Inhibition of poly(ADP-Ribose)polymerase:A promising strategy targeting pancreatic cancer with BRCAness phenotype
Keun-Yeong Jeong,Haejun Lee
Keun-Yeong Jeong,R&D Center,Metimedi Pharmaceuticals,Incheon 22006,South Korea
Haejun Lee,Department of Nuclear Medicine,Gil Medical Center,Incheon 21565,South Korea
Abstract The use of chemotherapeutic regimens for the treatment of pancreatic cancer is still limited because pancreatic cancer is usually diagnosed at an advanced stage as a refractory disease in which symptoms are difficult to recognize in the early stages.Furthermore,at advanced stages,there are important challenges to achieve clinical benefit and symptom resolution,even with the use of an expanded spectrum of anticancer drugs.Recently,a point of reduced susceptibility to conventional chemotherapies by breast cancer susceptibility gene(BRCA)mutations led to a new perspective for overcoming the resistance of pancreatic cancer within the framework of increased genome instability.Poly(ADP-Ribose)polymerase(PARP)-1 is an enzyme that can regulate intrinsic functions,such as response to DNA damage.Therefore,in an environment where germline mutations in BRCAs(BRCAness)inhibit homologous recombination in DNA damage,resulting in a lack of DNA damage response,a key role of PARP-1 for the adaptation of the genome instability could be further emphasized.Here,we summarized the key functional role of PARP-1 in genomic instability of pancreatic cancer with the BRCAness phenotype and listed clinical applications and outcomes of PARP-1 inhibitors to highlight the importance of targeting PARP-1 activity.
Key Words:Pancreatic cancer;BRCAness;Poly(ADP-Ribose)polymerase-1;PARylation;Poly(ADP-Ribose)polymerase-1 inhibitor
INTRODUCTION
Therapeutic perspectives in pancreatic cancer
Pancreatic cancer is usually diagnosed at an advanced stage as a refractory disease in which symptoms are difficult to recognize in the early stages.The 5-year survival rate is extremely low(less than 9%),and about two-thirds of all patients with pancreatic cancer die within one year of diagnosis[1].Furthermore,at advanced stages of the disease,there are major challenges to achieving clinical benefit and symptom resolution,even after expanding the range of anticancer drugs targeting pancreatic cancer,and to date,few options for treating pancreatic cancer have been proposed,such as gemcitabine alone,gemcitabine with nanoparticle albumin-bound paclitaxel(nab-paclitaxel),or gemcitabine in combination with capecitabine,fluorouracil,leucovorin,irinotecan,and oxaliplatin[2].The main cause of pancreatic carcinogenesis is genomic instability,and it is well established that cancer development is related to defects in DNA damage response[3].Recent genome-wide studies have made great strides in identifying distinct subpopulations of pancreatic cancer constituent cells with unstable genomic properties due to mutations in the DNA repair gene[3,4].Based on this background,there has been a focus on the high frequency of deleterious changes which lead to a truncated/faulty response to DNA damage in cancer cells.In particular,since breast cancer susceptibility genes(BRCA)mutations have been reported to decrease susceptibility to gemcitabine and platinum-based chemotherapy,a new perspective on the molecular mechanisms overcoming resistance in pancreatic cancer is required[5,6].Therefore,the recent approach targeting poly(ADP-Ribose)polymerase(PARP)-1 has emerged as an encouraging therapeutic strategy for inhibiting the pathogenesis of BRCAness pancreatic cancer within the framework of an increase in genome instability[7].
PARP-1 AND DNA DAMAGE RESPONSE IN PANCREATIC CANCER
PARP-1 is an enzyme that can regulate the intrinsic functions of several cytoplasmic and nuclear proteins based on inducing poly(ADP-Ribose)synthesis[8].In various cellular physiological functions led by PARP-1,the reaction to DNA damage is known as the most important biochemical function,and with its well-established crucial role in DNA damage repair,the upregulation of PARP-1 in cancer could lead to investigations into the potential for targeting this important enzyme[9].PARP-1 comprises a multi-domain structure that shares the catalytic domain showing structural homology with other ADP-ribosyl transferases for DNA damage repair[10].The N-terminal region contains a DNA-binding domain with three zinc fingers and an auto-modifying domain,and the C-terminal region comprises a protein interaction domain and a catalytic subdomain accountable for the poly ADP-ribosylation reaction[10,11].The construction of such domains enables genetic relations by catalyzing the covalent attachment of poly-ADP-Ribose polymers to DNA repair proteins and other receptor proteins,including transcription factors and chromatin modulators.Based on these structural interactions,PARP-1 can mediate ADP-Ribose synthesis and attach it to acceptor proteins[10,11].The PARP-1 signature motif includes an NAD+-binding site and comprises an acceptor of adenosine and the donor of nicotinamide wherein ADPRibose from NAD+ is transferred to target proteins for ADP-Ribose synthesis[11,12].It is an integrative and dynamic biochemical process defined as poly ADP-ribosylation(PARylation),and the hypothesis has recently been established that the synthesis process is determined by following potential pathways[11,12].PARP-1 catalyzes the transfer of ADP-Ribose units from NAD+ to compose the poly ADP-Ribose branches,which is negatively charged to several amino acid residues in PARP-1 or other receptor proteins[11].Besides,poly(ADP-Ribose)synthesis is based on the attachment of ADP-Ribose to the 2'-OH end of the growing chain by sequentially adding the next ADP-Ribose residues to the end of the ADP-Ribose moiety[11].The biochemical action of linking the long and negatively charged poly ADP-Ribose polymer to PARP-1 itself or a variety of acceptor proteins can be attributed to its primary function of repairing DNA damage during potential changes for cancer cell survival[11,13].In DNA damage repair,PARP-1 and PARylation are universally involved in both single-strand and double-strand DNA damage repairs,such as base excision repair,homologous recombination(HR),and non-homologous end-joining(NHEJ)[14].PARP-1 can functionally interact with X-ray repair cross-complementing protein 1,which plays a major role in signal pathways for single-strand DNA damage repair[14,15].The BRCA1 C-terminus directly binds to the poly ADP-Ribose chain and mediates early recruitment of DNA repair proteins to DNA lesions[16].Further,PARP-1 has been associated with HR-mediated repair and reactivation of stalled replication forks,thus promoting DNA replication for restarting stalled replication BRCA-dependent early double-strand DNA damage repair[17].Interestingly,the role of PARP-1 in an environment where germline mutations in BRCAs inhibit the HR-mediated repair of DNA double-strand breaks,thus resulting in a deficiency in the DNA damage response,can be further emphasized[6,18].
BRCANESS IN PANCREATIC CANCER AND PARP-1
BRCAness is defined as a set of traits in which BRCA1 or BRCA2 mutation phenocopies result in a lack of double-strand DNA damage repair,and a tumor cell has an HR obstruction with a germline BRCA1 or BRCA2 deficiency[19].The incidence of germline mutations of BRCAs that can be targeted for pancreatic cancer is estimated to be about 9%,but the incidence of these BRCA mutations(particularly BRCA2)in familial pancreatic cancer patients has increased to about 17%[20].Mutations in BRCA are responsible for causing genetic instability and worsening prognosis.BRCAness leading to the phenotype of HR deficiency is an indispensable marker for recognizing an increase in the pancreatic cancer risk,and the sensor defect of double-strand DNA break is an error-prone repair pathway,such as NHEJ,which accumulates increased genomic instability.In this context,the HR deficiency by BRCAness may rely on a process of overcoming genetic instability that is reliant on PARP-1 activation[21].As mentioned above,PARP-1 is an important nuclear enzyme in cellular homeostasis as it transforms various nuclear proteins by PARylation[8,11-14].The key feature of PARP-1 is the DNA repair responding to DNA damage by targeting the histone core and linker histone proteins in the nucleus[22].A serine group-binding ADP-ribose relies on a protein,histone PARylation factor 1(HPF1),which has been identified as a key protein that controls DNA damage-induced PARylation and is responsible for adaptation to genomic instability[23,24].Because PARP-1 continuously recruits DNA repair elements through PARylation in several receptor regions during genomic instability,HPF1 is used to regulate the excessive PARP-1 transformation to avoid apoptosis[14,15,24].Taken together,PARP-1 activity and PARylation may play an important role in adapting to genomic instability in pancreatic cancer in a tumor microenvironment undergoing persistent genomic instability by BRCAness[13-15,20,21,23,24].
CLINICAL STUDIES ON BRCANESS PANCREATIC CANCER BY PARP INHIBITORS
BRCAness is unstable NHEJ-dependent and drives distinctive DNA repair systems creating specific genotypic and phenotypic features[19].Therefore,it can be inferred that the sensitization of PARP-1 inhibitors has potential benefits for the treatment of BRCAness pancreatic cancer,and PARP inhibitors have recently emerged as a novel class of a targeted therapy specifically targeting BRCAness pancreatic cancer[18].To date,five PARP inhibitors have drawn significant clinical results targeting BRCAnesspancreatic cancer,and these agents bind to the catalytic domain of PARP and interfere with the base repair or suppress PARP synthesis[25].Olaparib is first approved for the treatment of advanced ovarian cancer;however,presently,it is also being administered to patients having pancreatic cancer with BRCA mutations.Niraparib is a functionally selective inhibitor of PARP used for the treatment of advanced pancreatic cancer with BRCA mutations.Veliparib is being studied for its applicability to treating non-small-cell lung cancer and breast cancer with BRCA mutations,as well as advanced pancreatic cancer.Rucaparib is a small-molecule PARP inhibitor targeting germline BRCA-mutated pancreatic cancer.Talazoparib is an orally bioavailable PARP inhibitor with the potential antineoplastic activity that targets pancreatic cancer with BRCA mutations[25,26].A pancreatic cancer olaparib ongoing(POLO)study was conducted on pancreatic cancer patients with BRCA mutations;these were the patients who did not show progression by platinum-based chemotherapy randomized to 92 patients in the phase 3 clinical trial.The results showed that median progression-free survival was increased to 31.3 mo in the olaparib group compared with 23.9 mo in the placebo group[27,28].Another phase 2 trial has also demonstrated the efficacy of targeting metastatic pancreatic cancer with the germline BRCA mutant.A total of 32 patients was recruited,with one-two showing the partial response(PR),and eleven showing the stable disease(SD)[29,30].Niraparib is undergoing a phase 2 clinical trial to test its safety and efficacy in patients with pancreatic cancer with HR deficiency,such as a BRCA mutation.This study is recruiting patients,and there are no interim reports[31,32].The combination effect of cisplatin and gemcitabine with or without veliparib was reported by a phase 2 study in pancreatic cancer patients with germline BRCA mutations.A total number of 52 patients were enrolled in the trial and were randomly assigned to be treated with triple combination(gemcitabine,cisplatin,and veliparib)or double combination(gemcitabine and cisplatin).The objective response rate(ORR)in the former was higher at 74.1% compared with 65.2% in the latter[33,34].A phase 2 trial of rucaparib in patients with pancreatic cancer with deleterious germline or somatic BRCA mutations was reported.In this study,19 patients were treated,and the confirmed ORR was 11%(1 PR and 1 complete response).The disease control rate(PR or SD for above 12 wk)was 32% in all patients[35,36].A doseescalation,phase 1 study was organized to validate the antitumor activity of talazoparib.This study reported clinical benefits in 4 of the 13 patients with pancreatic cancer.The tumor response rate was 15% PR and 15% SD,and the median progression-free survival was 5.3 wk[37,38].Table 1 presents a list of clinical trials for PARP inhibitors targeting BRCA mutant pancreatic cancer.However,while acknowledging the promising clinical outcomes of PARP-1 inhibitors,unexpected toxicity is an important concern to be considered.It can cause unacceptably high hematologic toxicity and adverse effects that are sporadically associated with acute myeloid leukemia.The combination of conventional chemotherapy,such as gemcitabine with veliparip or olaparip,was primarily associated with a marked increase in hematological toxicity above grade 3.Further,40% of pancreatic cancer patients who received only olaparib showed gastrointestinal disorders,fatigue,and lethargy,as well as hematologic toxicity(Table 1)[25,39-42].Therefore,potential solutions that can optimize treatment with sophisticated applied therapies through the development of new formulations are currently unmet medical needs.
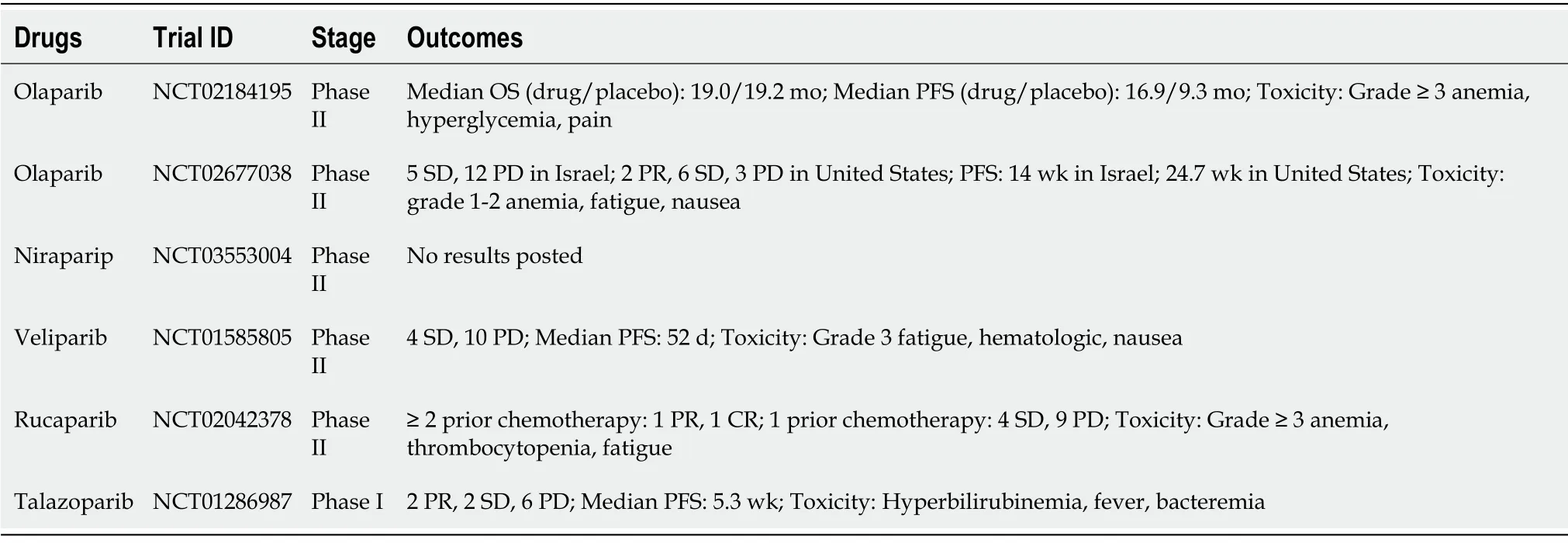
Table 1 Clinical trials of Poly(ADP-Ribose)polymerase-1 inhibitor for the treatment of breast cancer susceptibility gene mutant pancreatic cancer
CONCLUSION
The possibility that PARP-1 inhibitors effectively improve the prognosis by targeting pancreatic cancer with the BRCAness phenotype appears to deserve scientific attention,and the accumulation of such possibilities could be a key point in understanding whether PARP inhibitors can be used as a major therapeutic strategy as a single therapeutic agent or in combination with existing DNA damage agents to overcome resistance.
 World Journal of Gastrointestinal Oncology2021年11期
World Journal of Gastrointestinal Oncology2021年11期
- World Journal of Gastrointestinal Oncology的其它文章
- Hepatocellular carcinoma biomarkers,an imminent need
- Anatomical vs nonanatomical liver resection for solitary hepatocellular carcinoma:A systematic review and meta-analysis
- Atezolizumab plus bevacizumab versus sorafenib or atezolizumab alone for unresectable hepatocellular carcinoma:A systematic review
- Cell-free DNA liquid biopsy for early detection of gastrointestinal cancers:A systematic review
- Colorectal cancer in Arab world:A systematic review
- Induction chemotherapy with albumin-bound paclitaxel plus lobaplatin followed by concurrent radiochemotherapy for locally advanced esophageal cancer
