata
Peters,1871 (ZMB 6130,an adult male) in order to compare it with various populations of minervaryan frogs from the Western Ghats.While describing this taxon,Peters (1871) provided a brief comparison with“Rana limnocharis
Boie(
Rana gracilis
Wiegm.)”and“the South AfricanRana grayi
andRana fasciata
”,primarily distinguishing it based on very elongated limbs and reduced webbing between toes from the former,and well developed vomerine teeth from the latter species.Altogether,Peters (1871)described a number of morphological characters forRana brevipalmata
including head dimensions,position of nostril,tympanum diameter,presence of vomerine teeth,tongue shape,long limbs,relative length of fingers,extent of webbing on foot,skin texture (including dorsal skin with some short longitudinal folds and small rounded tubercles),body colouration and markings (including a bright mid dorsal line),and some body measurements,which were largely found to be matching with the available type.Boulenger (1890) discussed two characters distinguishing var.brevipalmata
fromRana limnocharis
:tibiotarsal articulation“reaching considerably beyond the end of the snout”and foot measuring“two thirds the distance between the end of the snout and the vent.”Boulenger (1920) also stated the first finger to be longer than the second inRana brevipalmata
.Later,Pillai (1980) studied populations from“Muthanga”,and“Chedleth”of Wayanad,Kerala state and“Anamalai”and“Valparai”of Anaimalai Hills,Tamil Nadu state,and provided further comparison betweenRana brevipalmata
fromRana limnocharis
mainly stating differences in reduced foot webbing,absence of dermal fringe along toe V,tympanum less than half the diameter of the eye,and the tympanum-eye distance equal to the tympanum diameter.We found most of the characters discussed by Boulenger (1890,1920) and Pillai (1980),as well as several characters discussed in the original description provided by Peters (1871),to be comparable with our multiple new collections from Idukki district of Peninsular India (Table S1),which were also recovered as a distinct and previously unnamed clade within the genusMinervarya
(Figure 1).Particularly notable among these are characters such as dorsal skin with relatively long longitudinal folds and small rounded tubercles,long hind limbs,reduced webbing between toes,and well developed inner metatarsal tubercle.Hence,building on the judgements made by previous researchers and the additional evidence gathered in the present study,we provisionally consider this taxon to have originated from the Western Ghats and identify live populations referable to this species (Table S1),thereby clarifying its taxonomic status.
(Rao,1937):rediscovery,neotypification,and synonymy.
This taxon was originally described asRana (Tomopterna) parambikulamana
Rao,1937 based on a single specimen from“Parambikulam forests”,with an accompanying illustration.This type specimen is considered lost(Dubois,1984) owing to which the species is currently known only from its original description.The generic placement of this species has been confusing due to its vague description with assignment to the subgenusTomopterna
(Rao,1937),where it continued to be placed by several subsequent authors (e.g.,Dutta,1997;Chanda,2002;Matsuiet al
.,2008).It was also proposed asincertae sedis
withinFejervarya
(Dubois,1987“1986”) or stated to be“invalid”(Purkayastha and Matsui,2012),both without discussion.These confusions probably stemmed from Rao’s observations (1937):“I have compared this specimen withR.rufescens,R.breviceps
andR.dobsoni
,from which it differs almost in every character,and generally resembles
R.tigrina
in external form,though differing in details both from this species andR.limnocharis
(Forma typica) through which this new species is derivable.”Nonetheless,even though the species appears to have been confused with members belonging to five different genera,its assignment toMinervarya
,as presently understood,can be considered more conclusive based on characters such as head longer than broad,snout pointed,projecting beyond the lower jaw;distinct tympanum;fourth toe considerably longer than the thigh or tibia;and presence of inverted“U”or“V”shaped skin fold,all of which are clearly comparable with the illustration of the type provided by Rao (1937).Within the genusMinervarya
,this taxon cannot be a member ofMinervarya rufescens
group due to its large snout-vent size,presence of“light vertebral band”,“Hind limb long,the tibio-tarsal articulation reaching far beyond the tip of the snout”,“inner metatarsal tubercle large smaller than the first toe”,head“distinctly longer than broad”,and“snout longer than eye”.Instead,the species can be assigned to theMinervarya mysorensis
group (see group definition in the section ‘3.4 Grouping of species in the genusMinervarya
’) due to its adult size“39.00”mm,“head longer than broad”,“Hind limb long,the tibio-tarsal articulation reaching far beyond the tip of the snout”,“fourth toe considerably longer than the thigh or tibia”,toes“¼ webbed”,“inner metatarsal tubercle large”,and dorsum with“a light vertebral band”and“cutaneous folds edged black”.Furthermore,our field studies at Parambikulam have resulted in the collection of a species of the genusMinervarya
that is comparable to many of the characters mentioned and illustrated in the original description ofM.parambikulamana
(Rao,1937).We provide a description of this new specimen and further find it necessary to designate the same as a neotype ofRana
(Tomopterna
)parambikulamana
Rao,1937 in accordance with Article 75 of the ICZN (International Commission on Zoological Nomenclature 1999,hereafter,The Code),owing to the following reasons:(1) The unavailability of its name bearing type (Dubois,1984;Biju,2001;present study).(2) In the absence of a type,direct evidence on the holotype ofRana
(Tomopterna
)parambikulamana
currently exists only from the original description and accompanying figure (Rao,1937).However,the description by Rao (1937) is imprecise and not particularly informative,except for a handful of characters such as presence of inverted“U”or“V”shaped skin fold on the dorsum and“inner metatarsal tubercle large smaller than the first toe”.These characters also largely overlap with other recognised and morphologically cryptic congeners,especiallyM.brevipalmata
(Peters,1871),M.mysorensis
(Rao,1920),M.sauriceps
(Rao,1937),andM.kudremukhensis
(Kuramotoet al.
,2008“2007”),some of which in addition have overlapping or close distribution ranges.(3) Further,while describing this species Rao (1937) stated its overall resemblance with two taxa that are now known to be morphologically unrelated,Rana tigrina
(=Hoplobatrachus tigerinus
) andR.limnocharis
(=Fejervarya limnocharis
).This adds to the confusion in diagnosingRana
(Tomopterna
)parambikulamana
solely based on its original description.(4)Moreover,the identity and the specific taxonomic status ofRana
(Tomopterna
)parambikulamana
itself have been previously doubted,with authors proposing it to be eitherincertae sedis
(Dubois,1987“1986”) or invalid (Purkayastha and Matsui,2012)probably due to the difficulty in assigning it to any known living population since its single original record.Hence,based on new evidence gathered in the present study,designation of a neotype is considered essential for defining this taxon objectively,in order to identify it properly from other close congeners,as well as to stabilise the taxonomic status of the nomen.We hereby designate BNHS 6113 from the original type locality“Parambikulam”as the neotype ofRana (Tomopterna)parambikulamana
Rao,1937 (=Minervarya parambikulamama
).The neotype description provided below is largely consistent with what is known of the former name-bearing type.
Synonymisationof Rao,1937.
Due to the comprehensive nature of our study,we also compared our new collections ofRana (Tomopterna)parambikulamana
Rao,1937 (=Minervarya parambikulamama
)with all other knownMinervarya
species sampled from regions across Peninsular India.Based on a detailed morphological comparison of the original description and new topotypic material,including the designated neotype,we find this taxon to be extremely similar toM.mysorensis
Rao,1922 with overlapping morphological traits,which do not support the two taxa to be reliably distinguished from each other.These include characters such as the adult size,snout sub-elliptical to pointed in dorsal and ventral view;short discontinued dorsal skin folds without a constant pattern;presence of an inverted“V”-shaped skin fold at the centre of the dorsum;foot including toes relatively long;and reduced webbing between toes.The skin texture disparity between Rao’s description ofM.parambikulamana
andM.mysorensis
is probably a misinterpretation due to the dehydrated condition of the described specimen,as evident from the illustration (Rao,1937,Figure 1).In addition,we also sequenced molecular data from the neotype specimen ofM.parambikulamana
and included it in our phylogenetic analyses to further clarify the genealogical affinities of this taxon.Our results findM.parambikulamana
to be nested together with various populations ofM.mysorensis
,includingM.kudremukhensis
andM.sauriceps
(both subsequently synonymised withM.mysorensis
),with shallow genetic distances of 0.8%–1.1%,which not only fall within the range of intraspecific distances observed for the variousMinervarya
species in our study (Table 1),but also do not correspond to any reliable morphological characters for interspecific comparison and may therefore be considered as intraspecific variations.Hence,in accordance with the Article 23 (Principle of Priority)of The Code we considerRana (Tomopterna) parambikulamana
Rao,1937 (=Minervarya parambikulamana
) as a junior subjective synonym ofRana (Rana) limnocharis mysorensis
Rao,1922 (=Minervarya mysorensis
).(Rao,1937):rediscovery,neotypification,and synonymy.
This taxon was originally described asRana (H ylorana) sauriceps
Rao,1937 based on“A number of specimens of all ages”from“Wattekole,Coorg,S.India”.However,the original description included measurements only for a single specimen with SVL measuring“30.00 mm”,along with illustrations (Rao,1937).Ever since,there have been no new reports or collections of this species,either from the type locality or other regions.Further,the original name-bearing type is considered lost (Dubois,1984),hence this nominal taxon was known only from its original description.Our surveys within the region of the type locality“Wattakole”(=Watekolli) resulted in collection of a ‘chocolate red’ coloured specimen belonging to a species of the genusMinervarya
(Figure 3) that is found to be comparable with the original description and accompanying illustrations ofRana(Hylorana) sauriceps
Rao,1937 with respect to several characters such as“hind limbs rather long,slender,tibio-tarsal articulation reaching the nostril”;“toes pointed;web not extending to the tip of the first phalangeal bone,rather stopping at the base”;“inner metatarsal about ½ the diameter of the eye”;“upper surface of the skin slightly granulate,with short interrupted longitudinal folds with a few tubercles”;a ∩-shaped mark found on the back behind the shoulders;dorsum chocolate red.We assign this new collection from the type locality as the neotype ofRana (Hylorana) sauriceps
Rao,1937,an action we consider necessary in accordance with Article 75 of The Code,owing to the following reasons:(1) The unavailability of its name bearing type (Dubois,1984;Biju,2001;present study) or any new referable material since the original description.(2) In the absence of a type,direct evidence on the holotype ofRana(Hylorana) sauriceps
currently exists only from the original description and accompanying figure (Rao,1937).However,the description by Rao (1937) is imprecise and not particularly informative.Several of the stated characters,particularly“Upper surface of the skin slightly granulate,with short interrupted longitudinal folds with a few tubercles”and“a ∩-shaped mark found on the back behind the shoulders”,also overlap with other recognised and morphologically cryptic congeners,especiallyM.brevipalmata
(Peters,1871),M.mysorensis
(Rao,1920),andM.kudremukhensis
(Kuramotoet al.
,2008“2007”),some of which in addition have overlapping or close distribution ranges.(3) Moreover,the specific taxonomic status ofRana (Hylorana)sauriceps
itself has been previously doubted with a proposal to consider it asincertae sedis
in the genusFejervarya
(Dubois,1987“1986”) that added to the confusion in assigning this nomen to any known living population ever since its original description.Hence,based on new evidence gathered in the present study,designation of a neotype is considered essential for defining this taxon objectively,in order to identify it properly from other close congeners,as well as to stabilise the taxonomic status of the nomen.We hereby designate BNHS 6114 from the original type locality“Wattekole”as the neotype ofRana (Hylorana)sauriceps
Rao,1937 (=Minervarya sauriceps
).The neotype description provided below is largely consistent with what is known of the former name-bearing type.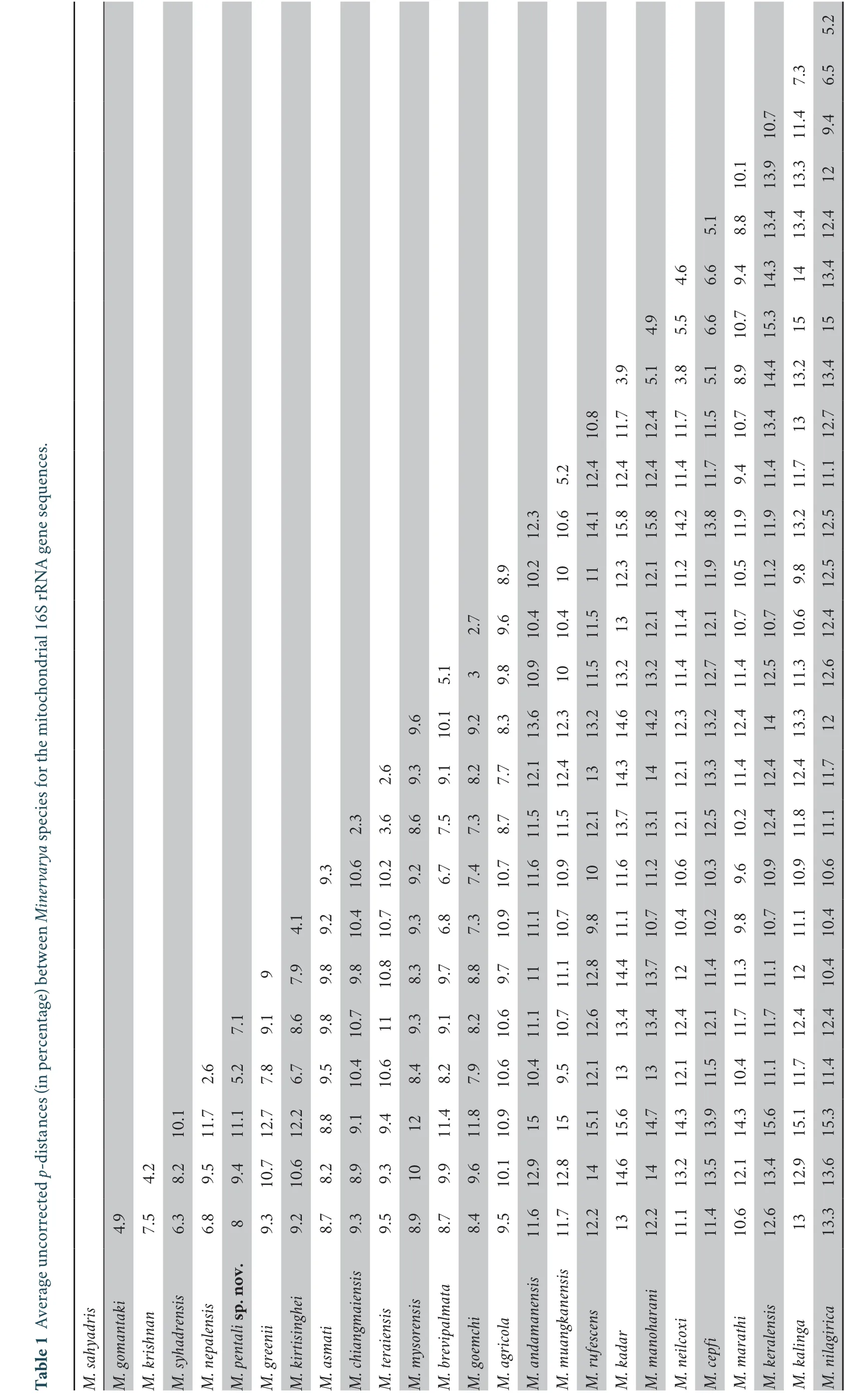
Description of neotypeof Rao,1937.
By present designation,Neotype:BNHS 6114 (Figure 3),from“Wattakole”(Watekolli),Karnataka state,India,collected by S.D.Biju on 17 August 2013.A medium-sized adult male(SVL 40.5);head longer (HL 14.5) than wide (HW 13.2);snout sub-elliptical to pointed in dorsal and ventral view,obtuse in lateral view,its length (SL 6.4) longer than horizontal diameter of eye (EL 3.9);loreal region flared and concave with indistinct canthus rostralis;interorbital space narrower (IUE 2.3) than upper eyelid width (UEW 2.8) and internarial distance (IN 3.4);tympanum oval,its horizontal diameter (TYD 2.1) nearly half (53.8%) of the eye diameter (EL 3.9);forearm (FAL 6.3)shorter than hand length (HAL 8.7);outer,inner,and middle metacarpal tubercles present on hand,well developed;thigh (TL 18.7) shorter than shank (SHL 20.0) and foot (FOL 23.4);foot webbing small:I1– 2II1–2III1–3IV3–1V;inner metatarsal tubercle prominent (IMT 2.0),oval,raised;outer metatarsal tubercle small,rounded.Dorsal skin shagreened to sparsely granular with short longitudinal skin folds;a faint inverted“V”-shaped ridge on anterior half of the dorsum,at the level of forearms;lateral surfaces of head shagreened;flanks shagreened to sparsely granular;fore-and hind limbs shagreened with a few scattered tubercles.Ventral surfaces shagreened,and posterior surfaces of thighs granular.Colour in preservation
.Dorsum light reddish-brown without any prominent markings;upper half of tympanum and inner margins of supratympanic fold light brown;flanks with faint blackish-brown patches;fore-and hind limbs without cross-bands;dorsal surfaces of posterior half of thigh light brown with buff-coloured reticulations.Ventral surfaces greyish-white with a yellow tinge.Synonymisationof Rao,1937.
We also compared the original description and our new collections of this taxon with all other knownMinervarya
species found in the Western Ghats.Evidence gathered from new morphological and molecular data findsRana (Hylorana)sauriceps
Rao,1937 (=Minervarya sauriceps
) to be conspecific withM.mysorensis
.Most morphological characters (including adult size) were observed to be extremely similar or overlapping between these two currently recognised taxa,barring some minor intraspecific variations.Furthermore,mitochondrial 16S rRNA sequence data from the neotype specimen ofM.sauriceps
nested together with various populations ofM.mysorensis
(Figure 1) with maximum genetic distances of up to 1.0%,which fall within the range of intraspecific distances observed for the variousMinervarya
species in our study (Table 1).Hence,in accordance with the Article 23 (Principle of Priority) of The Code we considerRana (Hylorana) sauriceps
Rao,1937 (=Minervarya sauriceps
) as a junior subjective synonym ofRana(Rana) limnocharis mysorensis
Rao,1922 (=Minervarya mysorensis
).(Kuramoto,Joshy,Kurabayashi,and Sumida,2008“2007”):re-examination of taxonomic status and synonymy.
This taxon was originally described asFejervarya kudremukhensis
from“Kudremukh”based on a male holotype (Figure 3) and three paratypes,including a female specimen (Kuramotoet al
.,2008“2007”).While describing this new species the authors compared and distinguished it mainly from four other known ‘large-sized’members of the genus:M.brevipalmata
,M.keralensis
,M.nilagirica
,andM.mudduraja
.Of these,the last was described as a new species in the same study asM.kudremukhensis
(Kuramotoet al
.,2008“2007”),while for the former three members the authors clearly stated that they did not collect any specimens and solely relied on museum specimens for largely morphometric comparisons.However,the authors did not specifically compare their new taxon withM.mysorensis
,M.parambikulamana
,andM.sauriceps
,assuming them to be smaller-sized species.Our extensive collections of minervaryan frogs from the Western Ghats show that various populations ofM.
‘kudremukhensis
’(including the examined type series) share overlapping size ranges and several other similar morphological characters with these three species.For example,adult size range,dorsal skin with short longitudinal skin folds and an inverted“V”-shaped ridge,snout subelliptical to pointed,tympanum about half of the eye diameter,hind limbs relatively long,relatively reduced foot webbing not extending beyond the second subarticular tubercle on either side of toe IV,mottling on posterior surface of thighs,and most specimens showing the presence of a middorsal vertebral line.Furthermore,since our study reports new collections ofM.mysorensis
,M.parambikulamana
,andM.sauriceps
,based on which their taxonomic identities have been clarified,there remains no doubt thatM.kudremukhensis
is conspecific with all of these taxa,and should hereafter be considered a junior subjective synonym ofM.mysorensis
,based not only on morphological but also conclusive phylogenetic evidence (Figure 1).Hence,in accordance with the Article 23(Principle of Priority) of The Code we considerFejervarya kudremukhensis
Kuramoto,Joshy,Kurabayashi,and Sumida,2008“2007”(=Minervarya kudremukhensis
) as a junior subjective synonym ofRana (Rana) limnocharis mysorensis
(Rao,1922)(=
Minervarya mysorensis
).(Jerdon,1853):taxonomic clarity and range extension.
This taxon was originally described asRana nilagirica
based on specimens from“marshes in the Wynaad and Neelgherries”,with a brief description (Jerdon,1853).However,the original name-bearing type of this nominal species was subsequently reported to be lost (Jerdon,1870).Most subsequent authors considered this taxon either as a subspecies or junior synonym ofFejervarya limnocharis
(e.g.,Annandale,1917;Boulenger,1882,1920).However,Dubois (1984) regarded it as a distinct species under his subgenusFejervarya
and designated a neotype (MNHNP 1984.2340) from“Governor Shola”,Nilgiris,Tamil Nadu,India (Figure 3).Thereafter,the species was treated as a member of the genusFejervarya
sensu lato(e.g.,Iskandar,1998;Feiet al
.,2002;Dineshet al
.,2015),until the recognition of the generic nameMinervarya
(sensu lato) for the predominantly South Asian radiation of what were formerly considered as fejervaryan frogs (Sanchezet al
.,2018).However,despite its largely undoubted status as a distinct species as well as availability of a name-bearing type,Minervarya nilagirica
is known only from the region of its original type locality.Our extensive collections of minervaryan frogs from the Western Ghats results in the following major findings:(1) significant extension of the geographical range ofMinervarya nilagirica
based on morphologically and genetically identified records from the Anaimalai hills (south of Palghat gap) to the hill ranges of Nilgiris,Siruvani,Wayanad,Coorg,and Chikmagalur,including Kempholey ghat,Shiradi ghat,and Kudremukh regions (north of Palghat gap),altogether encompassing the states of Tamil Nadu,Kerala,and Karnataka (Table S1;Figure 2);and (2) clarity on the identity and taxonomic status of two other nominal taxa from the Western Ghats—Rana murthii
Pillai,1979(=
Minervarya murthii
) andFejervarya mudduraja
Kuramoto,Joshy,Kurabayashi,and Sumida 2008“2007”(=Minervarya mudduraja
)—both of which are shown to be junior subjective synonyms ofMinervarya nilagirica
(Jerdon,1853),with formal actions of synonymy undertaken below in the subsequent sections.(Pillai,1979):re-examination of taxonomic status and synonymy.
This taxon was originally described asRana murthii
Pillai,1979 based on three male (including holotype) and two female specimens from“Naduvattom”,in the Nilgiris district of Tamil Nadu state.Pillai (1979) had discussed a single character to compare this species withFejervarya limnocharis
(referring toMinervarya nilagirica
),M.
brevipalmata
,and
M.greenii
,i.e.,“anterior part of lower jaw and two triangular patches on breast beset with small pearl-like papillae”.However,an examination of the male holotype ofM.murthii
does not show“pearl-like papillae”on the breast or the lower jaw (Figure 3).Given that the holotype ofM.murthii
and the neotype ofM.nilagirica
cannot be distinguished based on this character,the distinct status ofM.murthii
becomes doubtful.We instead observed one of the male paratypes ofM.murthii
as having granulations on the chest and lower jaw,which were absent in the holotype and the other male paratype.Hence,the presence of ‘pearl-like papillae’ appears to be a variable character even in reproductively active males.In this context,Kuramotoet al
.’s (2008“2007”) observation of“one exceptional individual”ofM.kudremukhensis
syn.nov.(=M.mysorensis
) having“six faint longitudinal series of small dots on the throat and breast”,suggests that this character could be found variably in other members of the genusMinervarya
.In our study,we also observed discolouration on the chest with sparse granulation in a few breeding males ofM.nilagirica
.Ruling out this character,we do not find any other reliable morphological differences between these two taxa.Although the size of the respective type specimens vary (M.murthii
,holotype,male:SVL 31.1 mm;M.nilagirica
,neotype,male:SVL 43.4 mm),it is considered a sampling disparity as multiple collections from our study show considerable size variation and overlap among specimens from Naduvattam (male:SVL 32–42 mm,N
=5 and female:SVL 34–49 mm,N
=5) and Governor Shola (male:SVL 28–47 mm,N
=4 and female:SVL 38–51 mm,N
=5).In addition to the lack of morphological differentiation,the type localities ofM.murthii
“Naduvattam”andM.nilagirica
“Governor Shola”lie in close proximity within the same geographical region (less than 20 km apart,with similar elevation and habitat),further suggesting these to be continuous populations of the same species.This is also congruent with molecular evidence presented in this study based on 16S mtDNA,which shows negligible genetic distance between these taxa.Hence,in accordance with the Article 23 (Principle of Priority) of The Code we considerRana
murthii
Pillai,1979(=Minervarya murthii
) as a junior subjective synonym ofRana nilagirica
Jerdon,1853 (=Minervarya nilagirica
).(Kuramoto,Joshy,Kurabayashi,and Sumida,2008“2007”):re-examination of taxonomic status and synonymy.
This taxon was originally described asFejervarya mudduraja
Kuramoto,Joshy,Kurabayashi,and Sumida,2008“2007”based on four female specimens,of which the holotype (Figure 3) is from“Talapu,Madikeri”and the paratypes from close vicinities in Kodagu district of southern Karnataka.While recognizing this species,the authors compared and distinguished it mainly from four other known ‘large-sized’ members of the genus:M.brevipalmata
,M.keralensis
,M.nilagirica
,andM.kudremukhensis
.As clarified under the discussion forM.kudremukhensis
,the authors did not collect any specimens of the former three taxa and relied on museum specimens for morphometric comparisons,largely with respect to relative proportions of body parts.For example,M.mudduraja
(females only) was distinguished from the closely relatedM.nilagirica
by longer hind limbs,foot,and hand;fromM.keralensis
by relatively smaller head width,tympanum,and inner metatarsal tubercle;fromM.brevipalmata
also by relatively smaller inner metatarsal tubercle and relatively larger eyelid width compared to inter-orbital distance;and fromM.kudremukhensis
by longer hands,fingers,and toes.Among these species,our further detailed morphological and molecular study revealsM.mudduraja
to be conspecific withM.nilagirica
.Based on examination of seven males and four females from the type locality Madikeri and surrounding regions,along with the holotypes ofM.mudduraja
andM.murthii
,and the neotype ofM.nilagirica
,we found that various quantitative and qualitative characters do not permit a reliable morphological distinction of the three species from one another.In addition,a genealogical comparison of our new 16S mtDNA data from topotypic collections ofM.nilagirica
andM.murthii
from Governor shola and Naduvattam,respectively,with the previously available sequences ofM.mudduraja
originating from the type specimens,shows maximum genetic distances of up to 0.8%,which fall within the range of intraspecific distances observed among populations of the same species in the genusMinervarya
(Table 1).Hence,in accordance with the Article 23 (Principle of Priority) of The Code we considerFejervarya mudduraja
Kuramoto,Joshy,Kurabayashi,and Sumida,2008“2007”(=Minervarya mudduraja
) as a junior subjective synonym ofRana nilagirica
Jerdon,1853 (=Minervarya nilagirica
).(Rao,1920):re-examination of taxonomic status and synonymy.
Another poorly known species,Minervarya modesta
,was originally described asN yctibatrachus sanctipalustris
var.modestus
Rao,1920 based on a holotype from“Jog,Shimoga,Mysore”.New specimens of this species have not been recorded ever since its original description.Bijuet al
.(2011) reviewed this taxon,discussed its taxonomic history,and formally transferred it to the genusFejervarya
(sensu lato) based on examination of the available holotype.In the present study,we collected several individuals of aMinervarya
species from Mavinagundi (near Jog falls)in Uttara Kannada district and Shimoga in Shimoga district of Karnataka state.Although the original description ofNyctibatrachus sanctipalustris
var.modestus
Rao,1920 is brief,ambiguous,and mostly includes a comparison with the originally assigned species,in addition to the holotype (ZSIC 19179) being severally damaged,we found our new collections to largely match with the available name-bearning type and some of the described characters,chiefly with respect to the adult snout-vent size (SVL 23.1 mm) and dorsal skin with“long longitudinal folds on the body and limbs”.Furthermore,our newly generated 16S mtDNA sequence from a topotypic specimen (SDBDU 2017.3677) nests together with various studied populations ofM.sahyadrensis
with negligible to maximum 0.8% intraspecific divergence.Hence,we proposeN yctibatrachus sanctipalustris
var.modestus
Rao,1920 (=Minervarya modesta
) to be considered as a junior subjective synonym ofMinervarya sahyadrensis
(Annandale,1919).(Bahuguna,2018):re-evaluation of generic placement.
Although this study focused onMinervarya
frogs of the Western Ghats,we sampled some populations of species with geographical ranges extending outside the region.During our surveys,we encountered a population morphologically identical toFejervarya jhilmilensis
(=Minervarya jhilmilensis
) (Sanchezet al
.,2018) at Haridwar,in close proximity to its type locality.This species was genetically close to members of the genusFejervarya
rather than members of the genusMinervarya
,with zero to maximum genetic distances of up to 2.5% fromF.
‘orissaensis
’ for the mitochondrial 16S gene sequences.Therefore,the species is removed from the genusMinervarya
and formally placed in the genusFejervarya
.However,we defer the evaluation of the specific taxonomic status ofFejervarya jhilmilensis
for a future study of fejervaryan frogs.3.3.Description of a new species
sp.nov.
Pental’s Minervaryan Frog
(Tables 1–2;Figures 1–2,4)
Etymology:
The species is named after Prof.Deepak Pental,a renowned Indian Plant Genetist and former Vice Chancellor of University of Delhi,in appreciation of his contributions to science.We also acknowledge his support and encouragement in setting-up of the Systematics Lab at Department of Environmental Studies,University of Delhi.The species epithetpentali
is treated as a noun in the genitive case.Holotype:
BNHS 6115,an adult male,from Nedumbassery,Cochin (10°09’42.5”N 76°23’22.4”E,11 m asl),Ernakulam district,Kerala state,India,collected by S.D.Biju and Sonali Garg on 29 July 2014.Paratypes:
BNHS 6116–BNHS 6119,four adult males,from Nedumbassery,Cochin (10°09’42.5”N 76°23’22.4”E,11 m asl),Ernakulam district,collected along with the holotype.Referred specimen:
SDBDU 2017.3628,an adult female from Kuriyode,Kollam district,Kerala state,collected by Sonali Gargand S.D.Biju on 1 August 2017.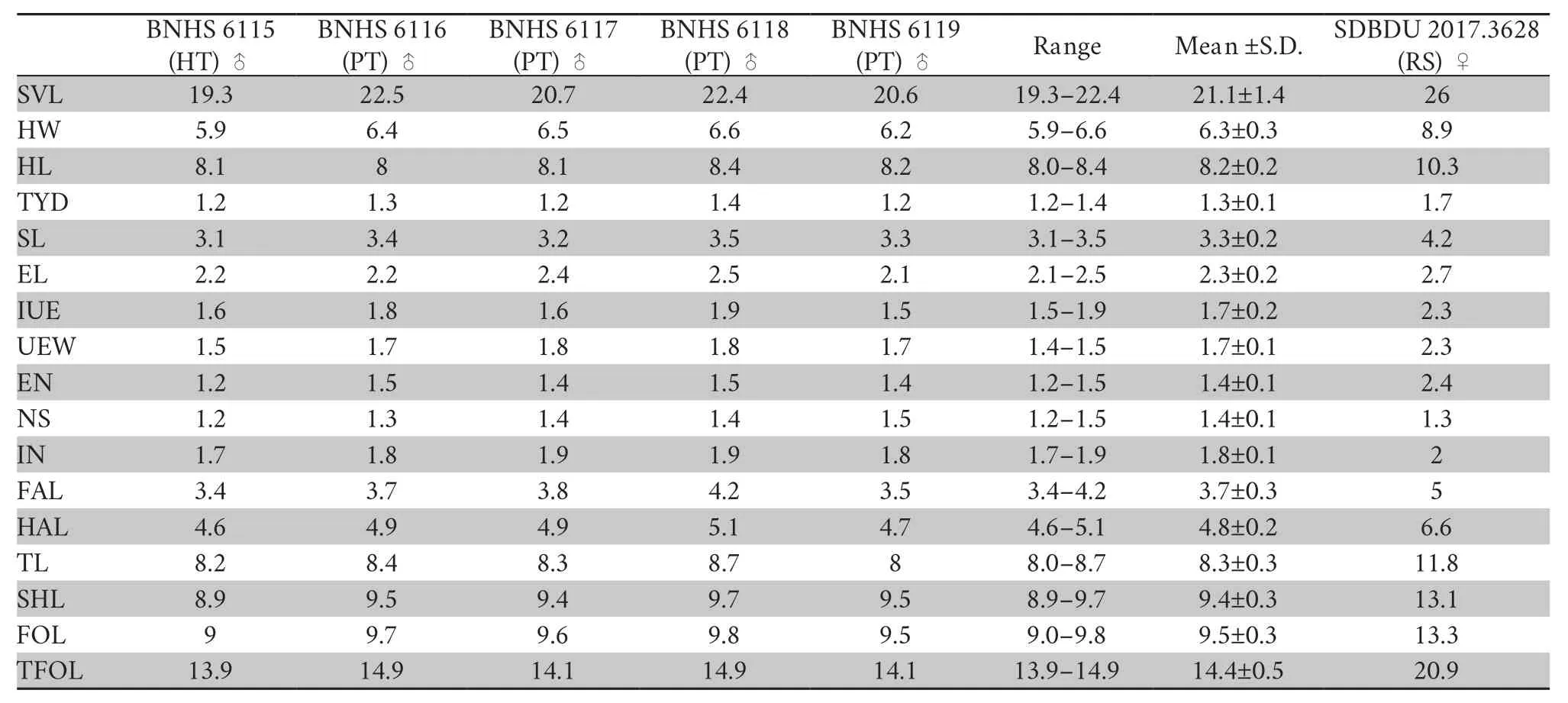
Table 2 Morphometric measurements for the type series and referred specimen of Minervarya pentali sp.nov. (in mm).
Description of holotype:
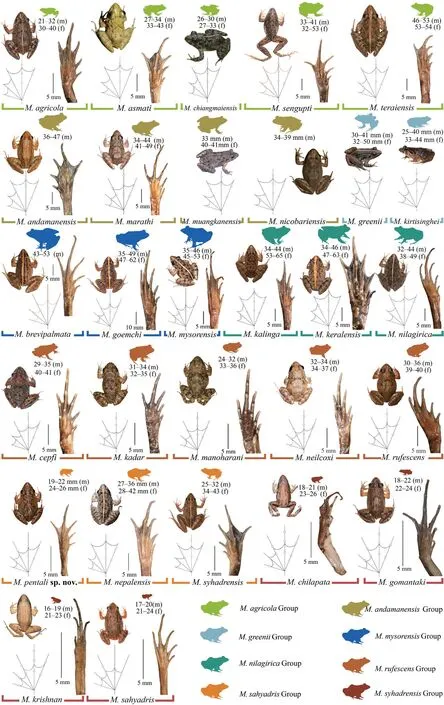
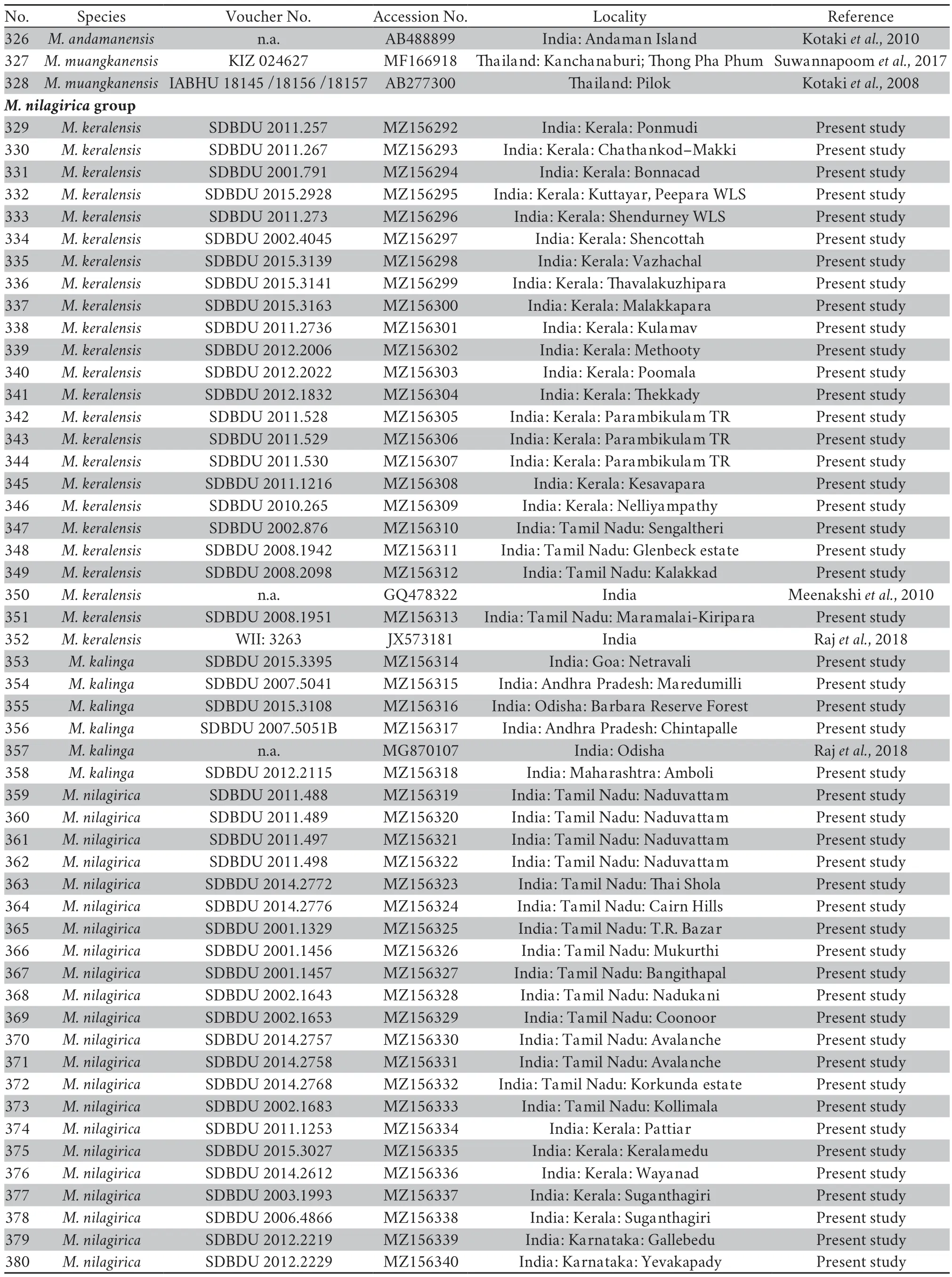
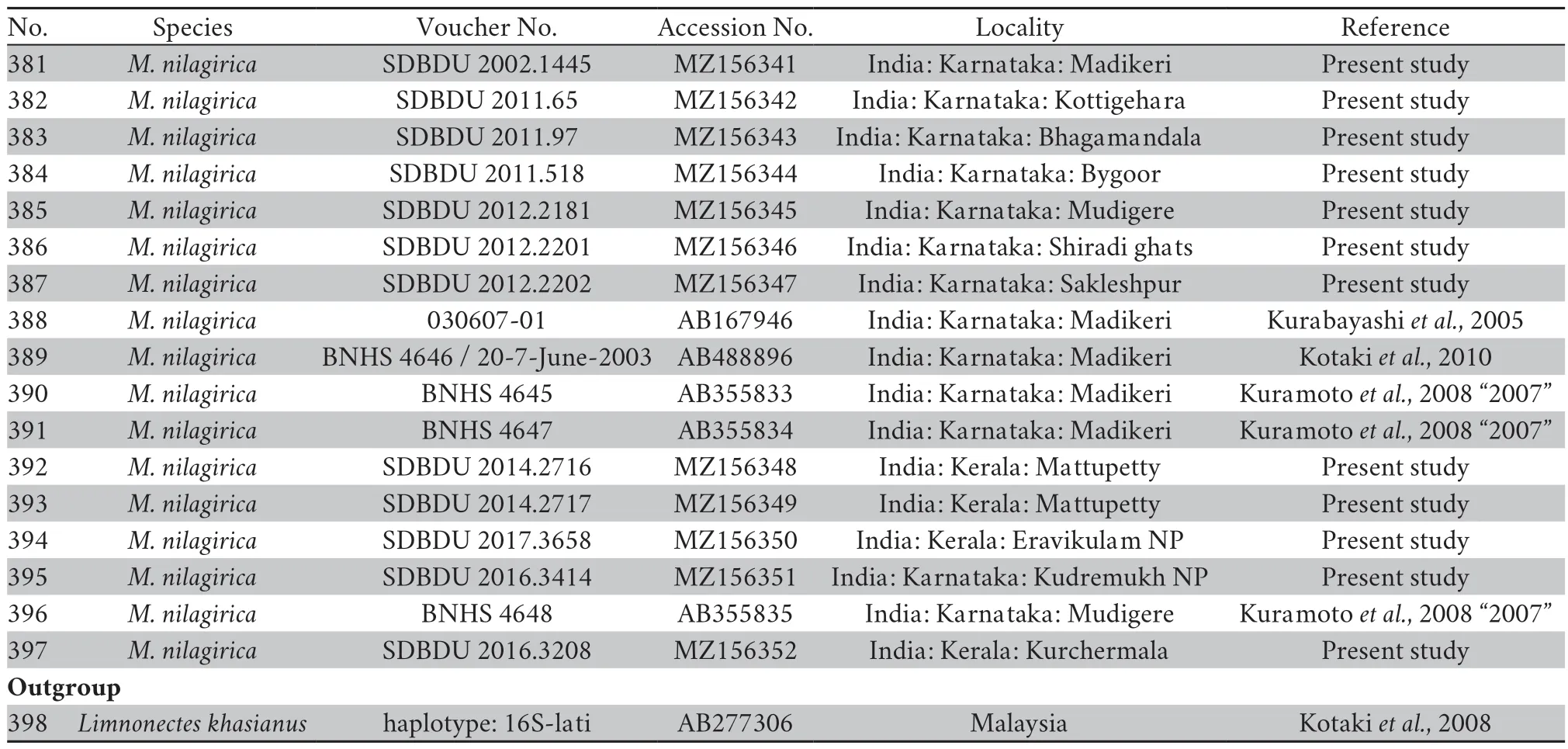
A small-sized adult male (SVL 19.3 mm),body rather slender;head longer (HL 8.1 mm) than wide(HW 5.9 mm;MN 6.2 mm;MFE 5.0 mm;MBE 3.1 mm);snout shape sub-elliptical in dorsal view,rounded in lateral view,slightly protruding,snout length (SL 3.1 mm) longer than horizontal diameter of eye (EL 2.2 mm);loreal region acute,rounded canthus rostralis;interorbital space flat,nearly equal to(IUE 1.6 mm) upper eyelid width (UEW 1.5 mm) and sub-equal to internarial distance (IN 1.7 mm);nostril oval,as close to the tip of snout (NS 1.2 mm) as to the eye (EN 1.2 mm);tympanum(TYD 1.2 mm) 54.5% of eye diameter (EL 2.2 mm);tympanum to eye distance (TYE 0.7 mm) 58.3% of the tympanum diameter;pineal ocellus present;supratympanic fold well developed,extends from posterior corner of the eye to near the shoulder;vomerine ridge present,bearing small teeth,at an angle of 45°to the body axis,as close to choanae as to each other;tongue moderately long,emarginated (Figure 4).Arms short,forearm length (FAL 3.4 mm) shorter than hand length (HAL 4.6 mm);fingers rather long,relative length of fingers II Hind limbs short,thigh (TL 8.2 mm) shorter than shank(SHL 8.9 mm) and foot (FOL 9.0 mm),distance from the base of tarsus to the tip of toe IV (TFOL 13.9 mm);toes long,relative length of toes I Skin of dorsum shagreened with scattered glandular projections and short longitudinal glandular folds;an interrupted inverse V-shaped ridge at the centre of dorsum;snout shagreened,upper eyelids sparsely tuberculate;upper and lower parts of flank shagreened with a few granular projections.Dorsal surfaces of forelimb,thigh,and shank shagreened with sparsely glandular projections.Ventral surfaces of throat,chest,belly,and limbs smooth;and posterior parts of thigh and region surrounding the vent sparsely granular;fejervaryan lines present on both sides of the belly;rictal glands present,just behind the labial commissure of the mouth (Figure 4). Colour in life Colour in preservation (alcohol) Figure 4 (A–G) Holotype of Minervarya pentali sp.nov. in preservation showing (A) dorsal view;(B) ventral view;(C) lateral view of head;(D) ventral view of hand;(E) ventral view of foot;(F) schematic illustration of foot webbing;(G) Size (SVL 19.3 mm) in comparison to the Indian five-rupee coin (23 mm diameter);(H–O) Minervarya pentali sp.nov. in life;(H–K) holotype showing (H) dorsolateral view;(I) dorsal view;(J) posterior view of thighs;(K) lateral view of groin;(L) paratype showing dorsolateral view;(M) paratype showing dorsolateral view;(N) paratype showing dorsolateral view;(O) paratype showing dorsolateral view;(P–S) male advertisement calls of Minervarya pentali sp.nov. showing (P) 10 second call segment with a single call type;(Q) 1 second call segment;(R) 0.2 second call segment;(S) spectrogram of the 0.2 second call segment shown in Figure 4R. Morphological variation: In preservation Secondary sexual characters: Male Female N Morphological diagnosis: Minervarya pentali sp.nov. N N N N N N N Morphological comparison: Minervarya pentali sp.nov. Minervarya vs. Minervarya sahyadris M.chilapata M.gomantaki M.krishnan M.sahyadris Minervarya sahyadris vs. vs. vs. Minervarya syhadrensis M.syhadrensis Minervarya pentali sp.nov. N N vs. N N vs. N N vs. N N vs. vs. Minervarya pentali M.nepalensis N N vs. N N vs. Minervarya pentali sp.nov. M.agricola vs. vs. M.andamanensis vs. vs. vs. M.greenii vs. vs. M.mysorensis vs. vs. M.nilagirica vs. vs. vs. M.rufescens vs. vs. vs. vs. vs. Minervarya pentali sp.nov. M.marathi M.andamanensis N N vs. N N Phylogenetic relationship: Minervarya pentali sp.nov. Minervarya syhadrensis M.nepalensis M.syhadrensis Vocalisation: Minervarya pentali sp.nov. Distribution and natural history: Minervarya pentali sp.nov. 3.4.Grouping of species in the genus Minervarya group Members included: Minervar ya agricola M.asmati,M.chiangmaiensis M.teraiensis M.sengupti Morphological definition: Phylogenetic relationship: Minervarya agricola Minervarya sahyadris Minervarya syhadrensis Distribution: group Members included: Minervar ya andamanensis M.muangkanensis M.marathi M. nicobariensis Morphological definition: Phylogenetic relationship: Minervarya andamanensis Minervarya rufescens M.marathi Distribution: group Members included: Minervarya greenii M.kirtisinghei Morphological definition: Phylogenetic relationship: et al et al et al et al et al et al et al Distribution: group Figure 5 Morphological characters (clock-wise:dorsal skin texture;ventral surface of foot showing the extent of webbing,and inner and our metatarsal tubercles;and schematic illustration of foot webbing) for twenty-nine recognised Minervarya species,arranged in eight species groups identified in the study.Frog silhouettes for each species indicate the species group by colour and the proportionate adult snout-vent size (SVL) in millimeters. Figure 6 Twenty-nine recognised Minervarya species depicted in life.Species are arranged in eight species groups identified in the study. Members included: Minervarya brevipalmata,M.goemchi M.mysorensis Morphological definition: Phylogenetic relationship: Minervarya mysorensis Distribution: group Members included: Minervarya kalinga,M.keralensis M.nilagirica Morphological definition: M.nilagirica M.nilagirica M.kalinga M.keralensis Phylogenetic relationship: Minervarya nilagirica Minervarya Distribution: group Members included: Minervarya cepfi M.kadar M.manoharani M.neilcoxi M.rufescens Morphological definition: Phylogenetic relationship: Minervarya rufescens M.marathi Minervarya andamanensis et al et al Distribution: group Members included: Minervarya pentali sp.nov., M.nepalensis M.syhadrensis Morphological definition: Phylogenetic relationship: Minervarya syhadrensis Minervarya agricola Minervarya sahyadris Distribution: group Members included: Minervarya chilapata M.gomantaki M.krishnan M.sahyadris Morphological definition: Phylogenetic relationship: Minervarya sahyadris Minervarya agricola Minervarya syhadrensis Distribution: Minervarya Minervarya et al Minervarya Fejervarya Sphaerotheca Fejervarya Minervarya Fejervarya et al et al Minervarya jhilmilensis Fejervarya et al Minervarya sahyadris Minervarya agricola Minervarya sahyadris Minervarya agricola Minervarya agricola Minervarya M.chiangmaiensis M.muangkanensis M.greenii M.kirtisinghei Appendix Table S1 Details of the mitochondrial 16S rRNA genetic samples included in the study.The list is arranged by the order of terminal nodes in the phylogenetic tree shown in Figure 1. Continued Table S1 Continued Table S1 Continued Table S1 Continued Table S1 Continued Table S1 Continued Table S1 Continued Table S1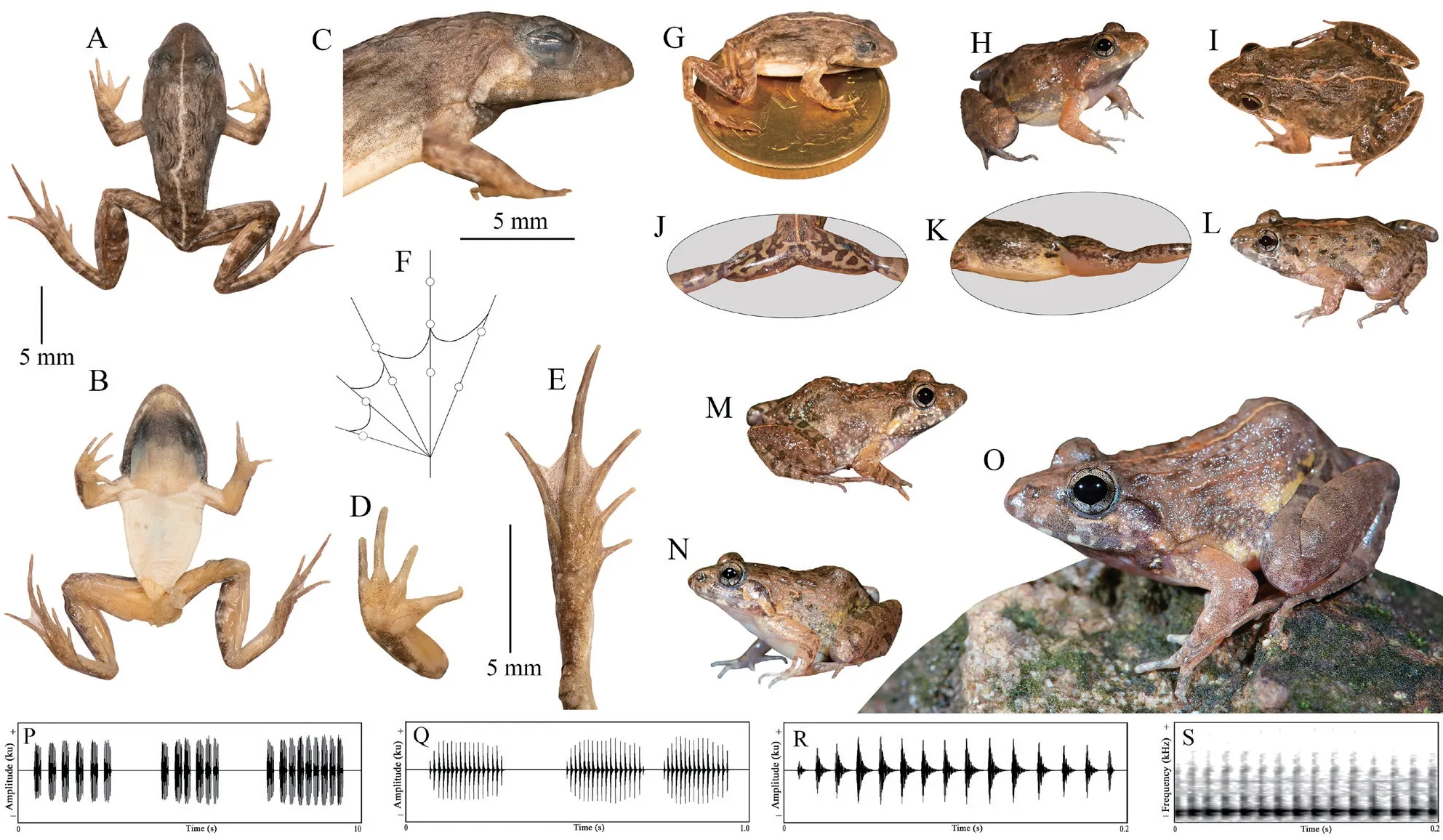

4.Discussion
Frogs of the genus


 Asian Herpetological Research2021年4期
Asian Herpetological Research2021年4期
