Effect of surface functionalization on the surface and interfacial properties of thermoplastic-coated carbon fibers
SU Ya-nan, JING De-qi, ZHANG Xing-hua, ZHANG Shou-chun,*
(1. National Engineering Laboratory for Carbon Fiber technology, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, China;2. Center of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences, Beijing 100049, China;3. Shanxi Engineering Research Center of Advanced Thermoplastic Composites, Chinese Academy of Sciences, Taiyuan 030001, China)
Abstract: Hydroxyl- and amino- functionalized carbon fibers (CF―OH and CF―NH2) were prepared by surface oxidation with an acid mixture (H2SO4∶HNO3 of 3∶1 v/v) followed by grafting with ethylenediamine. The functionalized CFs were sized with a sulfonated poly (ether ether ketone) (SPEEK) sizing agent to prepare CF―OH―SPEEK and CF―NH2―SPEEK materials. The effect of surface functionalization on the surface properties of the CFs and their interfacial properties in PEEK matrix composites were investigated. Results showed that the content of polar functional groups and wettability of the CFs increased significantly after surface functionalization. Chemical reactions between the modified CFs and the sizing agent, improved the interfacial adhesion between them. The interfacial shear strengths of CF―OH―SPEEK and CF―NH2―SPEEK reinforced PEEK matrix composites were increased by 6.2% and 14.0%, respectively, compared with that of the composites reinforced with desized-SPEEK CFs. The surface functionalization helps improve the interfacial adhesion of thermoplastic-coated CF/PEEK composites.
Key words: Carbon fiber;Poly (ether ether ketone);Surface functionalization;Surface properties;Interfacial shear strength
1 Introduction
Carbon fiber reinforced polymer composites(CFRPs) have been widely used in aviation,aerospace, and wind turbine blade owing to their low density, high strength and high modulus[1–3]. Among these CFRPs, the carbon fiber reinforced poly (ether ether ketone) (CF/PEEK) composite is expected to be used as structural components in aerospace owing to their good impact resistance, high toughness and easy recyclability. However, the interfacial adhesion between CFs and PEEK was affected by the inert surface of untreated CFs and inactive molecular chain of PEEK[4]. Therefore, it is necessary to modify the surface of CFs to further improve the performance of CF/PEEK composites and broaden their application field.
Many methods have been applied to modify the surface of CFs, including surface oxidation[5–6],plasma treatment[7–8], chemical vapor deposition[9–10]and sizing treatment[11]. Among them, sizing treatment can protect the CFs surface during preparation processing. Also, the interfacial compatibility between CFs and matrix is improved after sizing treatment.Thus, sizing treatment becomes the most widely used method.
After the sizing treatment, the effective load transfer of composites depends on the existence of interface layer between CFs and matrix. The interface layer consists of two parts: one is the interface between the CFs and the sizing layer, and the other is the interface between the sizing layer and the matrix.For CF/PEEK, the sizing agent mainly improves the interfacial adhesion between sized CFs and matrix by improving the interfacial compatibility, due to the inactive molecular chain of PEEK. However, there are three kinds of interactions between CFs and the sizing layer: chemical bonding, hydrogen bonding and mechanical interlocking, among which chemical bonding is the strongest interaction. Therefore, it is important to improve the chemical bonding between CFs and the sizing layer, which is beneficial to increasing the interfacial adhesion of composites. Zhang et al. used the polyurethane (PU) sizing agent to size the oxidized CFs and the functional groups on oxidized CFs could react with the PU sizing layer[12]. Yao et al. found that the interfacial shear strength (IFSS)between oxidized sizing CFs (CFox-Uni) and polycarbonate (PC) increased by 16.5% compared with the unmodified sizing CF (CFde-Uni)[13]. The increased IFSS is ascribed to the chemical interaction between the functional groups on the surface of CFoxand the sizing agent during the hot-pressing process. These results confirm that the surface functionalization of CFs is beneficial to improve the overall effect of thermoplastic sizing agent. Some methods have been proposed for the surface functionalization of CFs, including surface oxidation, plasma treatment, and chemical vapor deposition and so on. Meng et al. found that the polar functional groups and surface wettability of CFs were significantly improved after surface oxidation with KClO3/H2SO4system[14]. Wang et al. introduced sodium sulfonate group on CFs surface by diazotization grafting and the content of functional groups on the CFs surface increased significantly[15]. After diazotization grafting, there appeared cation-π bond interaction between CFs and sizing agent, which provide the good interfacial adhesion between CFs and polymer matrix. Sharma et al. also found that the content of polar functional groups on the CFs surface was significantly increased after plasma treatment[16].
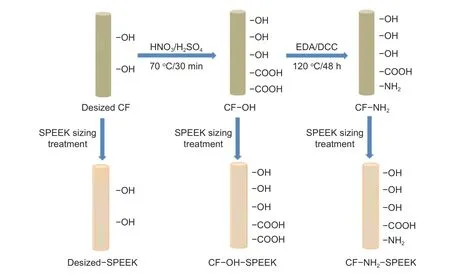
Fig. 1 The process of surface functionalization and sizing treatment.
Among these surface functionalization methods,surface oxidation and grafting are simple and controllable, and it could also avoid adverse effects on the performance of CFs. Therefore, in this work, mixed acid oxidation and ethylenediamine grafting were used to functionalize the CFs, then the functionalized CFs were sized with the sulfonated poly (ether ether ketone) (SPEEK) sizing agent. Finally, the effect of surface functionalization on the surface and the interfacial properties of thermoplastic coated CFs were investigated.
2 Experimental
2.1 Materials
The desized CFs used in this work are T700(12 K, Toray, Japan). PEEK powder was purchased from Victrex (UK). Ethylenediamine (EDA, 99.9%),sulfuric acid (H2SO4, 95%−98%), nitric acid (HNO3,65%−68%) and ethanol were supplied by Sinopharm Chemical Reagent Co., Ltd (China). N, N '- dicyclohexylcarbodiimide (DCC, 99.0%) was obtained from Aladdin Chemical Reagent Co., Ltd. (China). Deionized water was made in laboratory.
2.2 Surface functionalization of CFs
The process of surface functionalization and sizing treatment of CFs are shown in Fig. 1. The preparation process is as follows. First, the desized CFs were surface oxidized by a mixture of H2SO4and HNO3(3∶1v/v) at 70 °C for 30 min. After oxidization, the CFs were washed with deionized water and dried,which was donated as CF−OH. Then, CF−OH was added into the mixture of EDA and DCC and reacted at 120 °C for 48 h. After that, the CFs were washed with ethanol and deionized water, and then dried, which was donated as CF−NH2. Finally, the desized CFs,CF−OH and CF−NH2were sized with the SPEEK sizing agent with a spraying method. After sizing treatment, these CFs were donated as desized−SPEEK,CF−OH−SPEEK and CF−NH2−SPEEK, respectively.
2.3 Characterization
The morphologies of CFs after functionalization and sizing treatment were characterized by the field emission scanning electron microscopy (FE-SEM,JSM-7001F, JEOL, Japan) at an accelerating voltage of 5 kV. The surface roughness (Sa) of the CFs was analyzed by atomic force microscopy (AFM, NTMDT Prima, Bruker, Germany) with a non-contact mode in the scanning range of 3 μm × 3 μm. Three different areas on the CF surface were randomly selected for testing with a scanning rate of 1 Hz. The surface chemical composition of CFs was analyzed by X-ray photoelectron spectroscopy (XPS, AXIS ULTRA DLD, Japan) with an Al Kα X-ray source. The degree of sulfonation (DS) of the SPEEK was characterized by1H NMR (AVANCE III, Bruker, Germany),in which DMSO-d6was used as the test solvent.
The dynamic contact angle was measured by a dynamic contact angle instrument (k100, Krüss, Germany), as shown in Fig. 2. The preparation process of testing samples is as follows. Four fibers were randomly selected and fixed on the frame with an equal spacing. During the test, four fibers are controlled by a software to enter the test liquid at the same time,with an impregnation speed of 0.05 mm/s and an impregnation depth of 4.8 mm. The dynamic contact angle of CFs in deionized water (DI) and diiodiomethane (CH2I2) were measured by the Owens-Wendt method[17]. The polar component (γp) and dispersion component (γd) of CFs were calculated by Eq. (1) and(2), and the surface energy (γ) of CFs was calculated by Eq. (3). Theγ,γpandγdof the two test liquids are shown in Table 1.
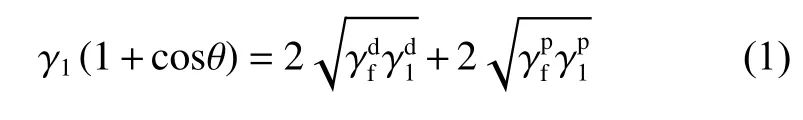
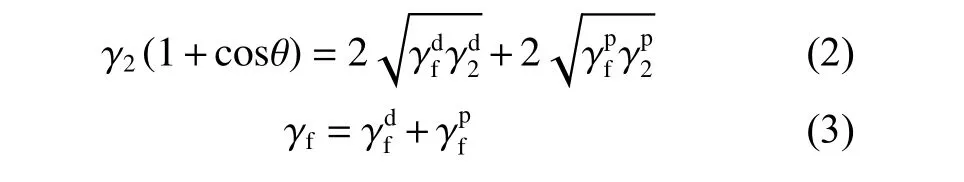
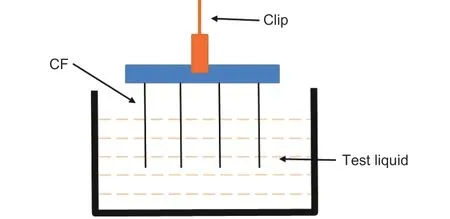
Fig. 2 The schematic diagram of measuring dynamic contact angle.
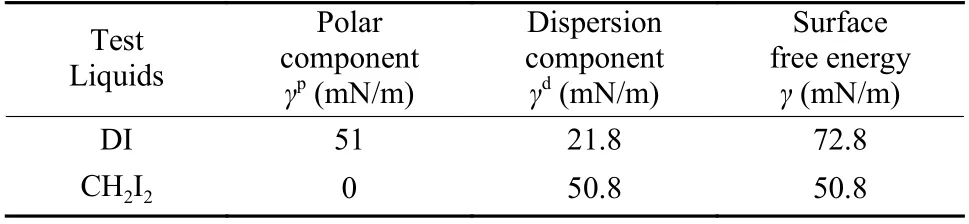
Table 1 The surface energy and its components of test liquids.
The tensile properties of CFs were analyzed by a single fiber stretching machine (YG001E, China) with a tensile rate of 2 mm/min. The preparation process of sample is shown in Fig. 3. At least 30 effective data were selected in this work.
The statistical distribution of CF tensile strength is usually analyzed by the Weibull theory as[18]Eq.(4):
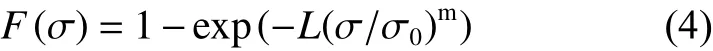
Eq.(5) was obtained by transforming Eq. (4),then the tensile strength data plotted in Eq. (5) were linearly fitted:
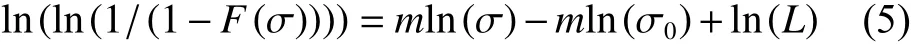
whereσis the tensile strength of CF,F(σ) is the cumulative distribution function of fracture strength,mis Weibull modulus, andσ0is the location parameter.
The interfacial shear strength (IFSS) of CF/PEEK was measured by micro-drop debonding test and the scheme is shown in Fig. 4. The single fiber was fixed on the frame, and the prepared PEEK dispersion was dropped on the single fiber to form micro-droplets. The resulting sample was melted at 380 °C for 15 min. The test was applied on an interfacial strength evaluation instrument (MODEL HM410, Japan) with a speed of 0.12 mm/min, and the IFSS of composites was calculated using Eq. (6):
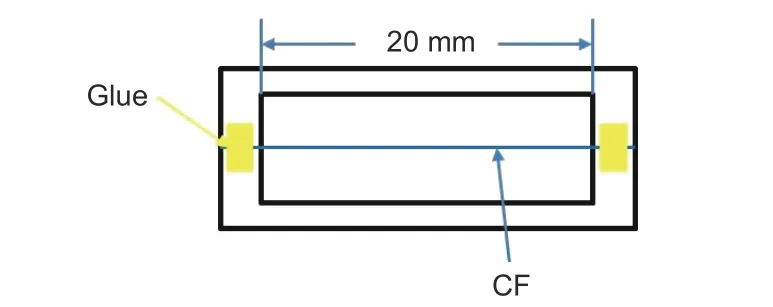
Fig. 3 The schematic diagram of monofilament tensile testing specimen.
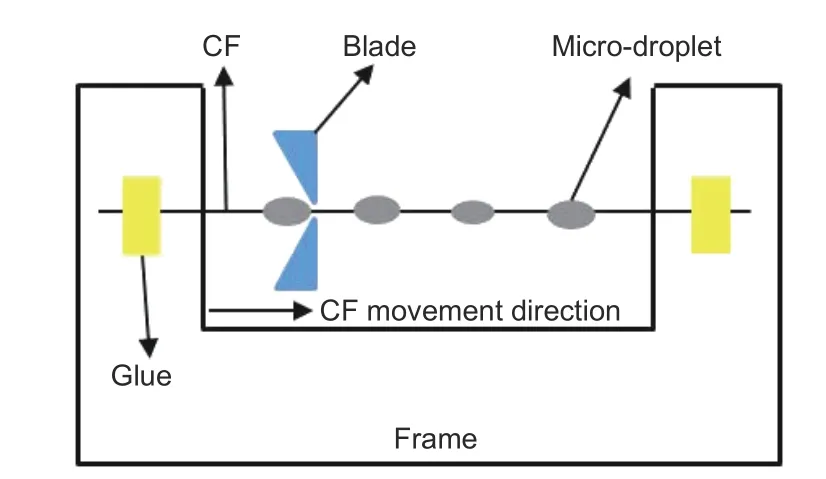
Fig. 4 The schematic diagram of micro-droplet debonding testing specimen.
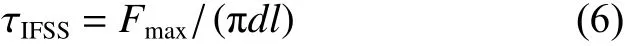
WhereFmaxis the maximum debonding force,dis the diameter of the CF, andlis the embedding length of CF by the PEEK droplet. At least 40 effective data was used in this work.
3 Results and discussion
The surface morphologies of desized CF,CF−OH, CF−NH2and the three types of CFs after SPEEK sizing treatment are shown in Fig. 5. The desized CFs (Fig. 5a) present a smooth surface with shallow grooves, which is the typical feature of CFs obtained from dry-jet wet spinning process. After the sizing treatment, the shallow grooves on the surface of desized-SPEEK are covered by a sizing layer (Fig. 5b).Compared with desized CF, the shallow grooves on CF−OH (Fig. 5c) and CF−NH2(Fig. 5e) are deepened due to the surface functionalization treatment.However, it is worth noting that the deepening degree is relatively small, indicating that surface functionalization treatment does not seriously etch the CFs. After the SPEEK sizing treatment, the groove depth on the surface of CF−OH−SPEEK (Fig. 5d) and CF−NH2−SPEEK (Fig. 5f) becomes shallower, as the SPEEK sizing agent covers the surface of CFs.
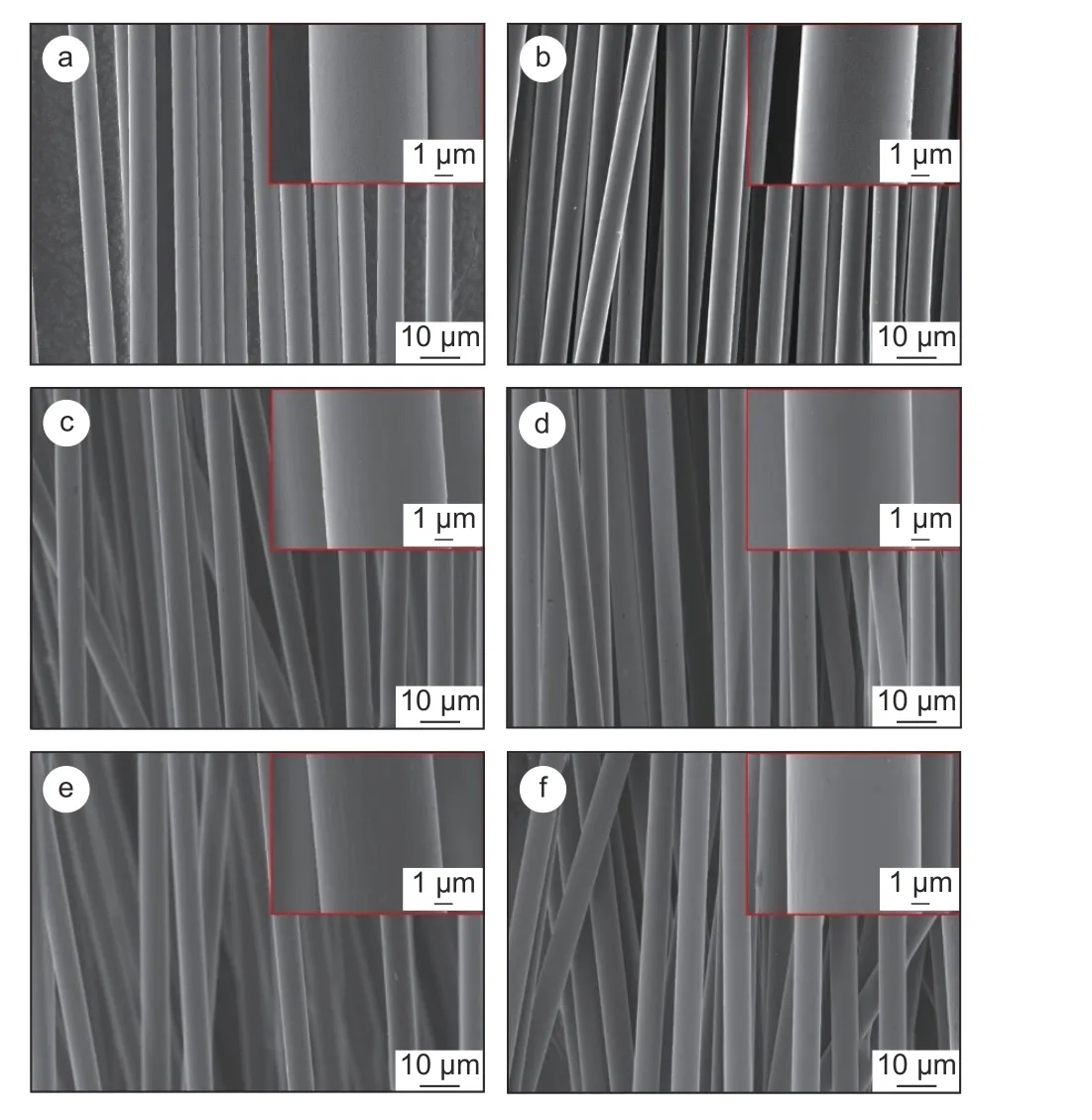
Fig. 5 Scanning electron microscope images of CFs: (a) desized CF, (b)desized−SPEEK, (c) CF−OH, (d) CF−OH−SPEEK, (e) CF−NH2 and(f) CF−NH2−SPEEK.
The surface roughness (Sa) of CFs was characterized by AFM and the results are shown in Fig. 6 and Table 2. As shown in Fig. 6, the surface of desized CF has the shallow groove morphology with theSaof 5.6 nm. After the sizing treatment, the shallow grooves on the surface of desized CF are covered by a SPEEK sizing layer. However, there are some uneven morphology due to the poor movement ability of SPEEK molecules. Thus, theSaof desized−SPEEK increases to 12.7 nm. For CF−OH and CF−NH2, the depth of shallow groove on the CF surface is deepened after surface functionalization, thus leading to the increase of theSato 23.4 and 27.2 nm, respectively. After sizing treatment, the surfaces of CF−OH−SPEEK and CF−NH2−SPEEK are coated with the SPEEK sizing layer, which would cover the grooves on the surface of CF. Besides, the uneven morphology of the sizing layer leads to the increase ofSa. Thus, combining these two factors, theSaof CF−OH−SPEEK and CF−NH2−SPEEK are 18.3 and 21.7 nm, respectively. The increasedSawould be conducive to the contact between CFs and matrix, and eventually beneficial to improve the interfacial adhesion of CF/PEEK composites.
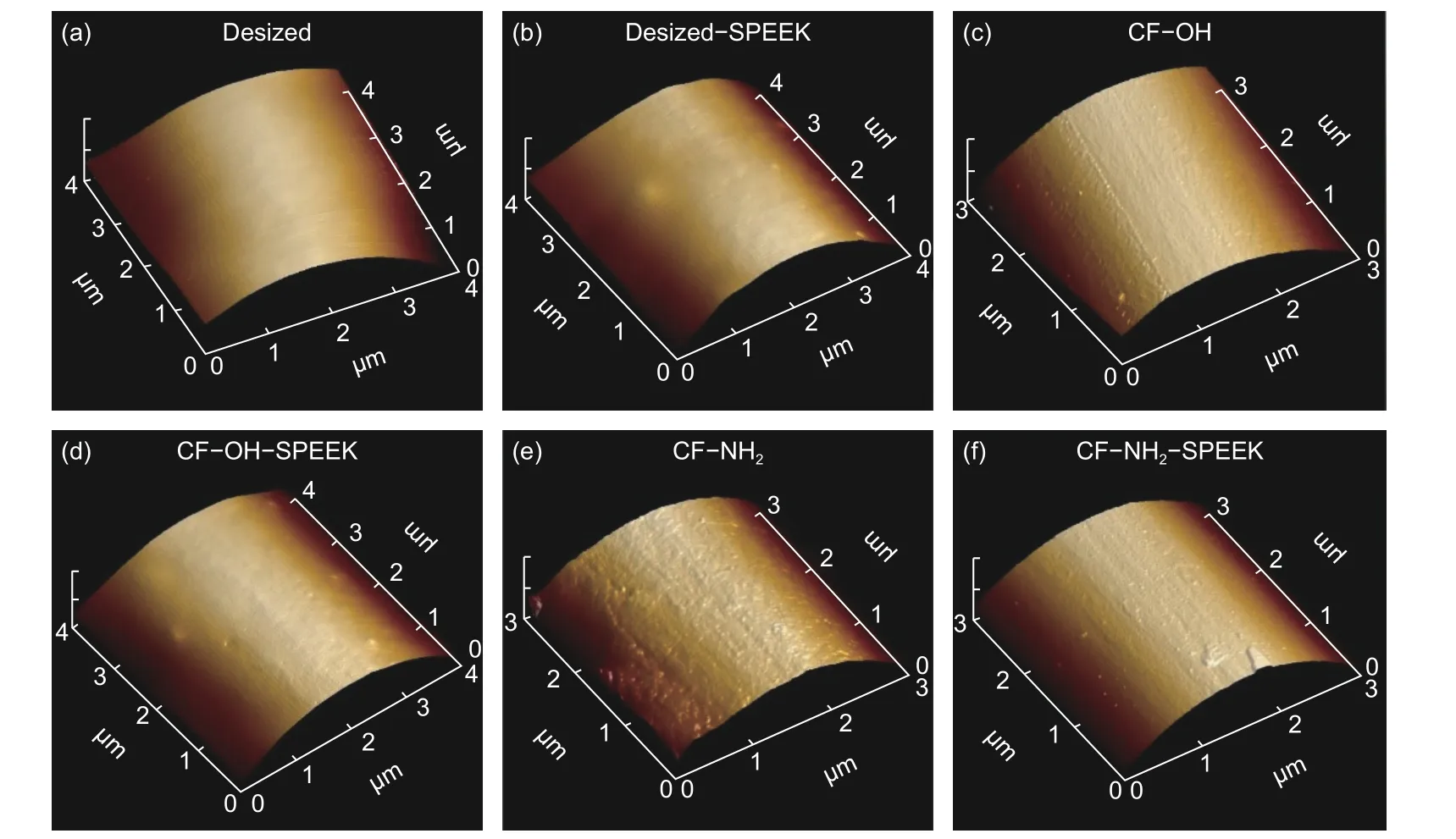
Fig. 6 AFM images of CFs: (a) desized CF, (b) desized−SPEEK, (c) CF−OH, (d) CF−OH-SPEEK, (e) CF−NH2 and (f) CF−NH2−SPEEK.
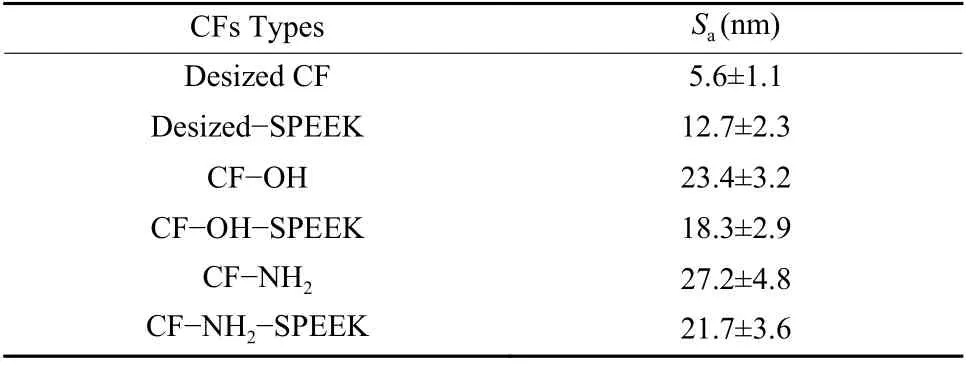
Table 2 Surface roughness of CFs.
XPS was applied to obtain the surface chemical compositions of CFs after the surface functionalization and sizing treatment. As can be seen from Fig. 7 and Table 3, the O content on the surface of CF−OH increases significantly with an increase of O/C ratio from 15.17% on the surface of the desized CF to 32.43% on the surface of CF−OH. After amino treatment, N element is introduced into the surface of CF−NH2, and the N content on CF−NH2surface increases significantly, whereas the content of O decreases a lot and the O/C ratio decreases from 32.43%in CF−OH to 28.13% in CF−NH2. After the SPEEK sizing treatment, the characteristic peaks of S appears on the surface of desized−SPEEK, CF−OH−SPEEK and CF−NH2−SPEEK, indicating that the SPEEK sizing agent is successfully coated on the surface of the three fibers.
The C1s spectra of these CFs can be fitted as follows: C—C (284.6 eV), C—N (285.5 eV), C—O(286.3 eV), C=O (287.3 eV) and O=C—O (288.9 eV).The results are shown in Fig. 8 and Table 4. Compared with the desized CF, the contents of C—O and O=C—O on the surface of CF—OH increase a lot,and the content of C—N on the surface of CF—NH2also increases. The contents of polar functional groups on the surface of CF—OH and CF—NH2increase from 22.10% to 32.94% and 33.22%, respectively,compared with the desized CF. After SPEEK sizing treatment, the C—S (286.8 eV) appears on the surface of desized−SPEEK, CF—OH—SPEEK and CF—NH2—SPEEK due to the introduction of the—SO3H group in SPEEK[19,20]. According to the XPS results, it can be seen that the surface functionalization significantly increases the polar functional group contents of CFs, which is conducive to the improvement of surface wettability of CFs and the chemical interaction between the CFs and the sizing layer.
The structure of SPEEK, a thermoplastic sizing agent used in this paper, is shown in Fig. 9. The—SO3H in SPEEK could react with —OH and —NH2on the surface of CFs. Therefore, to confirm the existence of this chemical reaction between the sizing layer and CFs, the1H NMR was applied to analyze the degree of sulfonation (DS) of soluble sizing agent on the CFs surface. As shown in Fig. 10, the appearance of the characteristic peak at around 7.51×10−6, corresponding to the Heproton due to the introduction of—SO3H, indicates that SPEEK sizing agent has been coated on the surface of CFs. The peaks of Haand Ha’,Hband Hb', and Hcand Hdare around 7.09×10−6and 7.00×10−6, 7.72×10−6and 7.80×10−6, and 7.03×10−6and 7.14×10−6, respectively. The DS of SPEEK used in this work is 73%, whereas the DS of soluble SPEEK in desized-SPEEK is only 38%. This result indicates that some —SO3H in SPEEK react with —OH and —NH2. The DS of soluble SPEEK on the surface of CF—OH—SPEEK and CF—NH2—SPEEK are 35% and 32%, respectively, which are lower than that of desized—SPEEK. The decrease of DS may be due to the increase of —OH, —NH2and other polar functional groups after surface functionalization which can react with —SO3H of SPEEK. Therefore, the chemical bonding degree between the sizing layer and CFs can be improved after surface functionalization.
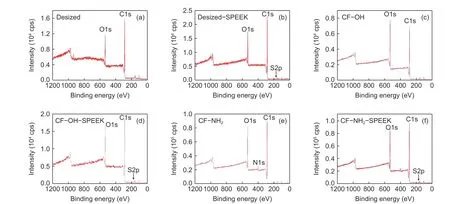
Fig. 7 XPS wide-scan spectra of CF surface: (a) desized CF, (b) desized−SPEEK, (c) CF−OH, (d) CF−OH−SPEEK, (e) CF−NH2 and (f) CF−NH2−SPEEK.
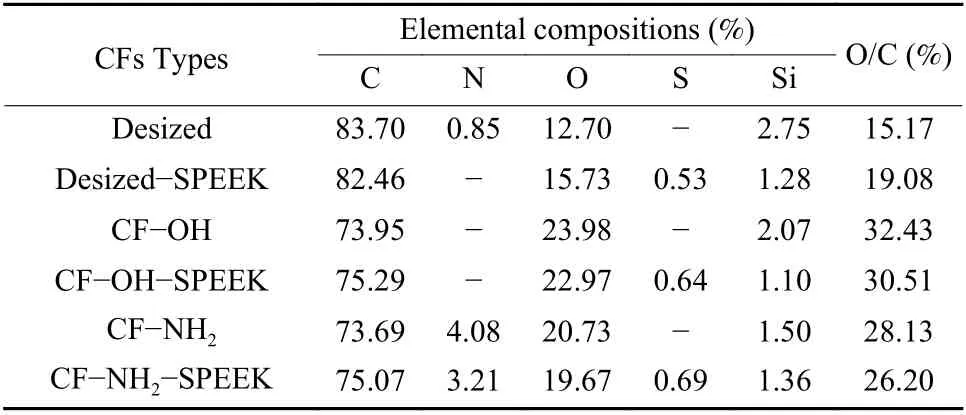
Table 3 Surface elemental compositions of CFs.
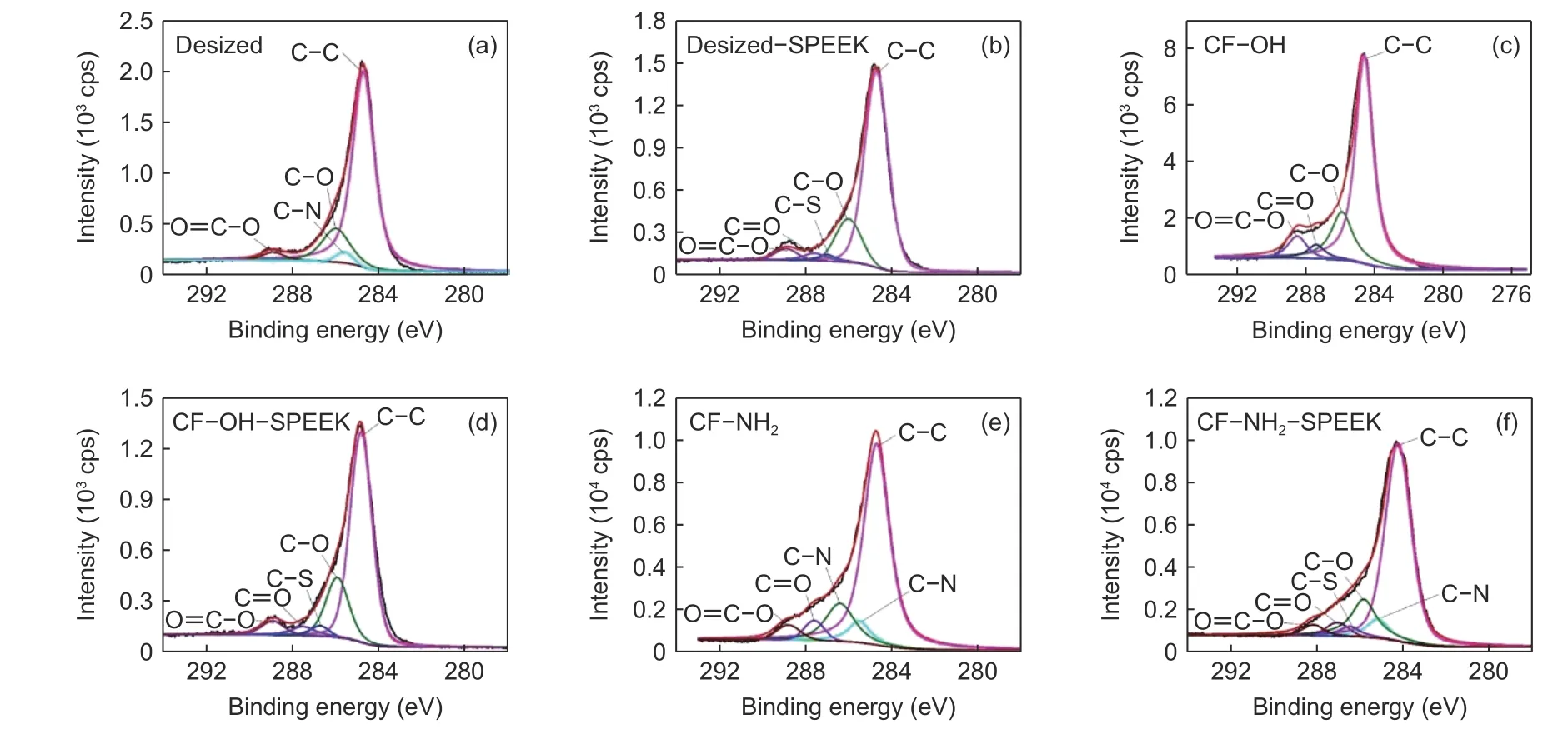
Fig. 8 XPS wide-scan spectra of CFs surface: (a) desized CF, (b) desized −SPEEK, (c) CF−OH, (d) CF−OH−SPEEK, (e) CF−NH2 and (f) CF−NH2−SPEEK.
Table 5 shows the dynamic contact angle resultsof several CFs. As listed in Table 5, the dynamic contact angles of desized CF in DI and CH2I2are 72.99°and 52.81°, respectively. After sizing treatment, the dynamic contact angles of desized-SPEEK in DI and CH2I2decrease to 59.28° and 36.66°, respectively.Compared with the desized CF, the contact angles of CF−OH and CF−NH2in DI and CH2I2decrease to 48.82° and 48.11°, and to 24.31° and 23.62°, respectively. The contact angles of CF−OH−SPEEK and CF−NH2−SPEEK in DI are 52.74° and 51.15° and in CH2I2are 29.22° and 27.15°, respectively.
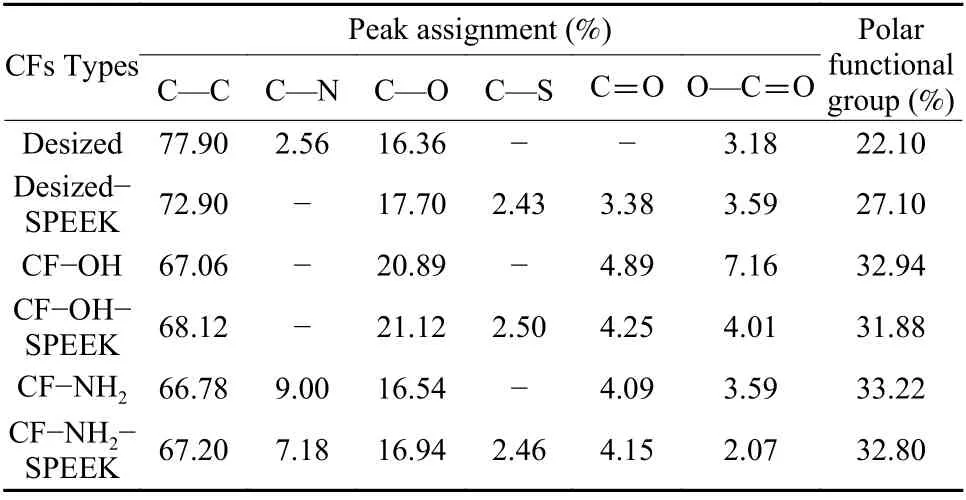
Table 4 The fitting results of C1s curves.
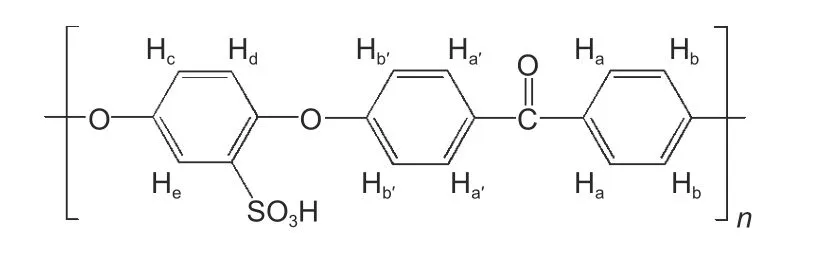
Fig. 9 The structure of SPEEK.
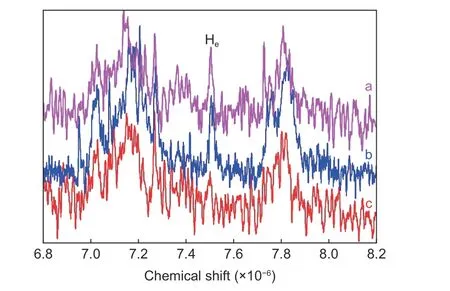
Fig. 10 1H NMR spectra of CFs: (a) desized −SPEEK, (b)CF−OH−SPEEK and (c) CF−NH2−SPEEK.
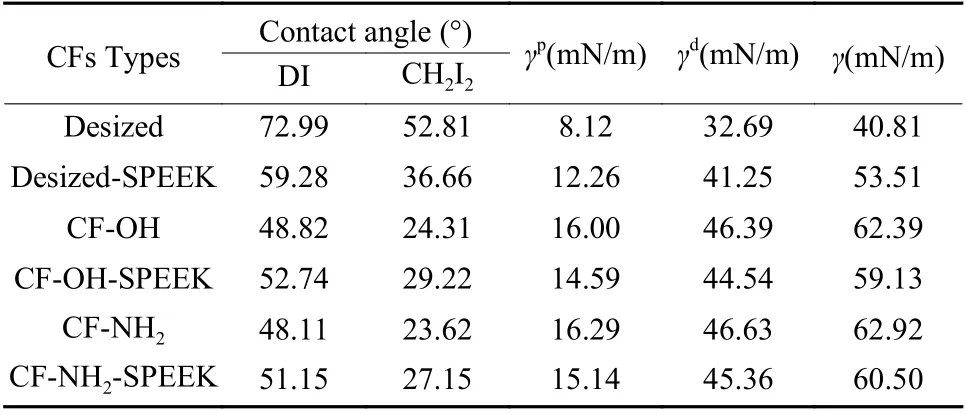
Table 5 The dynamic contact angles and surface free energy results of CFs.
It is well known that the contact angle between CFs and the test liquid is related to the surface free energy (γ) of CFs. Also, theγis calculated by the results of dynamic contact angle and the results are listed in Table 5. It can be seen that theγof desized CF is 40.81 mN/m, in which theγpandγdare 8.12 mN/m and 32.69 mN/m, respectively. After sizing treatment,theγdof desized−SPEEK increases to 41.25 mN/m with the increase of CF surface roughness. Meanwhile, the contents of polar functional groups increase after sizing, so theγpincreases to 12.26 mN/m,thus theγof desized−SPEEK is 53.51 mN/m. After surface functionalization, the contents of polar functional groups on the surface of CF−OH and CF−NH2increase significantly, so theγpincreases obviously,which are 16.00 mN/m and 16.29 mN/m, respectively.Meanwhile, theγdvalues of CF−OH and CF−NH2increase to 46.39 mN/m and 46.63 mN/m, respectively,with the increase of CF surface roughness. Thus, theγvalues of the CF−OH and CF−NH2are 62.39 mN/m and 62.92 mN/m, respectively. For CF−OH−SPEEK and CF−NH2−SPEEK, a SPEEK sizing layer is coated on their CF surface and the surface roughness of the fiber is slightly reduced. Therefore, theγdvalues are reduced to 44.54 and 45.36 mN/m, respectively.Meanwhile, some polar functional groups on the surface of the CF are covered by the sizing layer, which leads to the decrease of theγpto 14.59 and 15.14 mN/m for CF−OH−SPEEK and CF−NH2−SPEEK, respectively. Therefore, theγvalues of these two fibers,which are 59.13 and 60.50 mN/m, respectively, are lower than that of the unsized CFs. These results prove that the surface functionalization treatment used in this paper can improve the surface wettability of the CFs.
The influence of surface functionalization on the tensile properties of CFs was investigated and the dispersion degree of tensile strength of monofilament was deduced by Weibull modulus[21,22], and the results are shown in Fig. 11 and Table 6. It can be seen that the tensile strength and Weibull modulus of desized CF are 4.68 and 9.97 GPa, respectively. After functionalization, the tensile strengths of CF−OH and CF−NH2decrease to 4.36 and 4.37 GPa, along with the decrease of the Weibull modulus to 7.64 and 6.79,respectively. The decrease of tensile strength and the increase of dispersion degree of monofilament are due to the introduction of some defects on the surface of CFs after surface functionalization. It is worth noting that, the reduction of the tensile strengths of CF−OH and CF−NH2are less than 10% compared with the desized CF, illustrating that the surface functionalization method used in this paper is relatively mild and it could not induce serious damage to CFs.
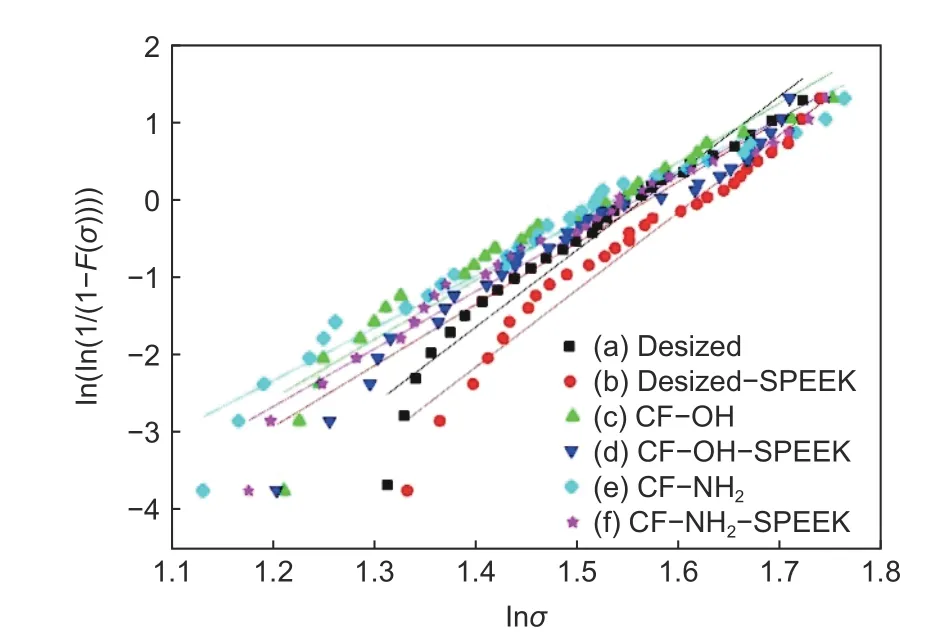
Fig. 11 Weibull distribution curves of CFs: (a) desized CF, (b) desized−SPEEK, (c) CF−OH, (d) CF−OH−SPEEK, (e) CF−NH2 and (f)CF−NH2−SPEEK.
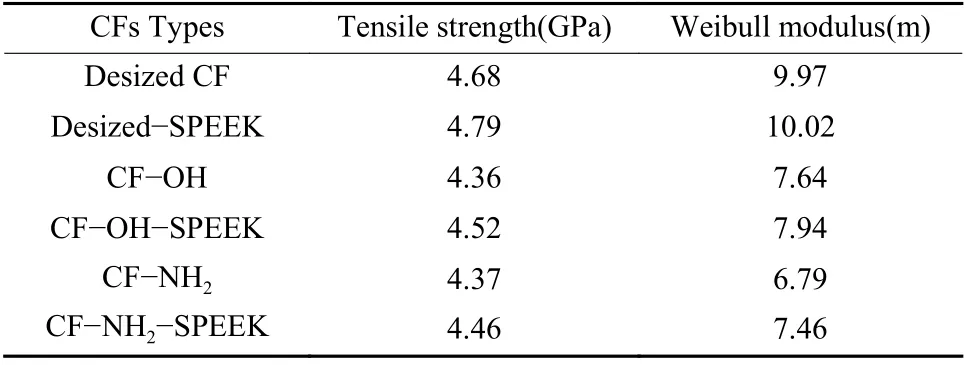
Table 6 The results of tensile strength and Weibull modulus.
After sizing treatment, the tensile strengths of desized−SPEEK, CF−OH−SPEEK and CF−NH2−SPEEK are 4.79, 4.52 and 4.46 GPa, respectively.Compared with the corresponding unsized CFs, the tensile strength of these CF increases a lot with the dispersion degree of tensile properties decreases a lot,which indicate that some defects of CFs are homogenized after sizing treatment.
In order to analyze the effect of surface functionalization treatment on the interfacial adhesion of CF/PEEK composites, the micro-drop debonding test was carried out on the composites with desized CF,CF−OH and CF−NH2. The results are shown in Fig. 12(a). It can be seen that the IFSS values of composites with CF−OH and CF−NH2are 65.4 and 68.7 MPa, respectively, which increase by 16.6% and 22.5%, compared with that of desized CF (56.1 MPa).After surface functionalization, the surface wettabilities of CF−OH and CF−NH2are improved, which is conducive to the wetting of CFs by polymer matrix. In addition, the surface roughness of these fibers are also increased, which increases the mechanical interlocking effect between CFs and polymer matrix. Thus, the IFSS values of composites with CF−OH and CF−NH2are obviously improved. It can also be seen from the AFM images in Fig. 6 that the surface roughness of CF−NH2is larger than that of CF−OH, so the IFSS of the composite with CF−NH2is greater than that with CF−OH.
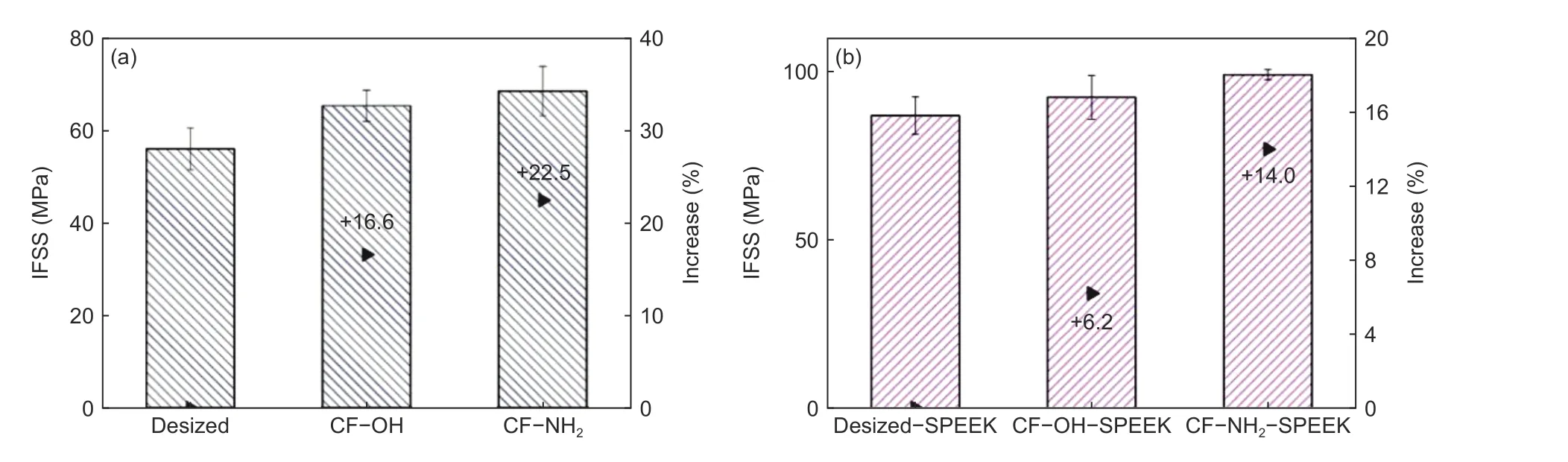
Fig. 12 The IFSS of CF/PEEK composites: (a) CFs without sizing and (b) CFs after SPEEK sizing.
The IFSS of the composites with CF−OH−SPEEK and CF−NH2−SPEEK are 92.4 and 99.1 MPa,respectively (Fig. 12(b)), which are increased by 6.2%and 14.0% as compared with that of desized−SPEEK(87.0 MPa). The reasons for the increased IFSS are as follows. First, the increase of polar functional group contents after surface functionalization can improve the chemical reaction between CFs and the sizing layer. Second, the increased surface roughness of CF−OH and CF−NH2is beneficial to the mechanical interlocking between CFs and the sizing layer. Third,the surface free energy of CF−OH−SPEEK and CF−NH2−SPEEK are higher than that of desized−SPEEK, which is conducive to the wettability of CFs by polymer matrix. Moreover, the greater chemical bonding degree between CF−NH2and the sizing layer,higher surface roughness of CF−NH2and greater wettability of CF−NH2−SPEEK, which make the IFSS of composites with CF−NH2−SPEEK higher than that of CF−OH−SPEEK. These results give additional evidences that the surface functionalization treatment can improve the interfacial adhesion of thermoplasticcoated CFs reinforced polymer composites.
4 Conclusions
The effect of surface functionalization on the surface properties of thermoplastic-coated CFs and the interfacial properties of composites were investigated.The results show that the surface roughness of CF−OH and CF−NH2increase from 5.6 to 23.4 and 27.2 nm, respectively. The contents of polar functional groups of CF−OH and CF−NH2increase by 10.84%and 11.12%, respectively, compared with the desized CF. The increase of surface roughness and the content of polar functional groups are beneficial to the improvement of wettability of CFs. Moreover, the surface functionalization method is also relatively mild and it could not damage the performance of CFs. As a result, the IFSS of CF−OH−SPEEK and CF−NH2−SPEEK increase by 6.2% and 14.0%, respectively, as compared with that of desized−SPEEK. In summary,the surface functionalization treatment is an effective method to improve the interfacial adhesion of thermoplastic-coated CFs reinforced polymer composites.
Acknowledgements
The Youth Innovation Promotion Association of the Chinese Academy of Science (CN) (Y201646);the Special Project of Strategic Leading Science and Technology of Chinese Academy of Sciences (A) Sub Topic (XDA17040519); Key R&D Projects of Shanxi Province (202003D111002, 201803D121042).
- 新型炭材料的其它文章
- 基于碳化钽涂层改性碳基材料的研究进展
- Two-dimensional layer materials for highly efficient molecular sensing based on surface-enhanced Raman scattering
- Coating a Na3V2(PO4)3 cathode material with carbon to improve its sodium storage
- Preparation of a N-P co-doped waste cotton fabric-based activated carbon for supercapacitor electrodes
- High-surface-area porous carbons produced by the mild KOH activation of a chitosan hydrochar and their CO2 capture
- Preparation of a porous carbon from Enteromorpha prolifera with excellent electrochemical properties

