Combination of oridonin and TRAlL induces apoptosis in uveal melanoma cells by upregulating DR5
Xin Hua, Peng Wu, Guo-Sheng Gao, Xiao-Lei Ye
1Department of Clinical Laboratory, Hwa Mei Hospital,University of Chinese Academy of Sciences, Ningbo 315010,Zhejiang Province, China
2Ningbo Institute of Life and Health Industry, University of Chinese Academy of Sciences, Ningbo 315010, Zhejiang Province, China
3Department of Clinical Laboratory, Renmin Hospital of Wuhan University, Wuhan 430060, Hubei Province, China
4College of Life Sciences, China West Normal University,Nanchong 637009, Sichuan Province, China
Abstract
● KEYWORDS: tumor necrosis factor-related apoptosisinducing ligand; oridonin; apoptosis; choroidal melanoma
INTRODUCTION
Choroidal melanoma (CM) is a primary ocular malignancy that adults, and eye removal is the most common surgical treatment. In recent years, since the curative effect of monotherapy is not always ideal, nearly 50% of patients will develop metastatic disease, the combination therapy using different means or combination drugs depending on the risk stratification of cancer patients has been concerned by the clinician[1-2]. The biological treatment of melanoma is a new direction, clinical studies using a high dosage of interferon combined with multi-targeted kinase inhibitors or vascular endothelial growth factor inhibitors are being performed and will continue in the future. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a member of the TNF family that can target tumor cell surface receptors (death receptor, DR) to induce apoptosis in a wide variety of tumor cells[3-4]. However, prolonged use of TRAIL may result in the decrease of DR4/DR5 on the cell membrane surface, resulting in decreased death-induced signalling complex (DISC)formation and decreased caspase activity, further causing target cells to be resistant to TRAIL[5-6]. Oridonin is a diterpenoid alkaloid with antioxidant, anti-inflammatory and antitumor efficacy[7-8]. Related studies showed that oridonin could induce the apoptosis of liver cancer HepG2 cells[9], oesophageal squamous cell carcinoma EC9706 cells[10], and L929 mouse fibroblast cells[11], but the specific mechanism is unclear.Oridonin combined with TRAIL can kill most of colon cancer cell lines such as SW480[12]. Some studies have shown that the analog of oridonin can also induce apoptosis in triple-negative breast cancer cells by up-regulating DR5[13].
Cancer cells have high heterogeneity, which means that different groups of cancer cells have various sensitivities to the same drug. Currently, clinical cancer treatment schemes prioritize treatments combining multiple drug applications;therefore, exploring the antitumor activity of combination chemotherapy has raised a future trend in cancer treatment.This study mainly explores whether the combination of oridonin and TRAIL in CM has inhibitory effects and preliminarily explores the mechanism of these combined applications.
MATERIALS AND METHODS
Reagents Soluble TRAIL protein (Novoprotein, Shanghai);oridonin (Chengdu Institute of Biology, Chinese Academy of Sciences); MTT (AMRESCO); RPMI1640 (Thermo Fisher);foetal bovine serum (FBS, GIBCO); DMSO (Life science);paclitaxel (Shanghai Yuanye Bio-Technology); penicillin (Life Technology); Streptomycin (GIBCO); DR5 antibody (Abcam);a-caspase-3 antibody (Abcam); x-linked inhibitor of apoptosis protein (XIAP) antibody (Abcam); β-actin antibody (Abcam).Cell Culture MUM-2B and C918 cell lines were purchased from Zhejiang Ruyao biotechnology Co., Ltd. Monolayer cells were cultured in RPMI 1640 medium containing 10% FBS,100 U/mL penicillin and 100 U/mL streptomycin at 37℃ with 5% CO2, and the cells were sub-cultured every 2-3d at a ratio of 1:3-1:5. Cell growth at the logarithmic phase was used in experiments.
MTT Assay Twenty-four hours before drug treatment,MUM-2B and C918 cells were trypsinized and collected, and the cell density was adjusted to 5×104cells/mL. The cells were inoculated in 96-well culture plates using 100 µL per well. For drug treatment, each concentration required three repeats. The oridonin group cells were treated with different concentrations of oridonin: 0.75, 1.5, 3, 6.25, 12.5, and 25 μmol/L, and the TRAIL groups cells were treated with different concentrations of TRAIL: 8, 16.1, 31.2, 62.5, 125, and 250 ng/mL. The combination groups were treated with 100 μL of a mixture of oridonin and TRAIL. The final concentrations of TRAIL were 8, 16.1, 31.2, 62.5, 125, and 250 ng/mL, and the final concentrations of oridonin were 0.8, 1.61, 3.12, 6.25, 12.5,and 25 μmol/L. The cells added 100 μL complete medium was used as the negative control group, and the cells added 5 μmol/L paclitaxel was used as the positive control group. After 48h,20 μL 5 mg/mL MTT was added to each well and incubated for 4h at 37℃. Then, discarded the supernatant, and added 150 μL DMSO to the wells. The OD value at 560 nm was recorded. Detected and recorded the OD value at 560 nm.Calculated the cell inhibition rate according to the following formula: cell inhibition rate = (1-ODmedicationadministrationgroup/ODcontrolgroup)×100%. The above experiment was repeated three times. Subsequently, MTT detection was also performed in si-DR5 cell lines to observe the effects of TRAIL and oidonin on cell activity. MTT detection was performed at 12, 24, 36, and 48h, respectively. The specific operation method is the same as above.
Morphological Analysis of Apoptosis MUM-2B cells were fixed with 70% precooled ethanol and stained with 1 μg/mL working concentration DAPI for 2-3min. The cell suspension was placed on the slide, and excess liquid was absorbed with filter paper. Morphological characteristics were immediately observed and captured using a fluorescence microscope at 360-400 nm excitation wavelength.
Cell-Cycle Distribution Analysis by Flow Cytometry The cell-cycle distribution after treatment with oridonin and TRAIL was determined by flow cytometry. Briefly, MUM-2B cells were plated in six-well plates and treated with different concentrations of oridonin and TRAIL for 48h. Control cells were treated only with the complete RPMI1640 medium containing 10% FBS+1% penicillin-streptomycin. After the treatment, the cells were trypsinized, collected from the plates,washed twice with cold phosphate buffered solution (PBS),and fixed with ice-cold 70% ethanol for at least 2h. The cell pellets were then collected by centrifugation (2500×g for 3min) and resuspended in 1 mL PI solution (50 mg/mL in PBS) containing 0.25 mg/mL RNase A. After incubation for 30min in the dark at 4°C, the cells analysed using an Attune®flow cytometer (Life Technology, Carlsbad, CA, USA).
Flow Cytometry Detection of Cell Apoptosis MUM-2B cells in the logarithmic growth phase were digested with 0.25% trypsin and resuspended in a complete medium. The cell density was adjusted to 5.0×104cells/mL, and the cells were inoculated into Petri dishes. The different treatment groups were as follows: blank control group; 3.12 or 12.5 μmol/L oridonin; 31.2 or 125 ng/mL TRAIL; 3.12 μmol/L oridonin+31.2 ng/mL TRAIL; and 12.5 μmol/L oridonin +125 ng/mL TRAIL. After incubation for 24h, the cells were digested with 0.25% trypsin. Then, the cell suspensions were collected and centrifuged at 1000×g for 5min. The supernatant was discarded and resuspended in PBS. Half of these cells were transferred to a 2 mL EP tube (Eppendorf Tube), and the rest were used for cell morphological analysis. The cell suspension was treated with RNase at 37℃ for 30min, and 300 μL of 1×binding buffer was added. The sample was stained with 5 μL PI and 5 μL FITC Annexin V, incubated in the dark for 15min at room temperature, and then detected with the flow cytometer. The stained cells were analyzed within 1h.
Si-DR5 Cell Lines Constructed DR5 siRNA was purchased from Origene (https://www.origene.com/). It is advisable to lay the cells in 24-well plates with a confluence of about 30%-50% during transfection. si-DR5 20 pmol or si-NC was dissolved in 50 μL Opti-Men serum-free medium. Lipo 2000 1 μL was dissolved in 50μL Opti-Men serum-free medium and transfected. During transfection, the medium was replaced with serum-free medium, 400 μL per well. After 4-6h, change to serum medium.
Western Blot Analysis Cells were treated with 0, 6.25 μmol/L oridonin, 62.5 ng/mL TRAIL and 6.25 μmol/L oridonin +62.5 ng/mL TRAIL for 48h, then the medium was discarded and washed with precooled PBS. After discarding the PBS,RIPA cell lysis buffer was added, and the cells were incubated in an ice bath for 5min. The total protein was collected and quantified by BCA assay. Each lane was loaded with 40 μg protein. After 10% SDS-page gel electrophoresis separation,the protein was transferred onto the PVDF membrane, blocked for 1h with 5% defatted milk at room temperature. Then XIAP, DR5, a-caspase-3, Bax, Bcl-2, and β-actin antibodies were added separately, and incubate at 4℃ overnight. Then,the membranes were washed with TBST 3 times, and the secondary antibody labeled with horseradish peroxidase (HRP)was added. The membranes were incubated for 1h at room temperature, washed three times with TBST, and detected using an ECL detection kit. β-actin was an internal reference protein.
Statistical Analysis All of the above experiments were repeated three times separately, and the data were expressed as mean±standard deviation. Statistical software SPSS22.0 was used to analyse whether there were differences between groups by one-way analysis of variance (ANOVA). The pairwise comparison was tested by LSD method.P<0.05 was considered statistically significant.
RESULTS
Effects of Oridonin and TRAIL on the Inhibition of Melanoma Cells MUM-2B and C918 are normal CM cells.Figure 1 indicated the effects of oridonin and TRAIL on MUM-2B cell proliferation after treatment for 48h. Figure 1B indicates the effects of oridonin and TRAIL on C918 cell proliferation for 48h. MUM-2B cell proliferation can be inhibited by the individual treatments of oridonin and TRAIL.However, the effect significantly increased when the treatments were combined. The inhibition rate in the combination group(25 μmol/L oridonin+250 ng/mL TRAIL) of MUM-2B cells was 77.26%±1.82%, which even exceeded the effect of 5 μmol/L paclitaxel, which was taken as a positive control and the inhibition rate was 67.02%±1.85%. Under the same conditions, the sensitivity of the C918 cells responding to these two compounds was significantly lower than that of MUM-2B cells. The results suggest that MUM-2B cells are more sensitive to the combination of TRAIL and oridonin than C918 cells.Thus, MUM-2B cells were chosen as the primary research object in subsequent experiments.
Individual and Combined Effects of Oridonin and TRAIL on the Morphology of Nuclei Figure 2 shows that the nucleus of the control group is relatively complete without a clear apoptotic cell. Typical apoptotic bodies without intact nuclei were observed in MUM-2B cells after treatment with paclitaxel Figure 2B. Figure 2C and 2D show that there was not a large number of apoptotic cells appeared under the low concentrations of oridonin, while in Figure 2E, many more apoptotic cells appeared under treatment with 12.5 μmol/L oridonin.As shown in Figure 2F-2H, the cellular state became fewer as the TRAIL concentration increased, but a large number of apoptotic cells were not observed. As shown in Figure 2I,fewer apoptosis cells appeared under the low concentrations of oridonin and TRAIL. However, as shown in Figure 2J and 2K, when high concentrations of oridonin and TRAIL were added to cells, a large number of apoptotic bodies appeared,indicating that the number of apoptotic cells increased.According to the results of DAPI staining, the number of cells undergoing apoptosis was relatively limited in the individual oridonin or TRAIL treatment groups, while the induction of a large number of apoptotic cells was observed in the combination group.
Effects of Oridonin and TRAIL on the MUM-2B Cell Cycle Figure 3 shows the cell cycle results after treatment with different concentrations of the compounds. Flow cytometry analysis showed that the proportion of cells in the G2 phase increased in the combination group compared with the individual oridonin and TRAIL treatment groups. The results of flow cytometry in the negative control group are shown as typical G0/G1, S, and G2/M peaks. The proportion of the G0/G1 phases was reduced after the combination treatment of the two compounds. Additionally, the proportion of cells in the G2/M phase increased as the concentration of the compounds increased, which may indicate the occurrence of G2/M arrest. The proportion of cells in the S phase did not change significantly compared with the control group (Figure 3K).
Individual and Combination Effects of Oridonin and TRAIL on Apoptosis Figure 4 shows that the induction of apoptosis in MUM-2B cells by oridonin is not evident, while the TRAIL treatment could significantly induce MUM-2B cell apoptosis(P<0.05). The MUM-2B cell apoptosis rate was significantly increased after the combined administration of TRAIL and oridonin; when 12.5 μmol/L oridonin and 125 ng/mL TRAIL were added, the cell apoptosis rate increased significantly (P<0.05) compared with TRAIL treatment alone.
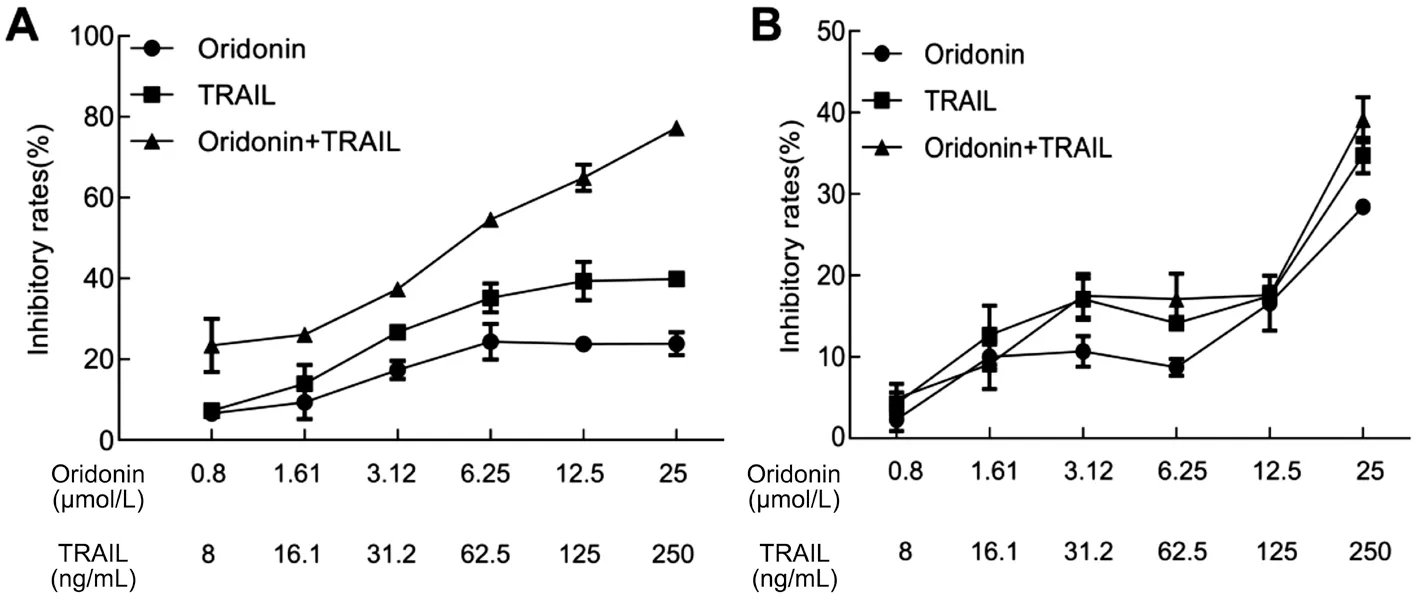
Figure 1 The effects of different concentrations of oridonin and TRAIL on MUM-2B cells (A) and C918 cells (B) proliferation in individual and combined treatments.
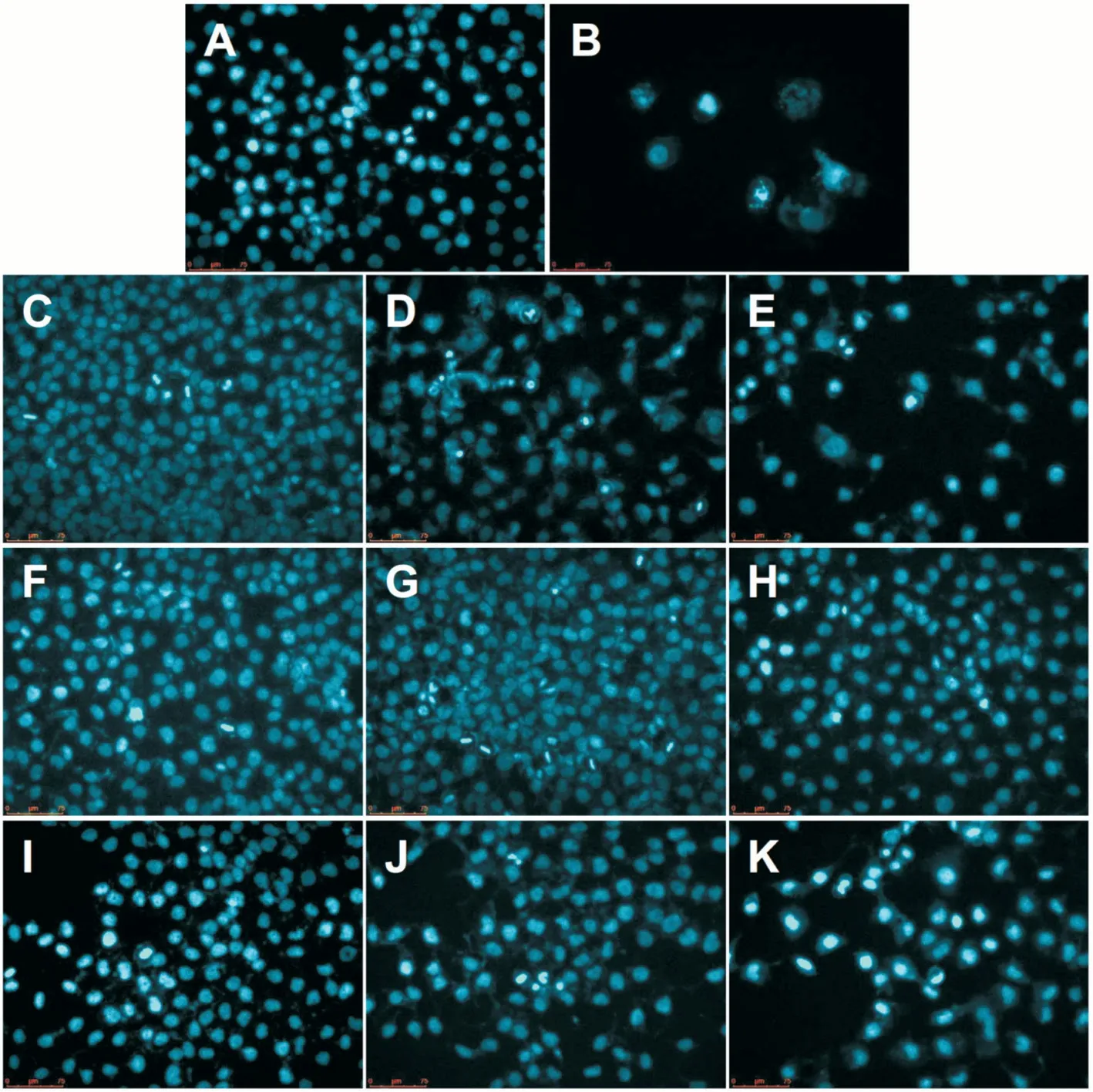
Figure 2 DAPI staining revealed the morphological characteristics the nuclei after treatment with different concentrations of oridonin and TRAIL after 48h A: Blank control group; B: 5 μmol/L paclitaxel positive control group; C: 3.12 μmol/L oridonin; D: 6.25 μmol/L oridonin; E: 12.5 μmol/L oridonin; F: 31.2 ng/mL TRAIL; G: 62.5 ng/mL TRAIL; H: 125 ng/mL TRAIL; I: 3.12 μmol/L oridonin+31.2 ng/mL TRAIL; J: 6.25 μmol/L oridonin+62.5 ng/mL TRAIL; K: 12.5 μmol/L oridonin+125 ng/mL TRAIL.
Individual and Combined Effects of Oridonin and TRAIL on Apoptosis-related Protein Expression Apoptosis-related protein expression levels were examined by Western blot. The results (Figure 5) showed that the protein expression of DR5,a-caspase-3 and BAX was enhanced when oridonin was used in combination with TRAIL, while the XIAP and BCL-2 proteins were down-regulated when oridonin was used in combination with TRAIL. si-DR5 cell line MUM-2B was constructed for the recovery experiment. Figure 6A shows the expression level of DR5 gene in cells transfected with DR5 siRNA by qPCR.Figure 6B shows the results of MTT assay. Compared with the control group, the activity of MUM-2B was significantly inhibited in the combination group (P<0.05). However, after the interference of DR5 gene expression, the inhibition of cell activity was reversed, and the activity was significantly increased compared with that of the single combination group(P<0.05). The results showed that the DR5 gene expression was down-regulated in si-DR5 group. Western blot results showed that the apoptosis-inducing effects of oridonin and TRAIL on normal MUM-2B cells were reversed after transfection with DR5 siRNA (Figure 6C-6D).
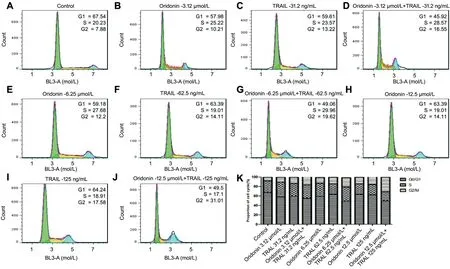
Figure 3 The cell cycle distribution after treatment with different concentrations of the oridonin and trail A-J: Cell cycle distribution after PI staining by flow cytometry; K: Histogram present the relative proportion of cell cycle in different drug treatment groups.
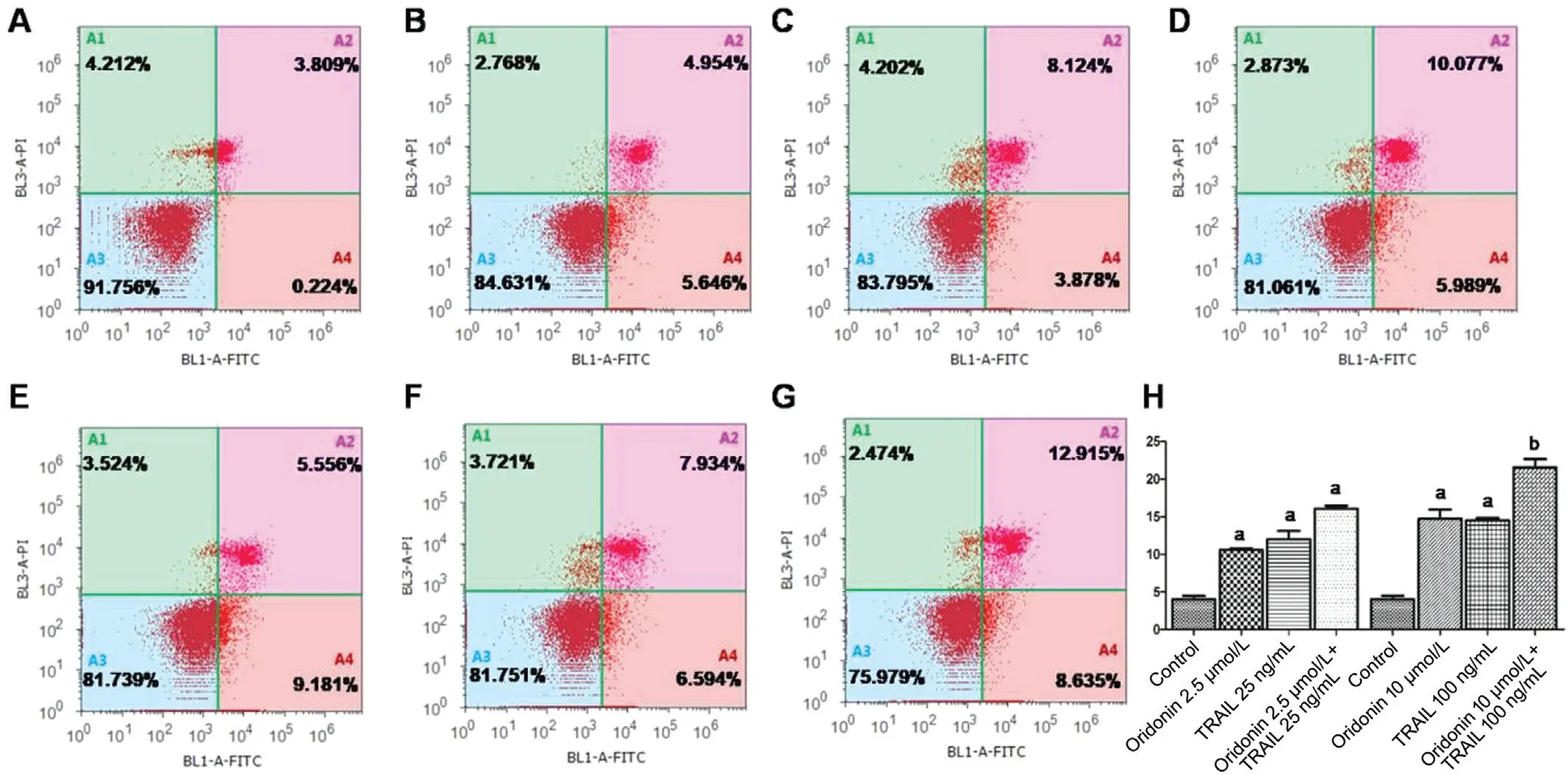
Figure 4 MUM-2B cell apoptosis induced by the combination of oridonin and TRAIL A-G: Flow cytometry analysis of cell apoptosis: A:Blank control group; B: 3.12 μmol/L oridonin; C: 31.2 ng/mL TRAIL; D: 3.12 μmol/L oridonin+31.2 ng/mL TRAIL; E: 12.5 μmol/L oridonin;F: 125 ng/mL TRAIL; G: 12.5 μmol/L oridonin+ 125 ng/mL TRAIL; H: Histogram present the proportion of apoptotic cells in each group,aP<0.05, bP<0.01, compared with control group.
DISCUSSION
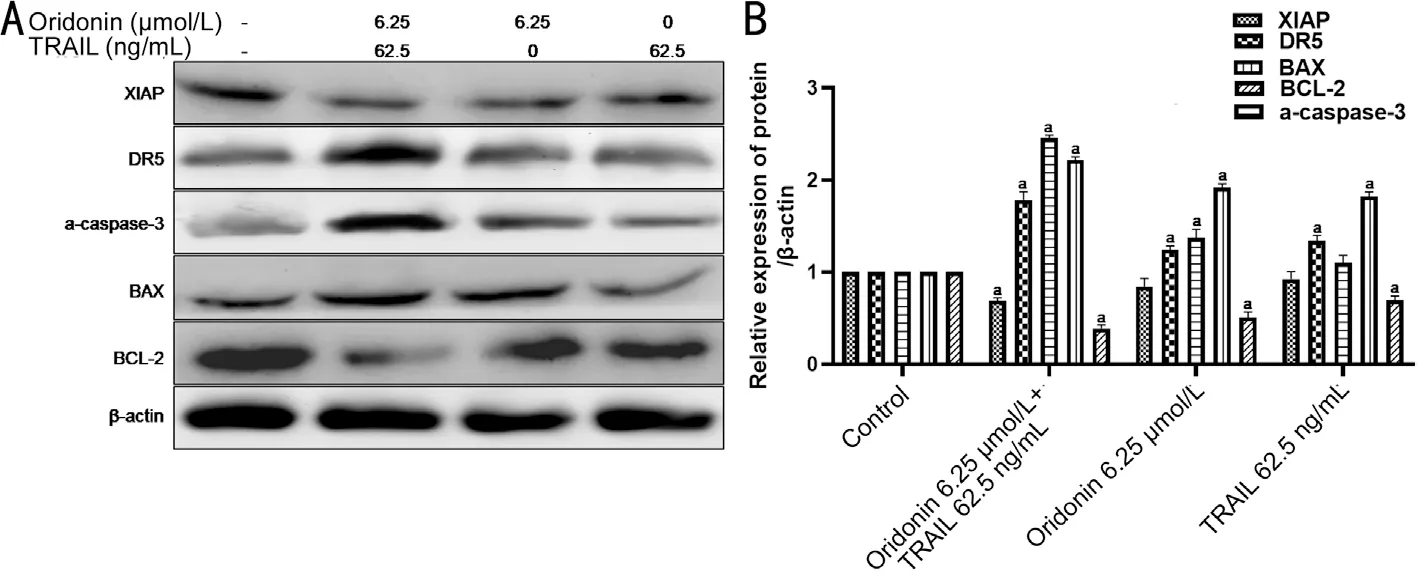
Figure 5 The expression level of apoptosis-related proteins in MUM-2B cells after the combined treatment of oridonin and TRAIL A: Western blot results suggested that caspase-3 activation and expression of DR5 were enhanced in the combination group; B: A histogram obtained by quantizing the gray value of the protein band in A. aStatistically significant difference compared with the control group, P<0.05.
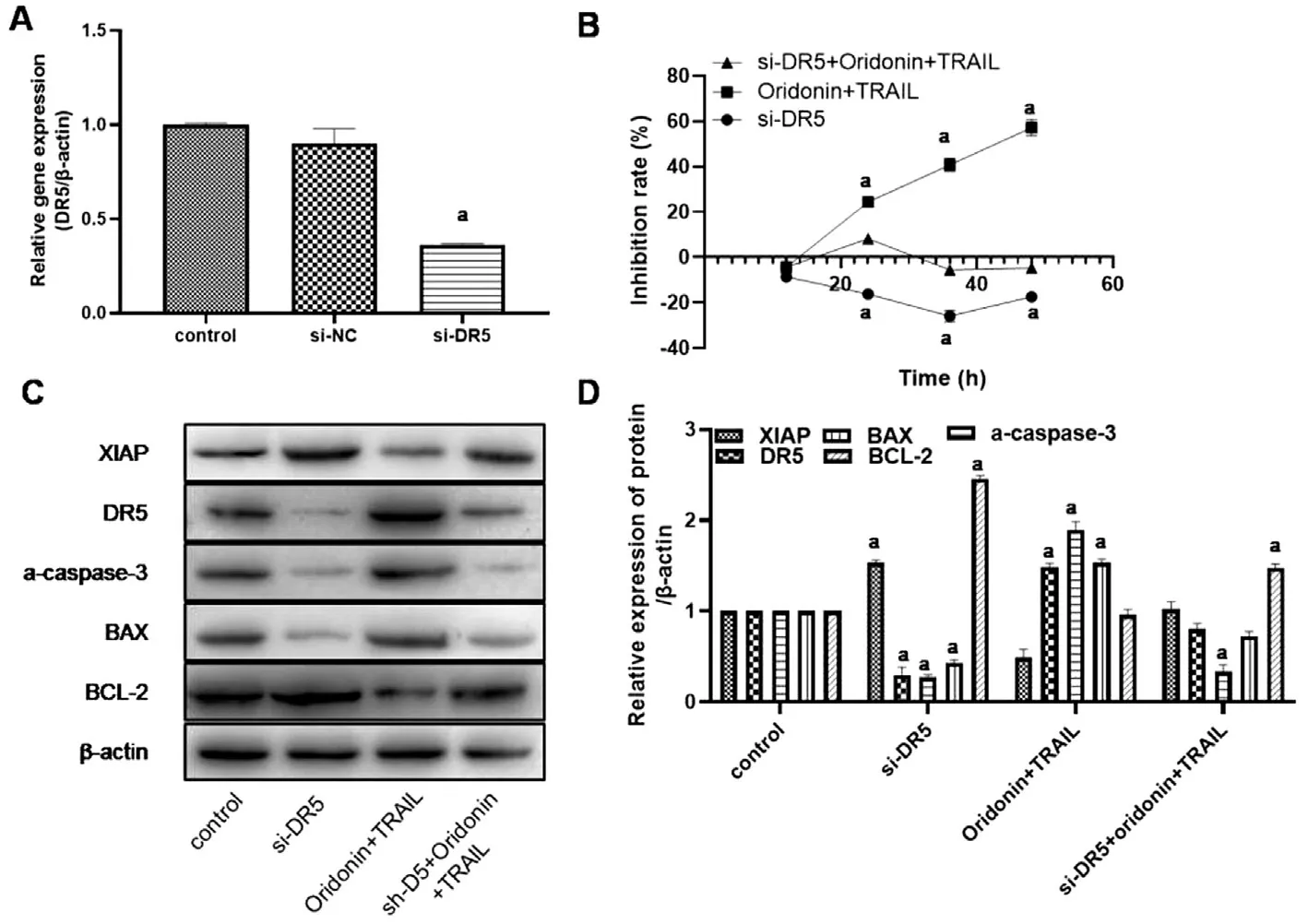
Figure 6 Silenced DR5 could reverse the apoptosis-inducing effect of oridonin and TRAIL combination on normal MUM-2B cells A: The relative expression level of DR5 gene after transfection with DR5 siRNA was detected by qPCR; B: The effect of TRAIL and oridonin on the activity of transfected si-DR5 cells was detected by MTT; C: Western blot was used to detect the expression levels of apoptosis-related proteins in each group; D: A histogram obtained by quantizing the gray value of the protein band in C. aStatistically significant difference compared with the control group, P<0.05.
CM is a severe threat to the patient’s health and a high degree of malignancy, rapid invasion and metastasis, and poor prognosis, resulting in primarily personalized treatment. The appropriate treatment method should be chosen according to the tumor size, location, morphology, growth rate, and so on.The effect of radiation is relatively good in clinical settings;however, there are still limitations. The patient’s condition should be considered when selecting a method with good curative effect, low recurrence rate, and few adverse reactions;all of the above represent the effective management of CM.
Oridonin, a tetranuclear diterpenoid with a kaurene skeleton,was discovered as one of the active ingredients extracted from traditional Chinese medicine (TCM), namedRabdosia Rubescens, which belongs toLipplectranthus. Oridonin has specific inhibitory effects on different types of cancer cells.Studies examining the effect of oridonin on non-small cell lung cancer showed that it could increase the expression level of the apoptosis-related protein BAX and reduce the expression of the apoptosis-inhibiting protein BCL-2 to promote lung cancer cell apoptosis and increase radiation sensitivity[14-15]. In a study of the mechanism of oridonin on HepG2 liver tumor resistance,it was found that oridonin may increase cell cycle-related proteins such as p-ATM, p-ATR, p-P53, p-Cdk1, p-CDC25C to activate the cell cycle checkpoint and then affect cell cycle progression, inhibiting the growth of tumor cells[16-17]. Studies on the inhibitory effect of oridonin on Hepatic stellate cells have shown that oridonin down-regulates the expression of nuclear transcription factors associated with inflammatory cytokines[18]. All of these studies indicated that oridonin might induce apoptosis by regulating apoptosis-related proteins.Moreover, oridonin may also regulate cell cycle-related proteins, and likely inducing cells to undergo apoptosis after blocking the cell cycle[19-21].
Since the TNF superfamily was discovered, its members have been gradually characterized. TRAIL is one of these proteins, and researchers found that TRAIL is homologous to the TNF and CD95L sequences. TRAIL inhibits tumors and is classified into the TNF superfamily when searching the DNA database. TRAIL is able to selectively target tumor cell by recognizing the DR on the cell surface. TRAIL signalling is not toxic to healthy cells and has specific inhibitory effects on a wide variety of cancers[3-4]. However, many types of cancer have been resistant to TRAIL in experimental applications[5],due to the functional loss of DR4 and DR5 on the cancer cell surface. One possible mechanism is the phagocytotic of the autophagosome in the tumor cells expressing DRs, which may lead to TRAIL resistance. When 3-MA was used to disrupt the formation of autophagosomes in cancer cells, the sensitivity of TRAIL-resistant cells to TRAIL can be restored after DR4/DR5 was expressed on the surface of the cell membrane.
Antitumor substances have always shown treatment effects only on certain types of cancer cells, and the inhibition effect is reduced after longer usage durations. Therefore, developing new substances or efficient combinations of drugs has become an inevitable trend. Current studies show that the combined use of various antitumor substances and methods may become a new treatment by successfully inhibiting tumors by restoring antitumor signalling pathways. For example,the combination of paclitaxel or cisplatin with neoadjuvant chemotherapy and radiotherapy has been proven to have a good effect on middle and early cervical cancer[22]. In addition,decades before, the combination of TRAIL and 5-FU was reported to inhibit colon cancer SW480 cellsin vitro[23]. In a recent report, when researchers studied the individual and synergistic action of B-9-3 (0.78 to 200.00 μg/mL), paclitaxel,vincristine, 5-fluorouracil, cisplatin and pharmorubicin(0.78 to 200.00 μg/mL) in LLC lung cancer cells, HT-29 colon cancer cells, HepG2 liver cancer cells and MCF-7 breast cancer cells after 48h, it was found that the combination of B-9-3, paclitaxel and vincristine could significantly reduce the survival rates of MCF-7 and HT-29 cells compared with B-9-3 alone; therefore, B-9-3 was thought to significantly inhibit the mobility of LLC cells, enhance the apoptosis rate and have a synergistic effect with different antitumor compounds[24]. The study hypothesized that the mechanism of the tumor suppressor plays a role in different nodes of the same signalling pathway to inhibit the proliferation of tumor cells.At present, the mechanism by which TRAIL combined with other drugs can enhance the ability to kill tumor cells is not yet precise, and the following two aspects probably contribute to this mechanism. Yanget al[25]reported that the combination of TRAIL and chemotherapy drugs could induce the level of the TRAIL receptors to increase in tumor cells significantly.Also, the apoptotic pathway may be enhanced due to some drugs inhibiting anti-apoptotic gene expression, resulting in cell death. Somasekharanet al[26]reported that restraining the expression of anti-apoptotic genes in tumor cells increased the sensitivity of chemotherapy drugs.
This study shows that the combination of TRAIL and oridonin can effectively kill the uveal melanoma cells C918 and MUM-2B,which are resistant to TRAIL. Regarding the mechanism of this effect, we hypothesized that oridonin might affect the autophagosomes in MUM-2B and C918 cells, leading to the redistribution of DR molecules on the surface of the tumor cells, allowing TRAIL to perform its antitumor function.
The DR5 is located on the surface of tumor cells and combines with TRAIL. The combined role of TNF and DR5 can induce apoptosis in cells, playing an essential role in the apoptosis of some cells. Its high expression may prompt cell apoptosis, and its low expression indicates that the cells are normal or exhibit DR inhibitor tolerance.
The caspase protein family causes cell apoptosis through specifically cleaving aspartic acid cysteine proteases. Caspases that begin this process (initiator caspases), such as caspase-8,play roles in early apoptosis, activating the caspase-3, as increased expression of the cleaved caspase-3. Another kind of caspase is a practitioner caspase (executioner caspases),such as cleaved-caspase-3, which cut the polymerase PARP polymer, may result in inhibiting DNA repair, reducing the ability of the cell to monitor genetic integrity, increasing the activity of Ca2+/Mg2+dependent endonuclease to DNA that is affected by the negative regulation of PARP, breaking the DNA between nucleosomes, and inducing apoptosis. As a protein whose function is to promote apoptosis, under normal circumstances, caspases exist in the cytoplasm in the form of non-active enzymes, which can only be activated after cells receive apoptosis signals. Afterward, the molecular expression level, especially the expression level of the terminal effector a-caspase-3, markedly increases. By detecting the expression level, the cell mass apoptosis can be determined. XIAP is the main member of the IAP family found in human embryonic brain cells in 1996 by Peter. The XIAP gene is located on chromosome X q25, and the encoded protein contains 497 amino acids. The human XIAP is a typical IAP, consisting of three BIR domains and three RING structures, which are the structural basis for inhibiting the activity of caspase. XIAP can directly combine with activated a-caspase-3, caspase-7 and caspase-9 to inhibit their activities, thus inhibiting tumor cell apoptosis. Detecting the status of apoptosis-related proteins can provide information about the activity of cell apoptosis.In conclusion, this experiment suggested that the combined application of oridonin and TRAIL has a stronger inhibitory effect in MUM-2B cells than in C918 cells by detecting many differences after treatment with different concentrations of oridonin alone or in combination with TRAIL. The combined inhibitory effect of oridonin and TRAIL was significantly stronger than their individual effects, as confirmed by observing cellular morphology, quantifying the cell cycle,measuring apoptosis-related proteins, and performing a series of other experiments. To determine the influence of these treatments on intracellular signal transduction pathways and gene expression levels in MUM-2B cells, we will apply siRNA transfection to cells to verify the above results, and other pathways involved need further study.
ACKNOWLEDGEMENTS
Authors’ contributions: Study concept and design: Ye XL,Wu P and Gao GS; Acquisition, analysis,or interpretation of data: Ye XL and Wu P; Drafting of the manuscript: Ye XL and Wu P; Critical revision of the manuscript for important intellectual content: Ye XL, Wu P, Gao GS and Hua X; Statistical analysis:Ye XL and Wu P; Study supervision: Gao GS and Hua X. All the authors have read and approved the manuscript.
Foundation:Supported by Ningbo Leader and Top Notch Person Training Project (No.20150012).
Conflicts of Interest:Hua X, None; Wu P, None; Gao GS,
None; Ye XL, None.
 International Journal of Ophthalmology2021年12期
International Journal of Ophthalmology2021年12期
- International Journal of Ophthalmology的其它文章
- Upregulation of ASPP2 expression alleviates the development of proliferative vitreoretinopathy in a rat model
- Mesenchymal stem cell-derived exosomes inhibit the VEGF-A expression in human retinal vascular endothelial cells induced by high glucose
- Protective effects of umbilical cord mesenchymal stem cell exosomes in a diabetic rat model through live retinal imaging
- New technique for removal of perfluorocarbon liquid related sticky silicone oil and literature review
- Quantitative analysis of retinal vasculature in normal eyes using ultra-widefield fluorescein angiography
- Evaluation of the long-term effect of foldable capsular vitreous bodies in severe ocular rupture
