A standardized crush tool to produce consistent retinal ganglion cell damage in mice
Pedro Norat, Jingyi Gao, Sauson Soldozy, Hao F. Zhang, Xiaorong Liu
In the mammalian central nervous system, neuronal loss induced by injuries or in neurodegenerative diseases is often irreversible (Quigley, 2016; Gan et al., 2018). Following the disease insult,the surviving neurons may continue to lose their functionality because their axons degenerate and fail to maintain proper synaptic connections, and the underlying molecular and cellular mechanisms remain to be investigated(Raff et al., 2002; Bei et al., 2016;Quigley, 2016). Retinal ganglion cells(RGCs), for example, the neurons conveying visual information from the retina to the brain via the optic nerve,is a great model system to study the neural circuit and function because of its relatively easy accessibility and manipulation of the tissue. Optic nerve crush (ONC) injury in mice, the mechanical damage of the RGC axons at 0.5–1 mm behind the eye globe (Li et al., 1999), is a widely used model to examine the neural degeneration and axonal regeneration (Nickells et al., 2012; Quigley 2016). Since the introduction of this mouse model in late nineties, major progress has been made to characterize the axon degeneration and regeneration, especially assisted by the powerful mouse genetic tools that allow examining the molecular mechanisms underneath (Duan et al.,2015; Yi et al., 2016; Feng et al., 2017;Tran et al., 2019).
Optic nerve crush injury usually induces the death of 50–80% of RGCs within 2–3 weeks, and the main challenge is to generate reproducible and consistent RGC damage among different research groups (Liu et al., 2020). To study axonal degeneration and regeneration, it is essential to injure all axons, because an incomplete lesion may lead to confusing results and misinterpretation of axonal rescue (Fischer et al., 2017). Different strategies have been developed to produce consistent axonal damage and to validate axon regeneration. For example, different fluorescent colors were used to label RGCs and their axons before and after the ONC injury in order to distinguish degenerated and spared axons, which also made possible to track the axons’ survival at different time points following ONC (Fischer et al., 2017). The use of a calibrated tool,on the other side, allows experimenters to apply a consistent force on the optic nerve. A large number of tools were tested in an attempt to achieve a standardized crush injury among different laboratories (see discussion in(Liu et al., 2020)). In our recent study,we designed a device to measure the force and duration exerted by a N-7 selfclosing forceps on the optic nerve in mice (Liu et al., 2020).
The device we designed has two miniature precision foil gauges coupled at optimal locations, one on each arm of the tweezer that allows the measurement of the force and duration applied by the experimenter (Figure 1; Liu et al., 2020). Throughout the study, the same experimenter crushed the optic nerves of multiple mice, with varied forces and different durations. We quantified the effects of crush duration and crush force on RGC survival, and our results revealed a reduction on axon density with the increased force pulse (newton-second). In other words,a longer crush with stronger force produced more axon loss (Liu et al.,2020). Moreover, this new tool allows the experimenter to adjust the force and duration of the crush which will eventually standardize their applied force (Liu et al., 2020). An improved consistency of axon injury could thus be achieved by different research groups in future.
At the same time, we did notice that the tweezers have a flat tip that do not fit perfectly around the nerve, and,consequently, may produce uneven force around the ON and also permit spared axons on the edges of the ON(Figure 1). The difference between spared and regenerated axons should be carefully identified. Fischer et al. (2017)warned that spared axons usually are linear and continuous beyond the injury site for long distances. Moreover, not all RGCs respond to the injury following the same pattern, which further increased the complexity of data analyses.
There are ~45 distinct types of RGCs in mice, with each exhibiting an unique morphology and physiological properties (Sanes and Masland, 2015).Quigley et al. (1987) had related a greater conservation of RGC fibers with a smaller diameter compared with the RGC fibers with larger diameter in a chronic glaucoma model of primates. In line with above, other studies, including ours, showed a great variation in the survival rates of RGCs following ONC(Duan et al., 2015; Feng et al., 2017;Tran et al., 2019). However, the results and interpretations of the survival rates following axons injury, even for the same type of RGC, may be inconsistent in different studies. This is probably due to limitations of different labeling techniques and diverse classification parameters. For example, Duan and his colleagues have shown that the α-RGC type was resilient to the ONC, while our studies suggested that a small subgroup of α-RGC type in fact were susceptible to the ONC (Duan et al., 2015; Feng et al., 2017). The recent publication of Tran et al. (2019) has used the single cell RNA sequence to create an atlas of RGC types and then tracked type-specific response to the ONC injury. They described four different types of α-RGC, where two are resilient and the other two are susceptible (Tran et al., 2019), which possibly explained the discordance mentioned above.
Interestingly, all five intrinsically photosensitive RGC (ipRGC) types were resilient after ONC (Tran et al., 2019).The ipRGC expresses melanopsin, which is a photopigment responsible for the intrinsic sensitivity to light (Do, 2019).The axons of ipRGC project to different brain areas such as the suprachiasmatic nucleus to synchronize the circadian clock to the environmental time cues (Do, 2019). It is remarkable that some of the ipRGC and the resilient α-RGC overlapped (Tran et al., 2019),demonstrating the complexity of the RGC classification and the interpretation of results. Duan et al. (2015) further demonstrated the expression of osteopontin and insulin-like growth factor receptor 1 in this specific subset of ipRGC and α-RGC promoted their regeneration. Taken together, in the presence of a distinct combination of molecular factors, each group of RGCs may behave differently following the crush insult, which adds another layer of variability to the data analyses.
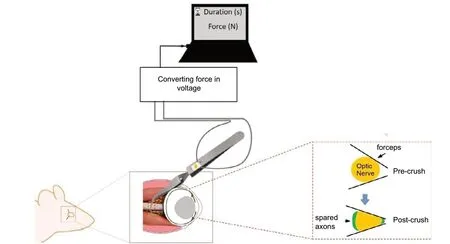
Figure 1|Schematic illustration of the optic nerve crush (ONC) injury in mice.
In summary, baseline inputs need to be established to induce consistent damage to the optic nerve with controlled force, duration, and location, in order to optimize the consistency of RGC damage. The issues with spared axons following the ONC can be addressed by developing a standardized and customized tool with tips that conform to the geometry of the ON, mitigating spared axons on its edges. More studies are needed to further understand the molecular variabilities among subtypes,and how these molecules may affect the survival and regeneration of specific types of neurons in neurodegenerative diseases.
We thank James Cole and Dr Mingna Liu for their helpful discussion.
This work was supported by NIH grants R01EY029121 (to XL and HFZ) and R01EY026286 (to XL).
Pedro Norat, Jingyi Gao,Sauson Soldozy, Hao F. Zhang,Xiaorong Liu*
Department of Biology, University of Virginia,Charlottesville, VA, USA (Norat P, Gao J, Liu X)
Department of Neurological Surgery, University of Virginia Health System, Charlottesville, VA, USA(Soldozy S)
Department of Biomedical Engineering,Northwestern University, Evanston, IL, USA(Zhang HF)
Department of Psychology, University of Virginia,Charlottesville, VA, USA (Liu X)
*Correspondence to:Xiaorong Liu, PhD,xl8n@virginia.edu.
https://orcid.org/0000-0002-7655-6342(Xiaorong Liu)
Date of submission:May 22, 2020
Date of decision:June 30, 2020
Date of acceptance:September 28, 2020
Date of web publication:December 7, 2020
https://doi.org/10.4103/1673-5374.301015
How to cite this article:Norat P, Gao J, Soldozy S,Zhang HF, Liu X (2021) A standardized crush tool to produce consistent retinal ganglion cell damage in mice. Neural Regen Res 16(7):1442-1443.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriatecredit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Herbert M. Geller, NIH, USA.
- 中国神经再生研究(英文版)的其它文章
- Clusterin: a multifaceted protein in the brain
- The future of adenoassociated viral vectors for optogenetic peripheral nerve interfaces
- Are mitochondria the key to reduce the age-dependent decline in axon growth after spinal cord injury?
- The molecular profile of nerve repair: humans mirror rodents
- Neuritogenic function of microglia in maternal immune activation and autism spectrum disorders
- A closer look at cannabimimetic terpenes, polyphenols, and flavonoids: a promising road forward

