Radiomics in hepatocellular carcinoma:A state-of-the-art review
Shan Yao,Zheng Ye,Yi Wei,Han-Yu Jiang,Bin Song
Shan Yao,Zheng Ye,Yi Wei,Han-Yu Jiang,Bin Song,Department of Radiology,West China Hospital,Sichuan University,Chengdu 610041,Sichuan Province,China
Abstract Hepatocellular carcinoma(HCC)is the most common cancer and the second major contributor to cancer-related mortality.Radiomics,a burgeoning technology that can provide invisible high-dimensional quantitative and mineable data derived from routine-acquired images,has enormous potential for HCC management from diagnosis to prognosis as well as providing contributions to the rapidly developing deep learning methodology.This article aims to review the radiomics approach and its current state-of-the-art clinical application scenario in HCC.The limitations,challenges,and thoughts on future directions are also summarized.
Key Words:Hepatocellular carcinoma;Radiomics;Deep learning;Artificial intelligence;Medical imaging;Predictive modeling
INTRODUCTION
Hepatocellular carcinoma(HCC)is the most common cancer with fast rising incidence in both males and females and the second major contributor to cancer-related mortality worldwide[1,2].Medical imaging has been playing a pivotal role in the entire diagnosis and management process of HCC,with the capacity to non-invasively provide multi-parameter,multidimensional,and multi-modality structural and functional information on lesion and peri-tissues on computed tomography(CT)and magnetic resonance imaging(MRI)[3-7].
Although the current diagnosis and treatment system continues to improve progressively,some crucial aspects such as the high heterogeneity and diverse biological behaviors of HCC tumors,which directly affect the prognosis and survival of patients,remain a concern and need to be addressed[8,9].
However,certain limitations of traditional imaging and report methods such as insufficient depth of imaging feature interpretations,the influence of subjective variability among observers,and unavailability to meet the needs of modern precise medicine may hinder comprehensive evaluations and personalized treatment of HCC.
In recent years,with rapid developments in big data mining and artificial intelligence(AI)fields,medical imaging in gastrointestinal and abdominal diseases has been empowered with more efficient combinations of data[10-12].Radiomics,a burgeoning technology that could transform potential pathological and physiological information from routine-acquired images into high-dimensional quantitative and mineable imaging data[13-15],has been demonstrating great potential in the diagnosis,classification and staging,clinical decision assistance,and prognosis and survival predictions of HCC.
Hence,this article reviews the radiomics approach and its current state-of-the-art clinical application scenario in HCC.Additionally,the limitations,challenges,and thoughts on future directions are summarized.
RADIOMICS BASIC WORKFLOW IN HCC
Radiomics is a multi-disciplinary technology that refers to extraction and analysis of a large number of advanced and quantitative image features from medical imaging such as CT,MRI,positron emission tomography(PET),or ultrasound(US),with high fidelity and high throughput[13,15,16].The core steps include data acquisition,image segmentation,feature extractions,analysis,and model building and validation.Most current research on radiomics in HCC was performed with the general procedure described above(Figure 1).
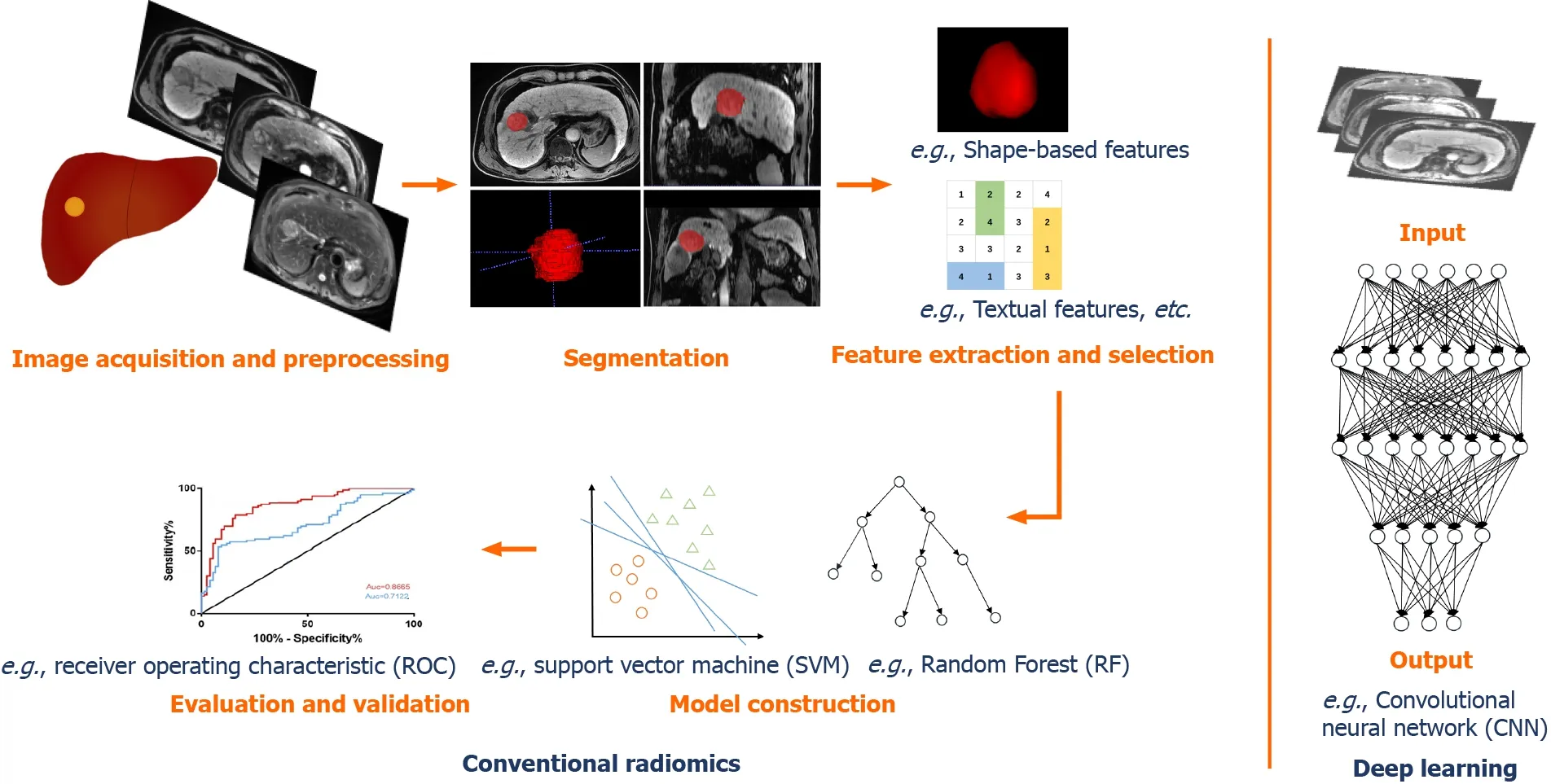
Figure 1 General workflow of radiomics and deep learning in hepatocellular carcinoma.
Image acquisition and preprocessing
At the beginning and as the basis of radiomics flow,medical images can be acquired using CT,MRI,US,or PET for single- or multi-center studies with retrospective or prospective cohorts and different task targets.CT and MRI-based,retrospective,single-center studies account for the vast majority of HCC radiomics publications.Given that the reproducibility and comparability of image characteristic analysis are influenced by facilities,platforms,parameters,and factors like those in clinical practice,there is a clear need for standardized image acquisition and reconstruction protocols[15,16].Besides,in order to avoid bias due to inconsistent pixels,gray levels,or variable resolutions,image preprocessing mainly using resampling and normalization is indispensable to ensure a feasible and repeatable subsequent analysis[17,18].
Segmentation
Segmentation of the regions of interest(ROIs)or the volumes of interest(VOIs)is normally performed in three ways:Manual,semi-automatic,and automatic,among which the first is used most often at present.Manual segmentation relies on the radiologists to identify and annotate lesions manually.It has the advantage of higher accuracy,although it is time-consuming with low efficiency and inter-operator variability.There is a great availability of open-source software for segmentation,such as ITK-SNAP(www.itksnap.org),3D Slicer(www.slicer.org),MIM(www.mimsoft ware.com),and ImageJ(https://imagej.nih.gov/ij/).In recent years,semi-automatic and automatic segmentations have been more developed with the assistance of a series of computer algorithms[19-23].
Feature extraction and selection
A number of features can be extracted from the 2D ROIs or 3D VOIs,which are attributed to the basis of radiomics analysis.Features can be divided into two types:“Semantic” and “agnostic”[15].The “semantic” features include qualitative features like location,size,shape,and vascularity.The “agnostic” features refer to mathematically quantitative descriptions of the invisible characteristics of lesions,which can be roughly classified into four types:(1)Morphologic features that are expressed as statistical values;(2)First-order features(histogram features)reflecting the distributions of different gray levels of lesion,mainly including the standard deviation,energy,entropy,kurtosis,sharpness,skewness,and variance;(3)Second-order features(textual features)that describe the tumor heterogeneity addressing the spatial relationships of pixels or voxels,commonly using a gray-level co-occurrence matrix and gray-level run-length matrix[24,25];and(4)Higher-order features that were extracted utilizing various filters,such as wavelet transforms,Laplacian filters,and Minkowski functionals.
However,several features are not desirable.Redundant and irrelevant features affect the accuracy and robustness of the model.In order to avoid overfitting and improve accuracy,it is necessary to select the most significant and informative features from a large number of extracted features for dimensionality reduction prior to modeling.This step has been commonly carried out in a variety of machine learning methods,such as filter-type methods like correlation or univariate regression,and embedding methods like least absolute shrinkage and the selection operator(LASSO)algorithm[26].
Model construction and validation
Clinical task-oriented models are built utilizing selected significant features,appropriately with the addition of some clinical indicators and laboratory indexes.In traditional machine learning,the commonly used methods are logistic regression,support vector machines(SVMs),decision trees,random forest(RF),K-nearest neighbor,and clustering analysis,etc.According to Parmaret al[26],the choice of modeling method has a dominant influence on the radiomics analysis results.Hence,various methods can be applied to select the model with the best performance in practice.
Taking into account the reliability and generalizability,each model must be evaluated and validated.The area under the receiver operating characteristic curve(AUC),decision curve analysis,and nomograms are commonly used for performance evaluations.Internal validation is indispensable,and external(multi-center)validation should also be conducted if conditions permit.However,most of the present studies are single-center studies with small samples,and by contrast,only a few omics models have been validated externally by multiple centers.
APPLICATIONS OF RADIOMICS IN HCC
Radiomics has been widely applied in diagnosis or differential diagnosis,pathological grading,aggressiveness evaluation,clinical treatment assistance,and recurrence and survival predictions of HCC.The tasks,methods,and results of some representative studies are listed in Table 1.
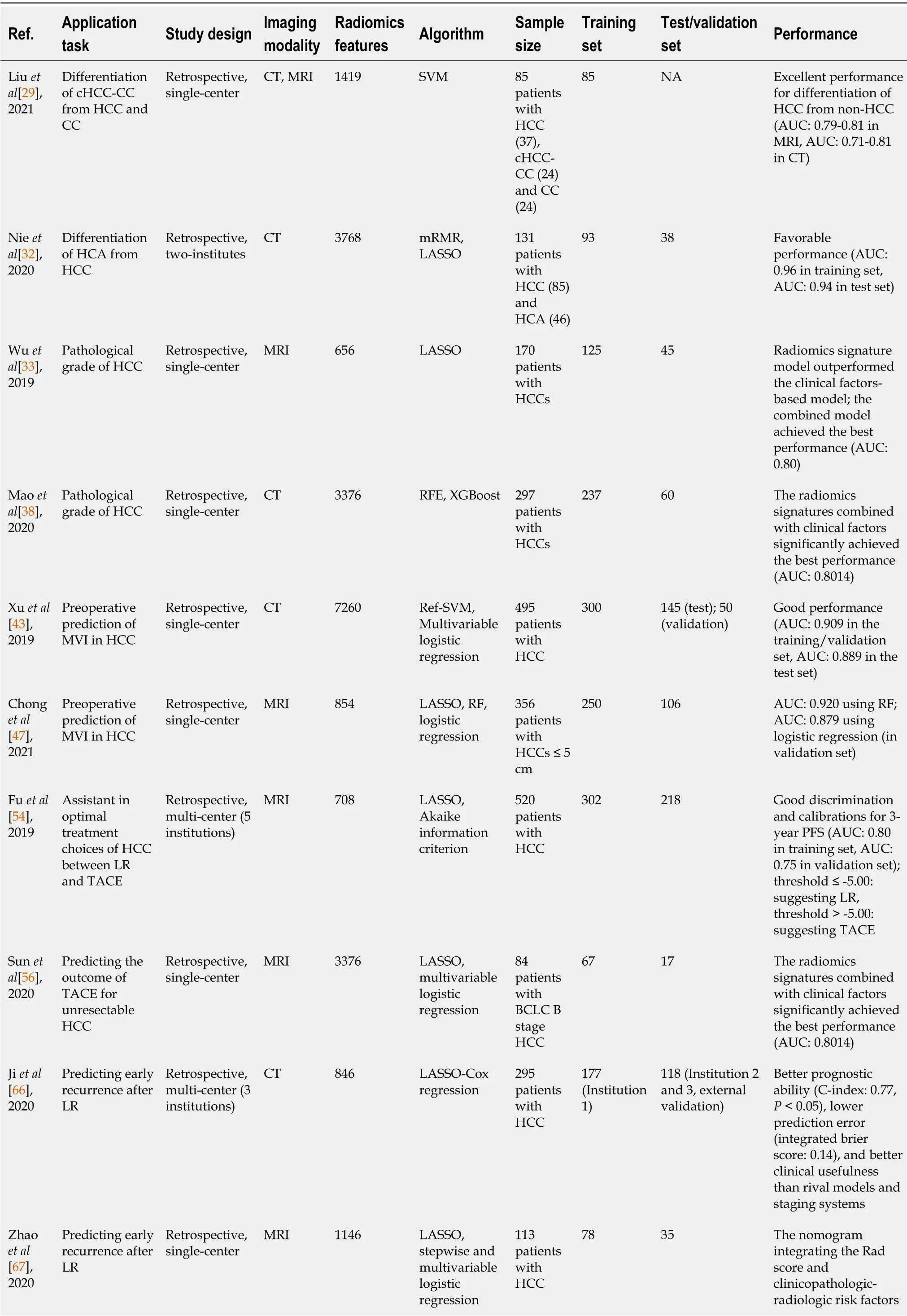
Table 1 Some representative studies of radiomics in hepatocellular carcinoma

cHCC-CC:Combined hepatocellular cholangiocarcinoma;NA:Not available;HCC:Hepatocellular carcinoma;CC:Cholangiocarcinoma;CT:Computed tomography;MRI:Magnetic resonance imaging;GLCM:Gray-level co-occurrence matrix;SVM:Support vector machine;AUC:Area under the receiver operating characteristic curve;HCA:Hepatic adenoma;mRMR:Maximal relevance and minimum redundancy;LASSO:Least absolute shrinkage and the selection operator;RFE:Recursive feature elimination;XGBoost:eXtreme gradient boosting;MVI:Microvascular invasion;Ref-SVM:Recursive feature selection support vector machine;RF:Random forest;LR:Liver resection;TACE:Transarterial chemoembolization;PFS:Progression-free survival;BCLC:Barcelona clinic liver cancer;C-index:Concordance index;RFS:Recurrence free survival.
Diagnosis and differentiation of HCC
Early and accurate diagnosis of tumors is decisive for clinical decision-making and treatments.As the most common primary liver cancer,HCC can be diagnosed based on medical imaging findings without histopathological confirmation according to clinical practice guidelines[27,28].
However,some lesions with similar imaging manifestations to HCC,such as combined hepatocellular cholangiocarcinoma(cHCC-CC),intrahepatic cholangiocarcinoma(ICC),hepatic adenoma(HCA),and hepatic hemangioma(HH),are still challenging regarding diagnosis in conventional imaging.Liuet al[29]investigated the differentiation of HCC from non-HCC tumors(cHCC-CC and CC)with MRI and CT radiomics features using an SVM machine learning algorithm.Their results showed that contrast-enhanced MRI(CE-MRI)phases were quite useful for differentiation of HCC from non-HCC with an AUC of 0.79-0.81,as well as pre-contrast and portal phase CT with an AUC of 0.81 and 0.71,respectively.Although the study was limited by inconsistent imaging protocols and a sample size that was too small to separate into training and validation cohorts.Lewiset al[30]used the histogram parameters of apparent diffusion coefficient(ADC)of diffusion weighted imaging(DWI)and liver imaging reporting and data system(LI-RADS)classifications to distinguish HCC from other primary liver cancers(ICC and cHCC-ICC).The results presented that the prediction model combined with gender,ADC fifth percentile,and LI-RADS classification obtained the best predictive performance with an AUC of 0.90[30].Regarding the distinction of HCA and HCC,Nieet al[31]reported that the CT-based radiomics nomogram was a potential tool to accurately differentiate HCA from HCC in the noncirrhotic liver with favorable performance(AUC of 0.96 in the training set and 0.94 in the test set).Similarly,this CT-based radiomics nomogram also achieved effective values in the preoperative differential diagnosis of FNH and HCC in the noncirrhotic liver(AUC of 0.979 in the training set and 0.917 in the test set)[32].Another study by Wuet al[33]developed and validated a radiomics signature using derived features from pre-contrast MR imaging sets to distinguish HCC and HH.The results witnessed an improved diagnostic performance of combination of in-phase,out-phase,T2 weighted imaging(T2WI),and DWI with logistic regression(AUC:0.86 in the training set and 0.89 in the test set),which outperformed the less experienced radiologist and was nearly equal to the experienced radiologist.These radiomics studies contributed potential supplements to accurate diagnosis and differentiation of HCC in medical imaging,but the results remain to be widely validated and amended in the clinical practice.
Pathological grading of HCC
The pathological grade is one of the vital factors affecting intrahepatic tumors recurrence,that is,high-grade tumors are associated with a high intrahepatic recurrence rate[34,35].The management of HCC varies with different pathological grades,and patients with higher intrahepatic recurrence rates require special treatments for surgery and follow-up compared with the lower-risk patients[6,36].Thus,accurate prediction of HCC pathological grade might promote clinical decision-making and formulation of the most appropriate treatment plan.Wuet al[37]built radiomics signatures on the basis of T1-weighted imaging(T1WI)and T2WI generated in LASSO,and assessed the predicted values of radiomics,clinical factors,and the combined models.The results showed that there were significant differences in categorization of high- and low-grade HCCs in MRI-based radiomics signatures(P<0.05).The predictive value of the radiomics signature model outperformed the clinical factorsbased model(AUC:0.74vs0.60,respectively),whereas the combined model incorporating both achieved the best performance with an AUC of 0.80[95% confidence interval(CI):0.65-0.90][37].Another similar study by Maoet al[38]aimed to predict the pathological grades of HCC preoperatively based on contrast-enhanced CT(CECT)-derived radiomics signatures.They established models using shape,first-order,second order,and higher-order features extracted from arterial phase(AP)- and venous phase-CECT imagesviarecursive feature elimination and eXtreme Gradient Boosting(XGBoost).They also found that combining radiomics signatures with clinical factorssignificantly improved the prediction performance at an AUC of 0.8014(95%CI:0.6899-0.9129)[38].It can be known that radiomics is a powerful tool for predicting the pathological grade of HCC closely related to the follow-up management,as well as extending the predictive value of clinical factors.
Aggressiveness evaluation of HCC
The aggressive tumor behavior is strongly linked to the prognosis of HCC patients.Microvascular invasion(MVI),defined as tumor cell nest in vessels lined with the endothelium that can only be determined on the postoperative histologic examination,is one of the crucial independent predictors of early recurrence(ER)of HCC patients after surgical treatment[39-41].So,it is of remarkable importance to accurately evaluate and predict the MVI of HCC preoperatively,so as to ensure and improve the prognosis of patients.Since Bakret al[42]pointed out the potential of a CT-based radiomics signature as a surrogate for MVI in HCC(AUC:0.76,95%CI:0.58-0.94)though in a small cohort,various researchers have explored an underlying association focusing on this field.Xuet al[43]developed a CT-based radiomics model integrating large-scale clinical factors and imaging features to predict the MVI and outcomes in surgically resected patients with HCC.The approach demonstrated good performance with an AUC of 0.909 in the training/validation set and 0.889 in the test set[43].A radiomics nomogram based on CECT established by Maet al[44]showed that portal venous phase(PVP)radiomics signatures exhibited better performance to predict MVI than AP and delay phase(DP)(AUC in validation sets:0.793vs0.684 and 0.490,respectively).Another study performed in two independent centers by Zhanget al[45]shared the same goal as those above,constructing CECT-based radiomics signatures in a LASSO algorithm and multivariable logistic regression.Enrolled patients from institution 1 were divided into the training and the test set,and patients from institution 2 served as an independent validation set,of which the AUC of MVI status predictions were 0.780,0.776,and 0.743,respectively,and the AUC of the final MVI risk classifier-integrated clinical stage reached 0.783,0.778,and 0.740,respectively[45].Regarding an MRI radiomics model for MVI prediction in HCC,Fenget al[46]first reported that the combined intratumoral and peritumoral radiomics model derived from gadolinium-ethoxybenzyl-diethylenetriamine(Gd-EOB-DTPA)-enhanced MRI showed effective value with an AUC of 0.83(95%CI:0.71-0.95)in the validation cohort along with a sensitivity of 90% and specificity of 75%[46].Additionally,specific to solidary HCCs ≤ 5 cm,Chonget al[47]built a multi-scale and multi-parametric radiomics nomogram based on Gd-EOB-DTPA MRI,and this also yielded favorable performance for preoperative MVI predictions,of which the AUC reached up to 0.920 (95%CI:0.861-0.979)using RF and 0.879(95%CI:0.820-0.938)using logistic regression in the validation set[47].Another study by Yanget al[48]indicated the helpful value of hepatobiliary phase(HBP)for predicting MVI,showing that HBP T1WI images and HBP T1 maps were independent risk factors for MVI and the model incorporating the clinicoradiological factors and HBP-derived radiomic features outperformed the former only in the training cohort(AUC:0.943vs0.850,P=0.002),though there was no statistical significance in the validation set(AUC:0.861vs0.759,P=0.111)[48].These studies provided new perspectives and approaches for aggressiveness evaluation of HCC and might help to improve the prognosis of patients and assist in the precise treatment plan making.
Clinical treatment assistance for HCC
Caution needs to be taken comprehensively when it comes to selecting the optimal treatment for HCC patients.In addition to the patients’ conditions and tumor stage,the trauma of the treatments which is associated with deterioration of liver function leading to death should be also given full consideration[49].For example,liver resection(LR)is curative to remove the tumor completely but highly traumatic.Transarterial chemoembolization(TACE)is minimally invasive while may leave some residual tumors.And their adaptation has expanded and even overlapped with the development of medical technologies[50-53].Focusing on this,Fuet al[54]proposed an individualized model to assist appropriate treatment choices for HCC patients between LR and TACE.They extracted radiomics features from CT images of HCC patients in five centers and combined them with clinical factors and radiological characteristics to construct a progression-free survival(PFS)model.The model yielded good discrimination and calibrations for 3-year PFS with an AUC of 0.80 in the training set and 0.75 in the validation set,outperforming the other four state-of-the-art models.And a nomogram was built to subdivide patients for optimal treatments by the threshold of the score difference.In the threshold ≤ -5.00 group,LR provided better PFS than TACE,which suggested LR to be a potential better option[hazard ratio(HR)=0.50,P=0.014 in the training set;HR=0.52,P=0.026 in the validation set].For the other patients,LR and TACE had similar PFS(HR=0.84,P=0.388 in the training set;HR=1.14,P=0.614 in the validation set).TACE seemed to be a better choice as it was less invasive and helped to control unnecessary trauma and risks[54].Moreover,for HCC patients who underwent hepatectomy,Caiet al[55]developed and validated a radiomics-based nomogram derived from PVP-CT images to predict posthepatectomy liver failure(PHLF)preoperatively,which exhibited superior discrimination with an AUC of 0.896(95%CI:0.774-1.000)in the validation set rather than other three methods[Child-Pugh,Model of End Stage Liver Disease(MELD),and albumin bilirubin].Furthermore,another 13 patients served for a pilot prospective analysis,and the radiomics nomogram predicted PHLF effectively with an AUC of 0.833(95%CI:0.591-1.000)[55].For unresectable HCC patients,Sunet al[56]established a radiomics model based on preoperative multiparameter MRI(mp-MRI)predicting early progression after TACE.The results identified the radiomics signature as an independent parameter of progressive disease(PD),and the mp-MRI signature achieved the greatest benefit with an AUC of 0.800 compared with the single ones[56].These studies demonstrated the guiding significance of radiomics in assisting clinical treatment selections for HCC,especially when there were more controversies,which could help patients and doctors weigh the advantages and disadvantages and choose the optimal personalized plan.
Recurrence and survival prediction in HCC
In routine clinical settings,LR is preferred as the first-line treatment option for HCC patients at an early stage and with preserved liver function,whereas liver transplantation(LT)is recommended for end-stage HCC patients with clinically proven portal hypertension and early-stage HCC meeting the Milan criteria.For patients who are not suitable for LR or LT(Barcelona Clinic Liver Cancer(BCLC)stage 0-A and some selected BCLC stage B),non-surgical local ablation techniques are considered as best choices[27,28,57,58].However,post-treatment recurrence remains a thorny problem that hinders clinical management progress and patient survival[59-65].Therefore,it is of emerging significance to preoperatively predict the recurrence risk after treatments.
Several radiomics studies based on preoperative CT or MRI have yielded favorable performance in post-LR ER predictions[66-72].In a recent multi-center study by Jiet al[66],recurrence-related radiomic features were extracted from preoperative CECT images of 295 surgically proven HCC patients from three independent institutions and then built with LASSO and Cox regression.The two radiomics-based models presented better prognostic ability[concordance index(C-index):0.77,P<0.05)],lower prediction error(integrated Brier score:0.14),and better clinical usefulness than rival models and staging systems[66].Another mp-MRI based radiomics study by Zhaoet al[67]established radiomics models deriving from in-out-phase T1WI,T2WI,DWI,and CE-MRI images.The combined nomogram integrating the Rad score and clinicopathologic-radiologic(CPR)risk factors showed better discrimination and clinical utility than the CPR and radiomics models alone(AUC:0.873vs0.742,respectively).For recurrence predictions for HCC after LT,Guoet al[73]also combined the CT-based radiomics signature and clinical risk factors to develop and validate a radiomics nomogram in LASSO and Cox regression algorithm,which achieved good predictive performance for recurrence-free survival with a C-index of 0.785(95%CI:0.674-0.895)in the training set and 0.789(95%CI:0.620-0.957)in the validation set.As for HCC patients who underwent ablation,Yuanet al[74]extracted radiomics features from three-phase preoperative CECT images(AP,PVP,and parenchymal phase),selected the significant features by mMRM,and then built a radiomics signature using LASSO and Cox regression.Similarly,the PVP-combined model adding the clinicopathological factors produced the best predictive performance to predict ER after curative ablation with a C-index of 0.792(95%CI:0.727-0.857)in the training set and 0.755(95%CI:0.651-0.860)in the validation set[74].
A radiomics approach has demonstrated encouraging results in survival analysis of post-treatment HCC patients[75-78].In a recent multi-center study,Wanget al[75]worked on predicting the 5-year survival of HCC patients after LR using an MRIbased radiomics model.They built radiomics signatures with an RF method and developed a combined model incorporating radiomics signatures and clinical risk factors,which obtained good calibration and satisfactory discrimination for survival prediction with an AUC of 0.9804 in the training set and 0.7578 in the validation set[75].Kimet al[77]predicted the overall survival(OS)of HCC patients who underwent TACE with the use of a pretreatment CT-based radiomics model.They applied LASSO-Cox regression algorithm for optimal survival-related feature selection and constructed a predictive model combining radiomics signature with clinical factors.The results suggested that the composite model can better predict the OS after TACE(HR:19.88,95%CI:6.37-92.02,P<0.001)compared with radiomics and clinical models only[77].In these studies,a substantial growth was observed in the performance of the state-of-the-art conventional models when adding the radiomics signature.They demonstrated the considerable value of radiomics approach to predict the ER risk and survival conditions of post-treatment HCC patients,which may facilitate personalized risk stratification and enlighten a new way for further clinical decision-making for HCC patients.
DEEP LEARNING BASED RADIOMICS
Deep learning,a ramification of machine learning algorithms developed from neural networks with multiple layers,has been widely used in medical image analysis with promising expectations[79,80].As a type of representation learning method,deep learning takes the strength of excellent self-taught ability which enables automatic learning and training of target-related features without manual segmentation and extraction(Figure 1).It has demonstrated deeper and more comprehensive data mining compared with radiomics based on traditional machine learning.Convolutional neural network(CNN)is the most popular model,meanwhile stacked autoencoders(SAEs),restricted Boltzmann machines(RBM),deep belief network(DBN),GAN,and U-net have been also applied[81-85].CNNs are mainly composed of three network layers,namely,the convolutional,the sampling,and the full connection layer,of which the core mechanisms include multi-layer stacking,local connection,weight sharing,and pooling.Automatic learning of informative features of medical images is accomplished without the need for manual segmentation and feature extraction.SAE is an unsupervised learning method,which trains the models by adjusting the advantage parameters of the encoder and layer.RBM,composed of visible units and hidden units,is a kind of generative stochastic neural network that learns probability distributions from input data sets.DBN can train the weights between its multiple neurons,which enables the whole neural network to generate training data according to the maximum probability.The models are chosen for different oriented tasks in HCC,including segmentation,tumor detection or classification,diagnosis and differentiation,aggressiveness evaluation,prognosis and survival analysis,and image quality improvement.The tasks,methods,and results of some representative studies of deep learning in HCC are presented in Table 2.
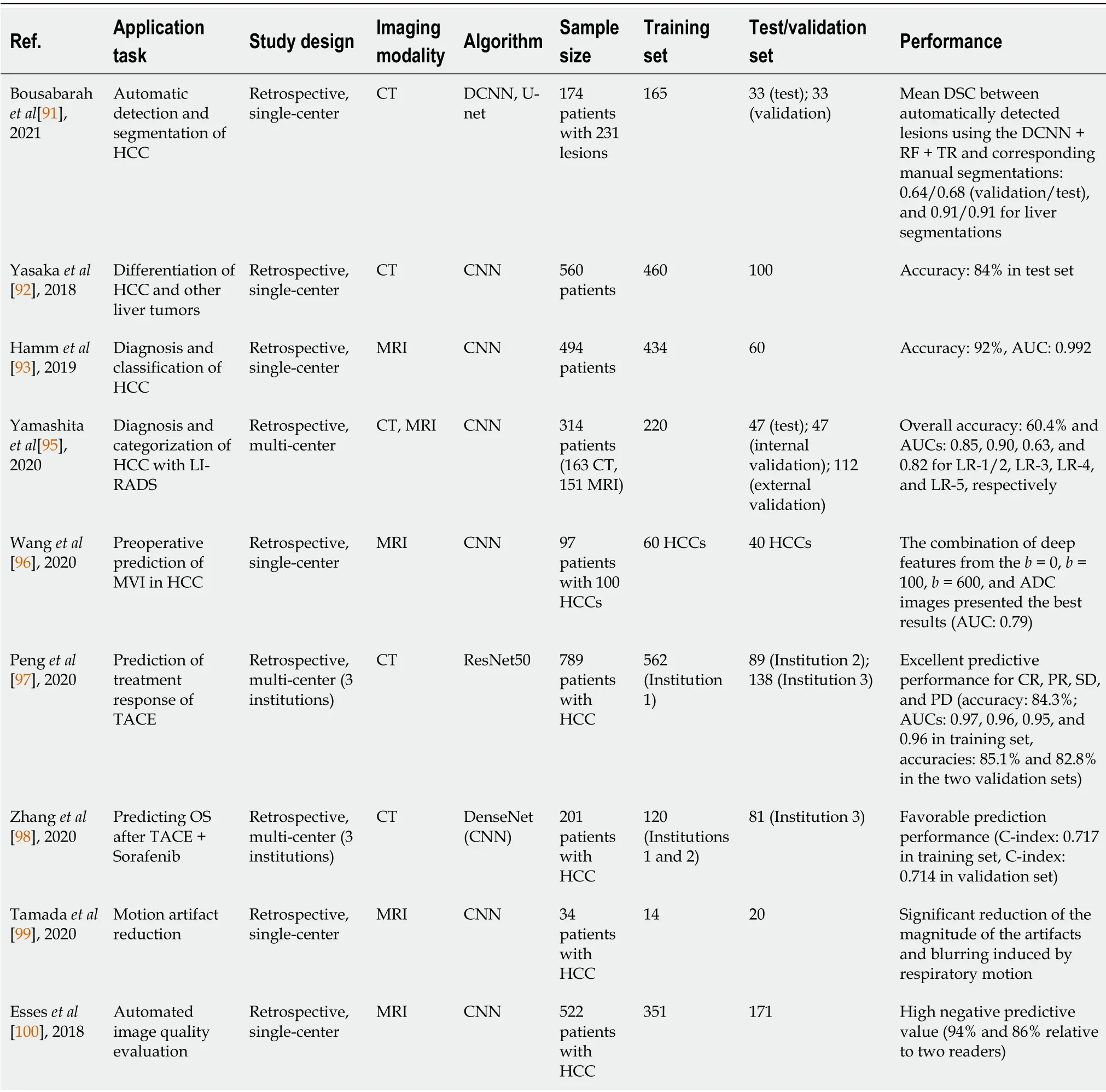
Table 2 Some representative studies of deep learning in hepatocellular carcinoma
APPLICATIONS OF DEEP LEARNING-BASED RADIOMICS IN HCC
Detection and segmentation of HCC
Manual segmentation is limited as it is time-consuming with low efficiency and interoperator variability.Thus,accurate and automatic liver and tumor segmentation methods are demanded in clinical practice.Deep learning algorithms,by contrast,enable automated segmentation and have been applied in various studies[86-91].Bousabarahet al[91]trained a deep CNN(DCNN)with a U-net architecture using multiphasic CE-MRI images and the dice similarity coefficient(DSC)was used to evaluate the performance.Their approach demonstrated the feasibility of automatically detecting and segmenting the liver and HCCs,and the mean DSC between automatically detected lesions using the DCNN + RF + thresholding and corresponding manual segmentations was 0.64/0.68(validation/test),and 0.91/0.91 for liver segmentations[91].However,most studies investigated the whole liver,liver tissues,or malignant tumors,whereas a few focused specifically on automatic segmentation of HCC,which should be developed and validated in further studies.
Diagnosis,differentiation,and classification of HCC
Yasakaet al[92]utilized a deep learning algorithm with CNN to differentiate HCCs and other liver masses based on three-phase CT images(pre-contrast,AP,and DP)and obtained an accuracy of 84% in the test set.Hammet al[93]designed a proof-ofconcept CNN-based deep learning system(DLS)for liver tumor diagnosis on the basis of mp-MRI.The DLS achieved an accuracy of 92% and an AUC of 0.992 for HCC classification.And they further indicated an “interpretable” DLS that could identify the correct radiological features of each test lesion on MR images with a positive predictive value of 76.5% and sensitivity of 82.9%[94].Another pilot study by Yamashitaet al[95]developed a CNN-based model with LIRADS to diagnose and categorize HCC on CT and MRI.It exhibited that the transfer learning model outperformed the custom-made model with an overall accuracy of 60.4% and AUCs of 0.85,0.90,0.63,and 0.82 for LR-1/2,LR-3,LR-4,and LR-5,respectively,whereas the external validation results were not accurate enough[95].Although the results were promising,those studies were preliminary and demonstrated the initial feasibility of deep learning in the diagnosis,differentiation,and classification of HCC.
Aggressiveness,treatment outcomes,and survival evaluation of HCC
The application of deep learning in aggressiveness behavior evaluation,treatment outcome prediction,and recurrence and survival analysis of HCC were not as sophisticated as those of conventional radiomics,but they also witnessed dramatic potential and clinical value.For MVI prediction,Wanget al[96]established a deep learning model with a CNN based on preoperative DWI and reported that the combination of deep features from theb=0,b=100,b=600,and ADC images presented the best results(AUC:0.79,P=0.002)[96].With regard to prediction of treatment responses,Penget al[97]trained and validated a deep learning model using ResNet50 on preoperative CT images of HCC patients who underwent TACE from three independent institutions.This multi-center study yielded excellent predictive performance for complete response,partial response,stable disease,and PD with an accuracy of 84.3% and AUC of 0.97,0.96,0.95,and 0.96,respectively,in the training set,and an accuracy of 85.1% and 82.8% in the two validation sets[97].Another multi-center study by Zhanget al[98]involved preoperative CECT images and they adopted a deep learningbased model utilizing DenseNet to predict OS of HCC patients treated with TACE plus sorafenib,which achieved favorable prediction performance with a C-index of 0.717 in the training set and 0.714 in the validation set.
Image quality improvement
Deep learning has been applied for image quality improvement,which helps with the diagnosis and interpretation of HCC and other liver lesions more accurately.For example,Tamadaet al[99]indicated a CNN-based method in Gd-enhanced MR images in the AP to improve the imaging quality,and the magnitude of the artifacts and blurring induced by respiratory motion were significantly reduced.Additionally,Esseset al[100]described an CNN-based method in T2WI liver MRI images for automated image quality evaluation,which yielded a high negative predictive value(94% and 86% relative to two readers)for screening diagnostic and nondiagnostic liver T2WI.The applications of deep learning for medical imaging technologies will be strikingly expanded in further explorations.
LIMITATIONS,CHALLENGES,AND FUTURE DIRECTIONS
Despite the encouraging achievements and progress of radiomics and deep learning in HCC,the prior studies also highlighted the limitations and challenges that must be addressed(Figure 2).First and most critically,the majority of current studies were retrospective with a small sample size performed in single center,lacking of uniform standards and external validation.The enrolled samples,imaging acquisition protocols,facilities,platforms,segmentation methods,modeling algorithms,and radiomics tools differed in various studies,which accounted for variations and poor generalizability.The studies based on radiomics quality score and Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis have also emphasized these insufficiencies[101,102].Getting with the consensus guidelines published by the image biomarker standardization initiative may help to cope with the problem[103].More importantly,prospective-design,multi-center,large-sample studies are urgently warranted in further investigation on HCC,along with intensive and standardized quality controls throughout the entire workflow.
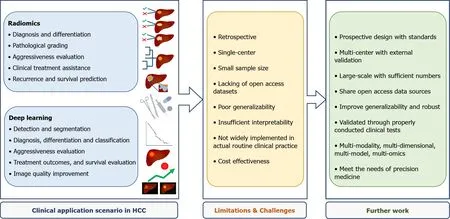
Figure 2 Summary of the clinical application scenario,limitations,challenges,and further work of state-of-the-art radiomics and deep learning in hepatocellular carcinoma.
Deep learning has been putting a brand-new step forward in radiomics,demonstrating superior potential in HCC-oriented tasks while requiring large-scale validation and long-term justification in further studies.Besides,the insufficient interpretability of these AI-medical imaging-combined approaches remains a concern,meaning that it is still quite challenging to adequately explain the underlying associations of radiomics analysis results and tumor heterogeneity and biological behaviors of HCC.
Moreover,few valuable datasets were shared with open access,which got in the way of accumulating sufficient numbers for statistical power.Therefore,it is an expecting choice to share open access database sources across institutions to strengthen the generalization ability and establish well-curated databases and networks,as the Quantitative Imaging Network(QIN)proposing[104].By the way,the cost-effectiveness of a radiomics or deep learning approach is also supposed to be weighted when applying it to a specific clinical situation of HCC,as it is procedure-complex,time-consuming,labor-intensive,and hardware- and software-demanding.
To date,radiomics and deep leaning have been applied in numerous HCC studies,but they have not been widely implemented into routine clinical practice,which requires to be extensively validated and optimized through further appropriate clinical trials.Radiogenomics,an encouraging field considered as a bridge connecting radiomics with genomics[105],is also of promising value in HCC whereas not in the scope of this review.Radiologists ought to get more involved to take full advantage of AI to improve the working efficiency and tackle problems driven by clinical demanding.For the foreseeable future,the multi-modality,multi-dimensional,and multi-model radiomics integrating clinical factors,laboratory information,and other omics has become the next trend of AI-driven medicine for novel evaluation and management of HCC.
CONCLUSION
To conclude,radiomics has enormous potential to become a powerful tool for HCC management covering detection and classification,diagnosis and differentiation,staging and grading,assessment of aggressive behavior and treatment responses,and prognosis and survival prediction.However,the underlying value of radiomics and deep learning based radiomics in HCC has not been fully investigated,as well as the applicability and generalizability in routine clinical practice.In the face of great opportunities albeit with challenges,the multi-modality,multi-dimensional,multimodel radiomics and multi-omics studies will become the most appropriate clinical research approaches,so as to meet the developing needs of precision medicine and enhance precision medicine initiatives.
 World Journal of Gastrointestinal Oncology2021年11期
World Journal of Gastrointestinal Oncology2021年11期
- World Journal of Gastrointestinal Oncology的其它文章
- Hepatocellular carcinoma biomarkers,an imminent need
- Anatomical vs nonanatomical liver resection for solitary hepatocellular carcinoma:A systematic review and meta-analysis
- Atezolizumab plus bevacizumab versus sorafenib or atezolizumab alone for unresectable hepatocellular carcinoma:A systematic review
- Cell-free DNA liquid biopsy for early detection of gastrointestinal cancers:A systematic review
- Colorectal cancer in Arab world:A systematic review
- Induction chemotherapy with albumin-bound paclitaxel plus lobaplatin followed by concurrent radiochemotherapy for locally advanced esophageal cancer
