Shuyu pills inhibit immune escape and enhance chemosensitization in hepatocellular carcinoma
Zhe Deng,Yong-Jie Teng,Qing Zhou,Zhao-Guang Ouyang,Yu-Xing Hu,Hong-Ping Long,Mei-Jie Hu,Si Mei,Feng-Xia Lin,Xin-Jun Dai,Bo-Yu Zhang,Ting Feng,Xue-Fei Tian
Zhe Deng,Xin-Jun Dai,Ting Feng,Xue-Fei Tian,College of Integrated Chinese and Western Medicine,Hunan Key Laboratory of Translational Research in Formulas and Zheng of Traditional Chinese Medicine,Hunan University of Chinese Medicine,Changsha 410208,Hunan Province,China
Yong-Jie Teng,Qing Zhou,Yu-Xing Hu,Mei-Jie Hu,The First Hospital of Hunan University of Chinese Medicine,Hunan University of Chinese Medicine,Changsha 410208,Hunan Province,China
Zhao-Guang Ouyang,Department of Preventive Dentistry,Guangzhou Key Laboratory of Basic and Applied Research of Oral Regenerative Medicine,Guangzhou Medical University,Guangzhou 510132,Guangdong Province,China
Hong-Ping Long,Experiment Center of Medical Innovation,The First Hospital of Hunan University of Chinese Medicine,Hunan University of Chinese Medicine,Changsha 410208,Hunan Province,China
Si Mei,Department of Physiology,Hunan University of Chinese Medicine,Changsha 410208,Hunan Province,China
Feng-Xia Lin,Department of Cardiology,Shenzhen Bao'an Traditional Chinese Medicine Hospital Group,The Affiliated Hospital of Guangzhou University of Chinese Medicine,Shenzhen 518133,Guangdong Province,China
Bo-Yu Zhang,College of Acupuncture and Massage,Hunan University of Chinese Medicine,Changsha 410208,Hunan Province,China
Key Words:Shuyu pills;Hepatocellular carcinoma;Tumour microenvironment;Immune escape;Chemoresistance
Abstract BACKGROUNDHepatocellular carcinoma(HCC)is characterized by dysregulation of the immune microenvironment and the development of chemoresistance.Specifically,expression of the programmed cell death protein 1(PD-1)/programmed cell death 1 ligand 1(PD-L1)axis,an immune checkpoint,may lead to tumour immune escape,resulting in disease progression.The latest research shows that tumour immune escape may be caused by the upregulation of PD-L1 mediated by hypoxia-inducible factor-1 alpha(HIF-1α),and simultaneous inhibition of HIF-1α and PD-L1 has the potential to enhance the host’s antitumour immunity.Moreover,inhibition of the PD-1/PD-L1 axis may mitigate tumour chemoresistance.Shuyu pills(SYPs)contain immunity-enhancing and antitumour components,making them a potential HCC treatment.AIMTo investigate the efficacy of SYPs for HCC treatment via simultaneous HIF-1α and PD-L1 inhibition and the mechanism involved.METHODSA subcutaneous xenograft tumour model was first established in BALB/c nude mice by the subcutaneous injection of 1 × 107 SMMC-7721 cells.Male mice(male,5 weeks old;n=24)were then randomly divided into the following four groups(n=6):Control(0.9% normal saline),SYP(200 mg/kg),SYP + cisplatin(DDP)(200 mg/kg + 5 mg/kg DDP weekly viaintraperitoneal injection),and DDP(5 mg/kg cisplatin weekly viaintraperitoneal injection).The dose of saline or SYPs for the indicated mouse groups was 0.2 mL/d viaintragastric administration.The tumour volumes and body weights of the mice were measured every 2 d.The mice were euthanized by cervical dislocation after 14 d of continuous treatment,and the xenograft tissues were excised and weighed.Western blot assays were used to measure the protein expression of HIF-1α,PD1,PD-L1,CD4+ T cells,and CD8+ T cells in HCC tumours from mice.Quantitative reverse transcription polymerase chain reaction was used for real-time quantitative detection of PD-1,PD-L1,and HIF-1α mRNA expression.An immunofluorescence assay was conducted to examine the expression of CD4+ T cells and CD8+ T cells.RESULTSCompared to mice in the control group,those in the SYP and SYP + DDP groups exhibited reduced tumour volumes and tumour weights.Moreover,the protein and mRNA expression levels of the oncogene HIF1α and that of the negative immunomodulatory factors PD-1 and PD-L1 were decreased in both the SYP and SYP + DDP groups,with the decrease effects being more prominent in the SYP + DDP group than in the SYP group(HIF-1α protein:Control vs SYP,P=0.0129;control vs SYP + DDP,P=0.0004;control vs DDP,P=0.0152,SYP + DDP vs DDP,P=0.0448;HIF-1α mRNA:control vs SYP,P=0.0009;control vs SYP + DDP,P <0.0001;control vs DDP,P=0.0003,SYP vs SYP + DDP,P=0.0192.PD-1 protein:Control vs SYP,P=0.0099;control vs SYP + DDP,P <0.0001,SPY vs SYP + DDP,P=0.0009;SYP + DDP vs DDP,P <0.0001;PD-1 mRNA:control vs SYP,P=0.0002;control vs SYP + DDP,P <0.0001;control vs DDP,P=0.0003,SPY vs SYP + DDP,P=0.0003;SYP + DDP vs DDP,P=0.0002.PD-L1 protein:control vs SYP,P <0.0001;control vs SYP + DDP,P <0.0001;control vs DDP,P <0.0001,SPY vs SYP + DDP,P=0.0040;SYP + DDP vs DDP,P=0.0010;PD-L1 mRNA:Control vs SYP,P <0.0001;control vs SYP + DDP,P <0.0001;control vs DDP,P <0.0001,SPY vs SYP + DDP,P <0.0001;SYP + DDP vs DDP,P=0.0014).Additionally,the quantitative and protein expression levels of CD4+ T cells and CD8+ T cells were simultaneously upregulated in the SYP + DDP group,whereas only the expression of CD4+ T cells was upregulated in the SYP group.(CD4+ T cell quantitative:Control vsSYP + DDP,P <0.0001,SYP vs SYP + DDP,P=0.0005;SYP + DDP vs DDP,P=0.0002.CD4+ T cell protein:Control vs SYP,P=0.0033;Control vs SYP + DDP,P <0.0001;Control vs DDP,P=0.0021,SYP vs SYP + DDP,P=0.0004;SYP + DDP vs DDP,P=0.0006.Quantitative CD8+ T cells:Control vs SYP + DDP,P=0.0013;SYP vs SYP + DDP,P=0.0347;SYP + DDP vs DDP,P=0.0043.CD8+ T cell protein:Control vs SYP + DDP,P <0.0001;SYP vs SYP + DDP,P <0.0001;SYP + DDP vs DDP,P <0.0001).Finally,expression of HIF-1α was positively correlated with that of PD-1/PD-L1 and negatively correlated with the expression of CD4+ T cells and CD8+ T cells.CONCLUSIONSYPs inhibit immune escape and enhance chemosensitization in HCC via simultaneous inhibition of HIF-1α and PD-L1,thus inhibiting the growth of subcutaneous xenograft HCC tumours.
INTRODUCTION
Hepatocellular carcinoma(HCC)is a common malignancy that ranks 6thin cancer incidence and 4thin cancer-related mortality worldwide[1].Elucidation of the molecular changes underlying HCC development should unveil novel molecular targets for the development of therapies aimed at controlling tumour progression and improving patient survival.Changes in the tumour microenvironment play a crucial role in tumour development and progression.One of the most important changes is the development of immunosuppressive mechanisms by tumour cells,which allow them to escape the host’s immune system,thereby enhancing their survival and proliferative,migratory,and invasive capabilities[2].An important mechanism that mediates the immunosuppressive microenvironment is the overactivation of immune checkpoints,a major one of which is formed by programmed cell death protein 1(PD-1)and its ligand programmed cell death 1 ligand 1(PD-L1).PD-L1 is expressed on the surface of many types of tumour cells,and PD-1 is expressed on the surface of tumourinfiltrating lymphocytes(TILs).The binding of PD-L1 to PD-1 molecules can inhibit the function of T cells,limiting their ability to destroy tumour cells.This promotes tumour immune escape and leads to disease progression[3].Additionally,PD-L1 overexpression is associated with a poor prognosis in many cancer types and development of tumour cell resistance to anticancer therapies[4].Therefore,inhibition of the PD-1/PD-L1 signalling pathway,which would restore the normal tumour cell surveillance and destruction activities of T cells,may represent an effective therapeutic strategy against HCC[5,6].
Hypoxia,which is associated with an imbalance in rapid tumour growth and an insufficient blood supply,is another common change that occurs in the microenvironment of solid tumours[7].Extensive research has demonstrated that the expression of hypoxia-inducible factor-1 alpha(HIF-1α),which plays a crucial role in tumour angiogenesis,invasion,and metastasis,is elevated in hypoxic tumours[8,9].Additionally,hypoxia has been shown to induce tumour chemoresistance[10,11].HIF-1,the major transcription factor mediating the adaptive response to hypoxia,is a heterodimeric complex consisting of the HIF-1α and HIF-1β subunits,with HIF-1α being the major functional protein[12].Recent studies have identified a significant positive correlation between HIF-1α and PD-L1 in a variety of tumour cell lines[13],where the upregulation of PD-L1 mediated by HIF-1α constitutes a tumour immune escape mechanism[14-17].In one study,knockdown of HIF-1α using small interfering RNA prevented the accumulation of HIF-1α protein,which inhibited the hypoxiamediated increase in PDL1 mRNA and consequently its protein expression on the cell surface[18].The molecular mechanism underlying this correlation was explored in a previous study,wherein hypoxia was shown to cause rapid,significant,and selective upregulation of PD-L1 in bone marrow mesenchymal stem cells,macrophages,dendritic cells,and tumour cells[19].This upregulation of PD-L1 was dependent on HIF-1α,an upstream regulator of PD-L1 mRNA and protein expression.HIF-1α binds directly to the transcriptional active site—the hypoxia response element—of the PD-L1 proximal promoter,leading to rapid PD-L1 accumulation and subsequent tumour immune escape[19].Therefore,simultaneous inhibition of PD-L1 and HIF-1α expression represents a promising novel strategy in cancer immunotherapy.
Traditional Chinese medicine is an important treatment modality for HCC.Shuyu pills(SYPs)are composed of a compound formulation that has long been used as a traditional Chinese medicine for improving energy metabolism and immune function.The pills exert myriad health-benefiting effects,such as boosting one’s immunity,and can be used as an adjunct cancer treatment.Yam polysaccharides,a component of SYPs,can enhance the antioxidative capacity and free radical-scavenging activities of the body and thereby reduce cellular oxidative damage[20].They can also improve the immunomodulatory activities of splenic lymphocytes and enhance immune function[21].Other components of SYPs,such as ginsenosides,can regulate signal transduction pathways associated with inflammation,oxidative stress,angiogenesis,and tumour cell metastasis[22].Ginsenosides also regulate the cell cycle and inhibit the multidrug resistance of cancer cells and are involved in cancer immunomodulation[23].Trichosanthin,another SYP component,can inhibit the growth of tumour cells and induce their apoptosis[24]and displays potent immunosuppressive activity[25].Thus,it is evident that the components of SYPs exert a multitude of effects that can contribute to antitumour activity.As a solid tumour characterized by hypoxia and immune dysfunction,HCC is also prone to developing resistance to chemotherapeutic drugs during clinical treatment.Therefore,in this study,we investigated the efficacy of the combination of SYPs and cisplatin(DDP)for the treatment of HCC and the mechanism involved.
The therapeutic efficacy and mechanism of action of SYPs against HCC were explored from the perspectives of immune escape in the tumour microenvironment and chemoresistance.We hypothesized that SYPs exert antitumour effects by simultaneously inhibiting HCC cellular expression of HIF-1α and PD-L1,which would improve the immunosuppressive state of the tumour microenvironment.We also hypothesized that the SYP and DDP combination would mitigate chemoresistance by inhibiting the PD-1/PD-L1 axis.
MATERIALS AND METHODS
The preparation of SYPs and DDP
Each SYP contained the following components:Rhizoma Dioscoreae,Radix Angelicae Sinensis,Ramulus Cinnamomi,Medicinal Fermented Mass,Radix Rehmanniae,Ginseng,Radix Glycyrrhizae,Rhizoma Chuanxiong,Radix Paeoniae Alba,Rhizoma Atractylodis Macrocephalae,Radix Ophiopogonis,Semen Armeniacae Amarae,Radix Bupleuri,Radix Platycodi,Poria,Colla Corii Asini,Rhizoma Zingiberis,Radix Saposhnikoviae,Radix Ampelopsis,andFructus Jujubaein an 8:2:2:4:2:4:4:2:2:2:2:2:1:2:2:1:2:2:8 radio.Granules of the traditional Chinese medicinal formula were used,and all medicinal substances were purchased from Guangdong Yifang Pellet Pharmaceutical Co.,Ltd.(Guangdong,China;Lot No.17043278).All study parameters fulfilled standard quality requirements.All medicinal substances were dissolved in warm water,and the mixtures were then vortexed into a suspension.Based on the pharmacological dose requirements of SYPs,the daily dose of SYPs for human adults was 156 g.Therefore,the equivalent dose for each nude mouse was 200 mg/kg according to the Table of Equivalent Dose Ratio Conversion between Human and Animal by Body Surface Area.DDP injections(Cat No.9E0214B02)were purchased from Qilu Pharmaceutical Co.,Ltd.(Shandong,China).The DDP dose was 5 mg/kg,administered intraperitoneally once a week.
The study of SYPs using high resolution mass spectrometry
SYPs were extracted using 100% methanol,and 5.000 g SYPs were extracted using 20.00 mL anhydrous methanol by ultrasonic extraction for 45 min.Then,the supernatant was centrifuged at 8000 RPM for 5 min.After centrifugation,the supernatant was filtered through a 0.22 μm microporous filter membrane,and the filtrate was analysed using UPLC-Q-TOF-MS(1290 UPLC-6540,Agilent Technologies Inc.,United States).We used an Agilent ZORBAX Eclipse Plus C18(3.0 mm × 100 mm,1.8 μm)column,and the mobile phase system consisted of acetonitrile(A)and water(containing 0.1% formic acid).The gradient elution procedure was used under the following conditions:0–10 min,5%–15% A;10–15 min,15%–20% A;15–25 min,25%–45% A;and 25–40 min,45%–80%.The flow velocity of the 1 μL sample volume was 0.4 mL/min.Mass spectrometry testing conditions were set to ionization mode and electrospray ionization,and accurate mass data correction were performed using electrospray ionization-L Low Concentration Tuning Mix(G1969–85000).Then,positive and negative ion analysis modes and MRE scan modes were adopted to analyse the samples.The sheath gas temperature was 350 °C,and the range of fullmass scanning was 100–1700 m/z.In addition,the capillary voltage was set to 4.0 KV.Nitrogen was chosen as the desolventizer gas,and the temperature was set at 325 °C with a flow rate of 6.8 L/min.Furthermore,to obtain an accurate analysis after primary scanning,secondary mass spectrometry was performed by dependent scanning,and the first three strengths were also selected for collision-induced dissociation.The range of secondary fragment scanning was 50–1000 m/z,and the fragment voltages were set to 10,20,and 30 kV.
Cells and animal grouping
A subcutaneous xenograft tumour model was established in BALB/c athymic nude mice(n=24,5 weeks old,18 ± 3 g)viathe subcutaneous injection of 1 × 107SMMC-7721 cells into the right side of the back of each mouse.Once the tumours were palpable in the mice(tumour volume,approximately 100 mm3),the animals were randomly divided into the following four groups(n=6):(1)Control(oral dose of 0.2 mL of 0.9% normal saline,daily);(2)SYP(oral dose of 200 mg/kg,daily);(3)DDP(intraperitoneal dose 5 mg/kg,once weekly);and(4)SYP(oral dose of 200 mg/kg,daily)+ DDP(intraperitoneal dose 5 mg/kg,once weekly).The tumour volume[calculated as the maximum tumour length × width(2 × 0.5)]and body weight of each mouse were measured every 2 d.Fourteen days after treatment,the mice were euthanized by cervical dislocation according to Animal Research:ReportingIn VivoExperiments.This study was approved by the Ethics Review Committee of Experimental Animal Welfare of Central South University and performed in accordance with the European Community Guidelines on the Use and Care of Laboratory Animals,with all laboratory animals being carefully attended to.
Western blot analysis
Protein extraction was performed for western blot analysis.In brief,mouse tumour tissue specimens were first lysed in ice-cold lysis buffer[150 mmol/L NaCl,20 mmol/L HEPES,1% Triton X-100,2 mmol/L EGTA,20 mmol/L glycerophosphate,1 mmol/L EDTA,and 10% glycerol plus protease inhibitor(ApplyGene,Inc.)].Then,the protein concentrations of the tissue lysates were measured using the bicinchoninic acid assay.Next,50 μg of the denatured protein was loaded onto a 4% sodium dodecyl sulphate-polyacrylamide gel for electrophoresis,after which the separated protein bands were electrotransferred to a PVDF membrane(EMD Millipore)for 2 h.After transfer,the membrane was soaked in a blocking solution(5% milk in 1 × TBST)at ambient temperature for 2 h and then at 4 °C overnight.The next day,the membrane was incubated with the primary antibodies at 37 °C for 60 min and then with the secondary antibodies at 4 °C overnight.The primary antibodies were as follows:Anti-PD-1(PD-1;Cat No.ab214421;dilution,1:1000;monoclonal antibody;Abcam);anti-PD-L1(PD-L1;Cat No.ab238697;dilution,1 μg/mL;monoclonal antibody;Abcam);anti-CD4(CD4;Cat No.MA1-146;dilution,1:1000;monoclonal antibody;Invitrogen Antibodies);anti-CD8(CD8;Cat No.ab209775;dilution,1:1000;monoclonal antibody;Abcam);anti-HIF-1α(HIF-1α;Cat No.ab1;dilution,5 μg/mL;monoclonal antibody;Abcam);and anti-p53(p53;Cat No.ab26;dilution,2 μg/mL;monoclonal antibody;Abcam).An anti-actin antibody(Cat No.60008–1-Ig;dilution,1:5000;monoclonal antibody;Proteintech Group,Inc.)was used as the protein-loading control.A horseradish peroxidase-conjugated polyclonal secondary antibody(dilution,1:5000;Proteintech Group,Inc.)was used for detection.SuperECL Plus detection reagent(Thermo Fisher Scientific,Inc.)was used as an enhanced chemiluminescent substrate to enable visualization of the protein bands.The protein signals could be visualized for 3 min and were exposed to Kodak Biomax XAR film(Kodak).ImageJ version 1.80 software(National Institutes of Health)was used to scan and quantify the intensity of each protein band.
Quantitative reverse-transcription polymerase chain reaction
Total RNA was extracted from SMMC-7721 cells using TRIzol reagent(Takara)according to the manufacturer’s instructions.Reverse transcription was then performed using cDNA reverse transcriptase.The polymerase chain reaction(PCR)a conditions were as follows:95 °C for 10 min,followed by 40 cycles of 95 °C for 15 s and 60 °C for 30 s.The following primer sequences were used for PCR:Actin,AC ATCCGTAAAGACCTCTATGCC(forward)and TACTCCTGCTTGCTGATCCAC(reverse);p53,CCCCTGTCATCTTTTGTCCCT(forward)and AGCTGGCAGAATA GCTTATTGAG(reverse);HIF-1α,TCCAGCAGACCCAGTTACAGA(forward)and GCCACTGTATGCTGATGCCTT(reverse);PD-1,GCACCCCAAGGCAAAAATCG(forward)and CAATACAGGGATACCCACTAGGG(reverse);and PD-L1,AAAGACGAGCATAGCCGAAC(forward)and GCCACACCAATCCAACACC(reverse).
Immunofluorescence assay
The tumour tissues were embedded into paraffin blocks using standard techniques,and the blocks were then sectioned into 4-μm slices.After the sections had been deparaffinized in water,they were subjected to heat-induced antigen retrieval in 0.01 M citrate buffer(pH 6.0).Then,once the sections had cooled to ambient temperature,they were washed 3 times with 0.01 M poly(butylene succinate)(PBS)(pH 7.2–7.6)for 3 min each time.The sections were then placed in sodium borohydride solution for 30 min at ambient temperature and thereafter rinsed with water for 5 min.Next,the sections were soaked in Sudan Black B staining solution at ambient temperature for 5 min,followed by rinsing with water for 3 min.Next,the sections were blocked in 10% normal serum/5% BSA for 60 min and then incubated overnight with appropriately diluted primary antibodies(CD4,CD8)at 4 °C.On the next day,the sections were rinsed 3 times with PBS for 5 min each time and then incubated with the secondary antibody(50–100 μL of anti-rat,rat-IgG-labelled fluorescent antibody)at 37 °C for 90 min.The sections were then rinsed 3 times with PBS for 5 min each.A working solution of 4',6-diamidino-2-phenylindole was used to stain the cell nuclei at 37 °C for 10 min,and the sections were then rinsed 3 times with PBS for 5 min each.Finally,the sections were mounted in buffered glycerol and stored in the dark until subsequent observation under a confocal fluorescence microscope.
Statistical analysis
All experiments were performed at least 3 times,and the results are expressed as the mean ± standard error of the mean.Data were analyzed for statistical significance using Student’st-test,withP<0.05 considered statistically significant.SPSS 17.0 software(SPSS,Chicago,IL,United States)was used for all statistical analyses.
RESULTS
Finger-print of SYPs
To elucidate the mechanism of action of SYPs against HCC,the primary components of SYPs were analysed by high-resolution mass spectrometry.The compounds were identified by extracting ion flow diagrams and comparing their molecular formulae with information in the literature and databases.A total of 20 compounds were analysed as follows:Amygdalin,albiflorin,paeoniflorin,prime-O-glucosylcimifugin,liquiritin,cimitin,5-O-methylvisammil glycoside,apigenin liquiritin,ginsenoside Rg1,ononin,isoliquiritin,platycodon D3,6-shogaol,glyyunnanprosapogenin D,glycyrrhizic acid,formononetin,licoricesaponine H2,ethylcinamate,glycyrrhisoflavanone,and ligustilide(Table 1).As shown in Figure 1,the 20 pharmaceutical ingredients matched in the fingerprints of SYPs were labelled,and the structure was analysed by mass spectrometry according to the chromatographic retention time.
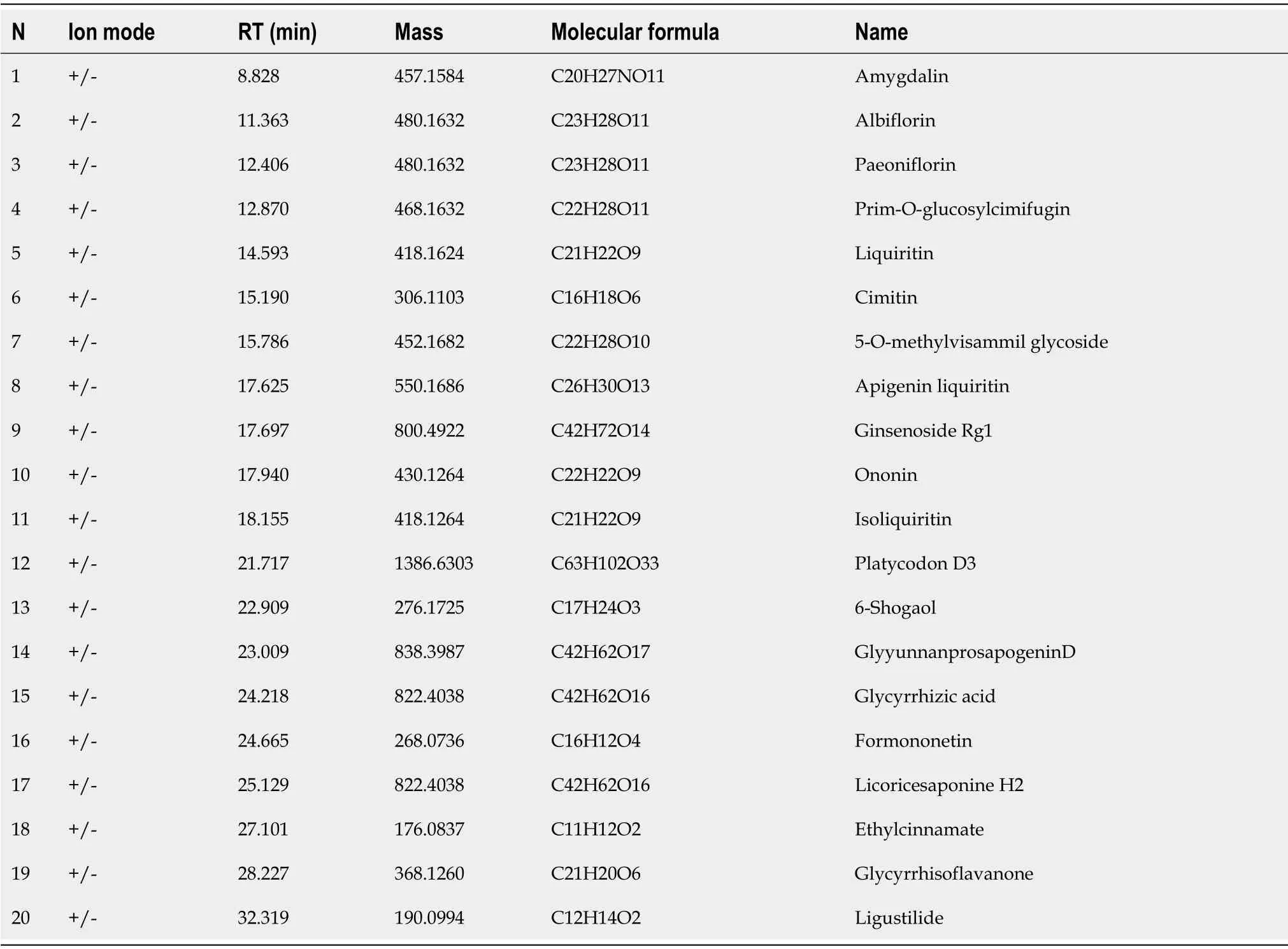
Table 1 Analysis information of 20 compounds in Shuyu pills
Effect of SYPs on the growth of subcutaneous xenografts of human HCC in nude mice
A subcutaneous xenograft tumour model was established in male BALB/c nude mice to validate whether SYPs could inhibit tumour growthin vivo.Tumour volume and body weight were measured every 2 d for 14 d.The mice were euthanized after 14 d of treatment,and the final tumour volumes and body weights were recorded.As observed from thein vivotumour growth curves,the SYPs and DDP inhibited the growth of the tumours(Figure 2A),with the tumour volumes and tumour weights observed in the SYP,SYP + DDP,and DDP groups being lower than those measuredin the control group.The inhibitory effect on tumour volume was more prominent in the SYP + DDP group than in either the SYP or DDP individual treatment groups(Figure 2B and C).The overall body weights of the mice did not decrease and were not significantly different among the four groups(Figure 2D).These findings indicate that SYPs inhibited the growth of human HCC tumours in nude mice,and their combined use with DDP exhibited a synergistic effect.
Expression of HIF-1α and PD-1/PD-L1
Next,we sought to investigate the antitumour mechanism of the SYPs.Western blot assays were used to measure the protein expression of HIF-1α,PD-1,and PD-L1 in tumours excised from the mice,whereas quantitative reverse-transcription polymerase chain reaction was used to measure mRNA expression of the three genes(Figure 3).Compared to levels in the control group,the protein and mRNA expression levels of the oncogene HIF-1α were significantly lower in the SYP,DDP,and SYP + DDP groups.Moreover,the expression of HIF-1α protein was significantly lower in the SYP + DDP group than in the DDP group,whereas the expression of HIF-1α mRNA was significantly lower in the SYP + DDP group than in the SYP group(HIF-1α protein:ControlvsSYP,P=0.0129;controlvsSYP + DDP,P=0.0004;controlvsDDP,P=0.0152,SYP + DDPvsDDP,P=0.0448;HIF-1α mRNA:ControlvsSYP,P=0.0009;controlvsSYP + DDP,P<0.0001;controlvsDDP,P=0.0003,SYPvsSYP + DDP,P=0.0192).Analysis of the immune checkpoint PD1/PD-L1 revealed that compared to its expression in the control group,protein expression of PD-1 was significantly reduced in the SYP and SYP + DDP groups,and the mRNA expression of PD-1 was significantly reduced in the SYP,SYP + DDP,and DDP groups.Moreover,the PD-1 protein and mRNA expression levels in the SYP + DDP group were significantly lower than its levels in the SYP and DDP groups(PD-1 protein:ControlvsSYP,P=0.0099;controlvsSYP + DDP,P<0.0001,SPYvsSYP + DDP,P=0.0009;SYP + DDPvsDDP,P<0.0001;PD-1 mRNA:ControlvsSYP,P=0.0002;controlvsSYP + DDP,P<0.0001;controlvsDDP,P=0.0003,SPYvsSYP + DDP,P=0.0003;SYP + DDPvsDDP,P=0.0002).Similarly,the protein and mRNA expression levels of the oncogene PD-L1 were significantly lower in the SYP,SYP + DDP,and DDP groups than in the control group.Moreover,the PD-L1 protein and mRNA expression levels were significantly lower in the SYP + DDP group than in the SYP and DDP groups(PD-L1 protein:ControlvsSYP,P<0.0001;controlvsSYP + DDP,P<0.0001;controlvsDDP,P<0.0001,SPYvsSYP + DDP,P=0.0040;SYP + DDPvsDDP,P=0.0010;PD-L1 mRNA:ControlvsSYP,P<0.0001;controlvsSYP + DDP,P<0.0001;controlvsDDP,P<0.0001,SPYvsSYP + DDP,P<0.0001;SYP + DDPvsDDP,P<0.0014).Notably,the protein and mRNA expression trends of HIF-1α,PD-1,and PD-L1 were similar.These findings indicate that SYPs and DDP simultaneously inhibit the expression of HIF-1α,PD-1,and PD-L1 in subcutaneous xenograft tumours in nude mice,with the combination of the two types of drugs displaying a synergistic effect.
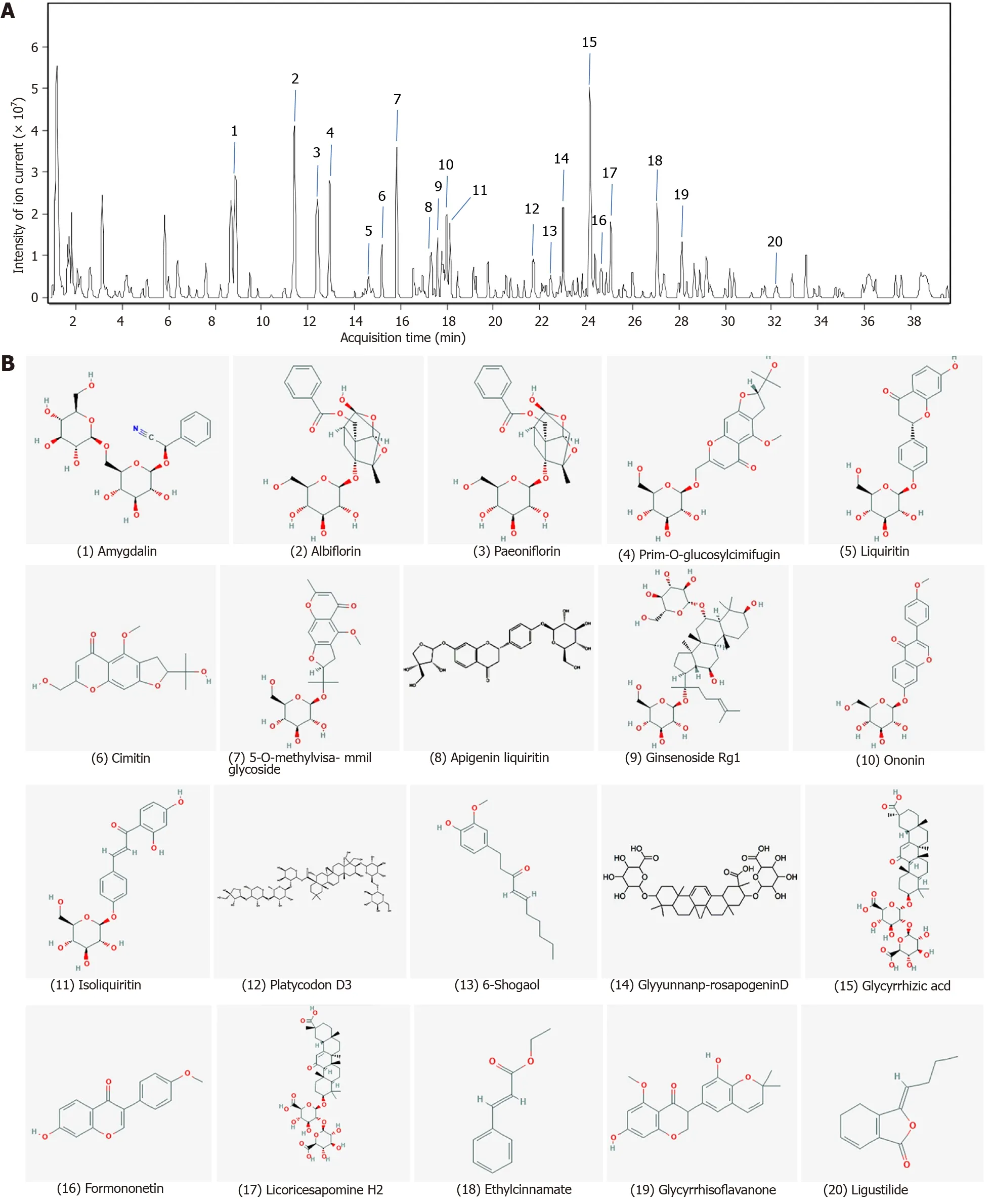
Figure 1 Finger-print of Shuyu pills.The finger-print of Shuyu pills was determined by high resolution mass spectrometry.A:The 20 pharmaceutical ingredients were labeled according to the chromatographic retention time and their structures were analyzed by mass spectrometry;B:Chemical structure formulae of 20 compounds.Chemical structures and formulae of 20 compounds from PubChem(https://pubchem.ncbi.nlm.nih.gov/),numbered according to the compounds information in Table 1.
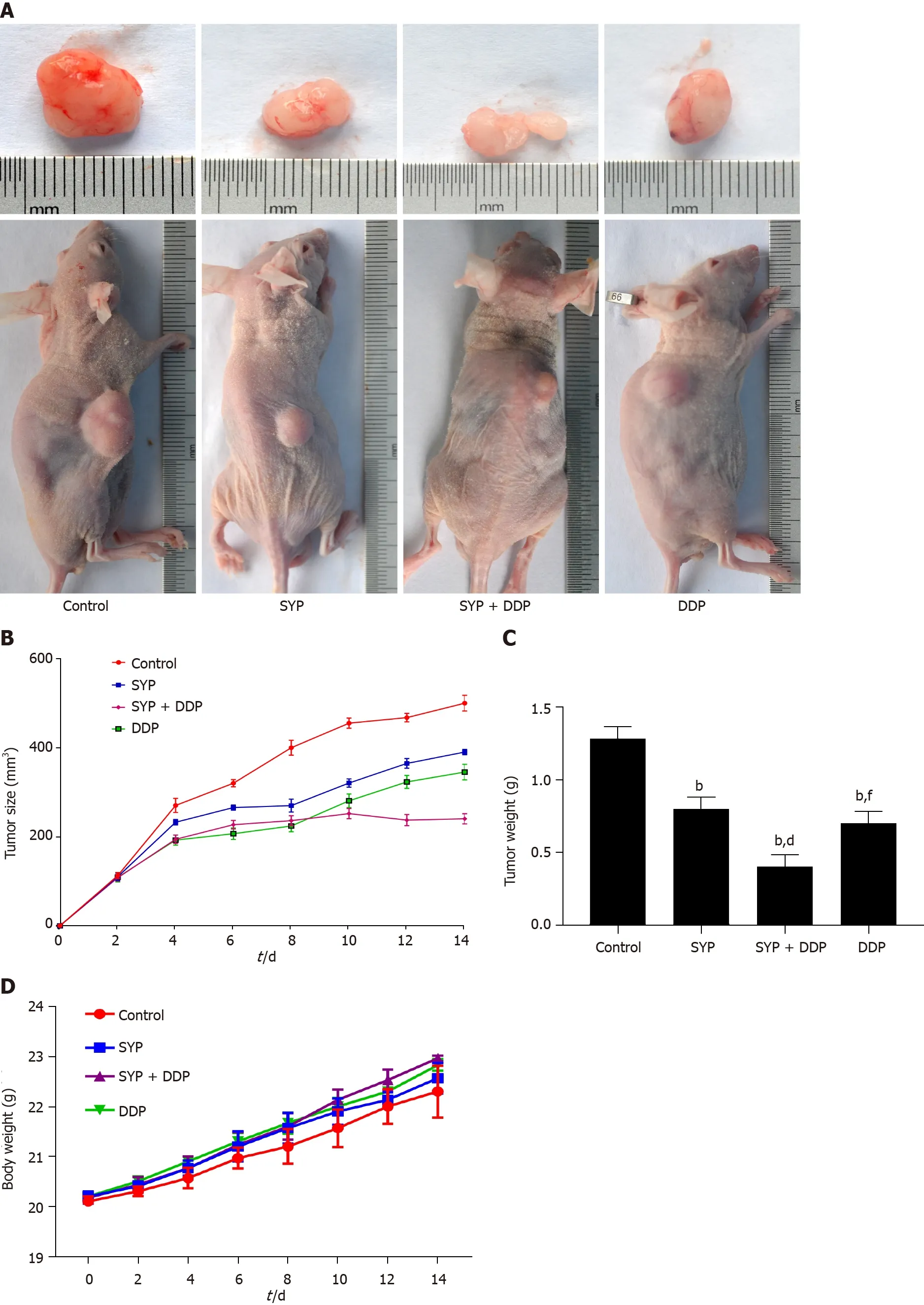
Figure 2 Shuyu pills inhibited the growth of hepatocellular carcinoma in vivo.The xenograft mouse model was established in BALB/c nude mice that were then randomly divided into four groups(n=6):Control group(0.9% normal saline,daily),Shuyu pills(SYP)(200 mg/kg,daily),Cisplatin(DDP)(5 mg/kg,once a week),and SYP(200 mg/kg,daily)+ DDP(5 mg/kg,once a week).The body weight of each mouse and the tumor volume were measured every 2 d,with the latter calculated as follows:Maximum tumor length × width(2 × 0.5).A:Representative images of the tumors at the end of treatment;B:Average tumor volumes,measured every 2 d;C:Tumor weights at the end of treatment;D:Average body weights of the mice,measured every 2 d.bP <0.01 vsControl group;dP <0.01 vs SYP group;fP <0.01 vs SYP + DDP group.SYP:Shuyu pills;DDP:Cisplatin.
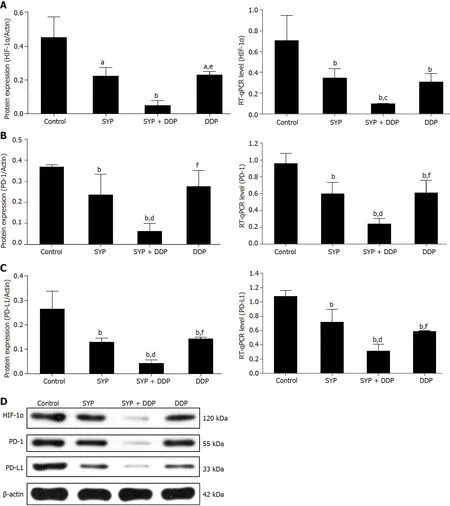
Figure 3 Shuyu pills inhibited the expression of hypoxia-inducible factor-1 alpha,programmed cell death 1,and programmed cell death 1 ligand 1.Nude mice injected subcutaneously with human hepatocellular carcinoma cells were treated with Shuyu pills(SYP),Cisplatin(DDP),or a combination of the two for 14 d,following which the tumor tissues were harvested as indicated.A–C:Western blot and quantitative reverse transcription polymerase chain reaction assays were used to respectively detect the protein and mRNA expression levels of hypoxia-inducible factor-1 alpha(HIF-1α),programmed cell death protein 1(PD-1),and programmed cell death 1 ligand 1(PD-L1)in the tumor tissue;D:Representative protein expression patterns of HIF-1α,PD-1,and PD-L1 as measured by western blot assay.Data are presented as the mean ± standard error of the mean,and comparisons between two groups were performed using the least significant difference test or Dunnett’s T3 method.aP <0.05 and bP <0.01 vs Control group;cP <0.05 and dP <0.01 vs SYP group;eP <0.05 and fP <0.01 vs SYP + DDP group.SYP:Shuyu pills;DDP:Cisplatin;HIF-1α:Hypoxia-inducible factor-1 alpha;PD-1:Programmed cell death 1;PD-L1:Programmed cell death 1 ligand 1.
Expression of CD4+ T cells and CD8+ T cells
To determine the effect of SYPs on immune function,immunofluorescence assays were conducted to determine the levels of CD4-expressing(CD4+)T cells and CD8-expressing(CD8+)T cells in subcutaneous xenograft tumours.Images were acquired under a fluorescence microscope for quantitative analysis of the fluorescence signals,and protein expression was determined using the western blot assay(Figure 4).Figure 4A shows immunofluorescence images of CD4+ T cells and CD8+ T cells,where quantitative analysis revealed that expression of CD4+ T cells was higher in the SYP + DDP groups than in the control group,and expression levels were significantly higher in the SYP + DDP group than in the SPY group or DDP group(SYP + DDPvscontrol,P<0.0001,SYPvsSYP + DDP,P=0.0005;SYP + DDPvsDDP,P=0.0002).Moreover,quantitative expression of CD8+ T cells exhibited similar results(controlvsSYP + DDP,P=0.0013;SYPvsSYP + DDP,P=0.0347;SYP + DDPvsDDP,P=0.0043)(Figure 3B).The western blot results showed that expression of CD4+ T cell protein was significantly higher in the SYP,SYP + DDP,and DDP groups than in the control group(controlvsSYP,P=0.0033;controlvsSYP + DDP,P<0.0001;controlvsDDP,P=0.0021).Moreover,expression levels were significantly higher in the SYP + DDP group than in the SYP or DDP group(SYPvsSYP + DDP,P=0.0004;SYP + DDPvsDDP,P=0.0006).In contrast,protein expression of CD8+ T cells was significantly higher in the SYP + DDP group than in the control,SYP,or DDP group(controlvsSYP + DDP,P<0.0001;SYPvsSYP + DDP,P<0.0001;SYP + DDPvsDDP,P<0.0001).These findings indicate that SYPs and DDP upregulated the expression of CD4+ T cells and CD8+ T cells in HCC xenografts in nude mice,whereas the use of SYPs alone only upregulated the expression of CD4+ T cells.
DISCUSSION
HCC is one of the most commonly diagnosed malignant diseases worldwide.Despite the significant progress made in its diagnosis and the advanced developments in cancer treatment modalities(e.g.,surgery,chemoradiotherapy,and targeted therapy),the prognosis for patients with HCC remains poor owing to the high recurrence and metastatic potential of the cancer cells[26].The ability of tumour cells to avoid immune destruction(immune escape)and their development of resistance to chemotherapeutic drugs[27]are key hurdles to the effective control of tumour progression[28,29].Tumour immune escape is an important mechanism that involves interactions between PD-1 molecules on cytotoxic T lymphocytes(CTLs)and PD-L1 molecules on tumour cells or other immune cells in the body.The PD-1/PD-L1 axis,which is one of several immune checkpoint modulators,primarily acts by inhibiting the adaptive Tcell response.Physiologically,it is implicated in self-tolerance and limitations in the immune response duration and magnitude[30].Tumour cells exploit the PD-1/PD-L1 immunomodulatory mechanism by activating the PD-1/PD-L1 axis,which deactivates CTLs and leads to their exhaustion,apoptosis,and reduced cytokine production,thereby suppressing the adaptive antitumour response[31].TILs play a crucial role in the antitumour immune response of the tumour host by specifically binding to and killing tumour cells or inducing their apoptosis[32].PD-L1 is highly expressed on tumour cells,whereas PD-1 is highly expressed on TILs.The binding of PD-L1 to PD-1 inhibits the activation of TILs and induces their apoptosis[33],which in turn inhibits the host’s antitumour immune response,resulting in tumour immune escape[34,35].CD8,a glycoprotein on the T-cell surface that contributes to antigen recognition by Tcell receptors(TCRs),is also involved in signal transduction during T-cell activation and is known as the coreceptor of TCRs.Upon activation,CD8+ T cells differentiate into CTLs,which can specifically recognize tumour-associated antigens presented on MHC class I molecules on the tumour cell surface.CTLs can also destroy tumour cells directly[36,37].A higher level of CD8+ T-cell infiltration in a tumour generally indicates a stronger immune response against the tumour and thus a more favourable prognosis[32,38].CD4+ T cells are equally important immune cells in the human body,where they secrete cytokines that help to initiate and maintain the CD8+ T-cellmediated antitumour response[39,40].However,because activation of the PD1/PD-L1 signalling pathway can specifically induce CTL apoptosis,this reduces their ability to kill tumour cells and promotes tumour immune escape.Therefore,the inhibition of PD-1/PD-L1 expression enhances the abilities of CD4+ T cells and CD8+ T cells to kill tumour cells or to induce tumour cell apoptosis,thereby preventing immune escape.
Recently,ample evidence has emerged showing that hypoxia,a common feature of many solid cancers,including HCC,contributes to tumour immune escape through multiple mechanisms[41].Notably,hypoxia significantly increases the expression of PD-L1 on myeloid-derived suppressor cells,macrophages,dendritic cells,and tumour cells,conferring them with additional resistance to CTL-mediated lysis.This is primarily due to the accumulation of HIF-1α[42]the factor that participates in the hypoxic response of tumour cells.HIF-1α activates myriad genes essential for HCC angiogenesis,proliferation,glucose metabolism,invasion,metastasis,and resistance to radiotherapy and chemotherapy[43-45].Hypoxia confers cells with resistance to CTLmediated lysis by upregulating HIF-1α and PD-L1,making these two proteins promising molecular targets for cancer treatment.A positive correlation between PDL1 and HIF-1α has also been identified in HCC tissues,and patients with HCC tumour tissues overexpressing both of these proteins exhibited a significantly increased risk of recurrence,metastasis,or death[42].The combination of HIF-1α inhibitors with PD-L1 blockade to target tumour hypoxia may represent a novel immunotherapy for overcoming weakened antitumour cytotoxicity and strengthening the immune system of patients with cancer.However,it remains unclear whether the simultaneous inhibition of HIF-1α and PD-L1 can suppress tumour immune escape in HCC.
Our study revealed that SYPs inhibited the growth of HCC subcutaneous xenograft tumours in nude mice,and the combined use of SYPs with DDP displayed a synergistic effect.With regard to the mechanism,SYPs simultaneously inhibited the expression of HIF-1α and the PD-1/PD-L1 axis and increased the expression of CD4+ T cells,and these effects were synergistic in the presence of DDP.Furthermore,expression of HIF-1α was positively correlated with expression of PD-1 and PD-L1 and negatively with that of CD4+ T cells and CD8+ T cells.These findings suggest that SYPs may inhibit the activation of HIF-1α,which in turn inhibits the expression of the PD-1/PD-L1 axis,thereby promoting the tumour-killing effects of CD4+ and CD8+ T cells and preventing immune escape in the tumour microenvironment.This novel immunotherapeutic approach of simultaneously inhibiting HIF-1α and PD-L1 expression exerts an antitumour effect in HCC.
It is worth noting again that the combination of SYPs and DDP displayed a synergistic effect in the present study.Research indicates that the interaction between PD-1 and PD-L1 contributes to the resistance of tumour cells to conventional chemotherapeutic drugs.Studies usingin vivotumour models have shown that anti-PD-L1 therapies that inhibit the PD-1/PD-L1 axis enhance the efficacy of conventional chemotherapy in preventing metastasis.Therefore,blockade of the PD-1/PD-L1 axis is an effective strategy for targeting immune checkpoints.Additionally,the combination of chemotherapy and immune checkpoint blockade is a novel treatment approach that can reduce tumour drug resistance and improve the effectiveness of chemotherapies[4].Based on these findings,we speculate that the synergistic effect of the SYP and DDP combination may be due to blockade of the PD-1/PD-L1 immune checkpoint,which mitigates the resistance of HCC cells to DDP and sensitizes the cells to this chemotherapeutic drug.Moreover,local hypoxia in the tumour microenvironment induces adaptations of tumour cells and enhances their chemoresistance[46].Solid tumours are dynamic and heterogeneous structures in which the oxygen tension is significantly lower than that in adjacent normal tissues.Since the vascular system cannot provide sufficient oxygen for the growing tumour,the long diffusion distance between the hypoxic regions and the tumour blood vessels limits the distribution of drugs.Many chemotherapeutic drugs,including platinum,require oxygen as an electron acceptor to induce cell death[47].Therefore,the regulation of HIF-1α expression can reduce the chemoresistance caused by hypoxia in the tumour microenvironment[48].Furthermore,we speculate that the synergistic effect of the SYP and DDP combination may also be due to the SYP-mediated improvement of the hypoxic state in the tumour microenvironment,which inhibits the hypoxia-induced chemoresistance of HCC cells and enhances their sensitivity to chemotherapy.Taken together,our data provide valuable insights for future research on HCC chemosensitization.
CONCLUSION
SYPs inhibit immune escape and enhance chemosensitization in HCCviasimultaneous inhibition of HIF-1α and PD-L1,thus inhibiting the growth of subcutaneous xenograft tumours with HCC.
ARTICLE HIGHLIGHTS
Research background
Hepatocellular carcinoma(HCC)is characterized by dysregulation of the immune microenvironment and the development of chemoresistance.The latest research shows that the simultaneous inhibition of hypoxia-inducible factor-1 alpha(HIF-1α)and programmed cell death protein 1(PD-1)has the potential to enhance the hosts antitumour immunity.Moreover,inhibition of the PD-1/programmed cell death 1 ligand 1(PD-L1)axis may mitigate tumour chemoresistance.
Research motivation
Shuyu pills(SYPs)contain immunity-enhancing and antitumour components,making them a potential HCC treatment.The motivation of this research was to study the effect and mechanism of SYPs on HCC.
Research objectives
To investigate the efficacy of SYPs for HCC treatmentviasimultaneous HIF-1α and PD-L1 inhibition and the mechanism involved.
Research methods
The subcutaneous xenograft tumours model was established in BALB/c nude mice.The male mice(male,5 weeks old;n=24)were then randomly divided into the four groups(n=6):Control group(0.9% normal saline),SYP group(200 mg/kg),SYP + cisplatin(DDP)group(200 mg/kg + 5 mg/kg weeklyviaintraperitoneal injection),and DDP group(5 mg/kg weeklyviaintraperitoneal injection).The tumour volumes and body weights of the mice were measured every 2 d.The mice were euthanized by cervical dislocation after 14 d of continuous treatment,and the xenograft tissues were excised and weighed.The western blot assay was used to measure the protein expression of HIF-1α,PD-1,PD-L1,CD4+ T cells,and CD8+ T cells in the HCC tumours from the mice.Quantitative reverse transcription polymerase chain reaction a was used for the real-time quantitative detection of PD-1,PD-L1,and HIF-1α mRNA expression.The immunofluorescence assay was conducted to examine the expression of CD4+ T cells and CD8+ T cells.
Research results
Compared with the mice in the control group,those in the SYP and SYP + DDP groups had lower tumour volumes and tumour weights.Moreover,the protein and mRNA expressions of the oncogene HIF-1α and that of the negative immunomodulatory factors PD-1 and PD-L1 were decreased in both the SYP and SYP + DDP groups,with the decrease effects being more prominent in the SYP + DDP group than in the SYP group.Additionally,the quantitative and protein expressions of CD4+ T cells and CD8+ T cells were simultaneously upregulated in the SYP + DDP group,whereas only the expressions of CD4+ T cells were upregulated in the SYP group.Finally,the expression of HIF-1α was found to be positively correlated with that of PD-1/PD-L1 and negatively correlated with the expression of the CD4+ T cells and CD8+ T cells.
Research conclusions
SYPs inhibit immune escape and enhance chemosensitization in HCCviasimultaneous inhibition of HIF-1α and PD-L1,thus inhibiting the growth of subcutaneous xenograft tumours with HCC.
Research perspectives
SYPs inhibit immune escape and enhance chemosensitization in HCC.It is a potential adjuvant drug for the treatment of HCC.
 World Journal of Gastrointestinal Oncology2021年11期
World Journal of Gastrointestinal Oncology2021年11期
- World Journal of Gastrointestinal Oncology的其它文章
- Hepatocellular carcinoma biomarkers,an imminent need
- Anatomical vs nonanatomical liver resection for solitary hepatocellular carcinoma:A systematic review and meta-analysis
- Atezolizumab plus bevacizumab versus sorafenib or atezolizumab alone for unresectable hepatocellular carcinoma:A systematic review
- Cell-free DNA liquid biopsy for early detection of gastrointestinal cancers:A systematic review
- Colorectal cancer in Arab world:A systematic review
- Induction chemotherapy with albumin-bound paclitaxel plus lobaplatin followed by concurrent radiochemotherapy for locally advanced esophageal cancer
