Mitochondrial H2Sn-Mediated Anti-Inflammatory Theranostics
Won Young Kim, Miae Won, Seyoung Koo, Xingcai Zhang, Jong Seung Kim✉
ABSTRACT The insistent demand for space-controllable delivery, which reduces the side effects of non-steroidal antiinflammatory drugs (NSAIDs),has led to the development of a new theranostics-based approach for anti-inflammatory therapy.The current anti-inflammatory treatments can be improved by designing a drug delivery system responsive to the inflammatory site biomarker, hydrogen polysulfide(H2Sn). Here, we report a novel theranostic agent 1 (TA1), consisting of three parts: H2Sn-mediated triggering part, a two-photon fluorophore bearing mitochondria targeting unit (Rhodol-TPP), and anti-inflammatory COX inhibitor (indomethacin). In vitro experiments showed that TA1 selectively reacts with H2Sn to concomitantly release both Rhodol-TPP and indomethacin. Confocal-microscopy imaging of inflammation-induced live cells suggested that TA1 is localized in the mitochondria where the H2Sn is overexpressed. The TA1 reacted with H2Sn in the endogenous and exogenous H2Sn environments and in lipopolysaccharide treated inflammatory cells. Moreover, TA1 suppressed COX-2 level in the inflammatory-induced cells and prostaglandin E2 (PGE2) level in blood serum from inflammation-induced mouse models. In vivo experiments with inflammation-induced mouse models suggested that TA1 exhibits inflammation-site-elective drug release followed by significant therapeutic effects, showing its function as a theranostic agent, capable of both anti-inflammatory therapy and precise diagnosis.Theranostic behavior of TA1 is highly applicable in vivo model therapeutics for the inflammatory disease.
KEYWORDS Theranostics; Anti-inflammation; Mitochondria; Hydrogen polysulfides
1 Introduction
Inflammation is a biological response of body tissues to deleterious stimuli including injury, infection, damaged cells,pathogens, and irritants [1]. The immune system removes the injurious stimuli and initiates tissue repair through the inflammatory process [2]. Inflammation can be classified into two types: acute and chronic. Acute inflammation is an initial response to the harmful stimuli of the immune system, during which the movement of both plasma and white blood cells from the bloodstream to damaged tissues is increased, causing many biochemical events. This stage is accompanied by characteristic symptoms such as heat, pain, redness, swelling, and loss of tissue function in due course. Chronic inflammation, however, is long-term and leads to whole-body inflammation, producing steady and low-leveled inflammation throughout the body, which can develop from continuous acute inflammation [3]. This lasting chronic inflammation can eventually start markedly damaging tissues and organs, leading to the development of various chronic inflammatory diseases such as cardiovascular and bowel diseases, diabetes, arthritis, and cancer [4, 5].Notably, it has been estimated that 15% of human cancers are associated with chronic inflammation [6].
Non-steroidal anti-inflammatory drugs (NSAIDs) exhibit excellent anti-inflammatory effects and are commonly prescribed medications to reduce pain, decrease fever, and prevent blood clots. NSAIDs reduce inflammation by inhibiting the activity of the cyclooxygenase enzymes (COX) involved in the synthesis of prostaglandins, which are key biological mediators in regulating inflammation [7]. Most NSAIDs are non-selective between COX-1 and COX-2. The NSAIDs inhibit COX-1 dependent generation of mucosal-protective gastric prostaglandins, thus considerably damaging the stomach and increasing the risk of gastrointestinal ulcers and bleeding, which are serious adverse effects of using NSAIDs. COX-2 selective inhibitors, on the other hand,exhibit lesser gastrointestinal side effects but cause thrombosis and substantially increase the risk of cardiovascular diseases [8]. These serious adverse effects are ascribed to the different roles and tissue localization of each COX isozyme.Due to these side effects of NSAIDs, there is an increasing demand for their selective delivery to specific inflammatory areas [9].
Hydrogen polysulfides (H2Sn,n> 1) have drawn much attention because they are known as a fundamental regulator in biological redox-processes. Recent studies have suggested that the H2Snmolecules engage in various physiological functions such as cellular signal transduction, redox biology,and cytoprotective processes [10—12]. Endogenous H2Sncan be easily produced from the reaction of H2S with reactive oxygen species (ROS) [13, 14]. In the body, the enzymes,cystathionine γ-lyase (CSE) and cystathionine-β-synthase(CBS), are able to produce H2S, which can also generate persulfides that could, in turn, be converted into H2Sn[15].The H2Snthus generated is stored in mitochondria and can be released in response to physiological conditions such as oxidative stress and inflammation [16]. When inflammation occurs, the released H2Snplays a crucial role in cytoprotection by scavenging ROS and also promoting nuclear localization of Nrf2 (Nuclear factor erythroid 2-related factor 2), which is one of the regulators that enhance anti-oxidant genes [17, 18].
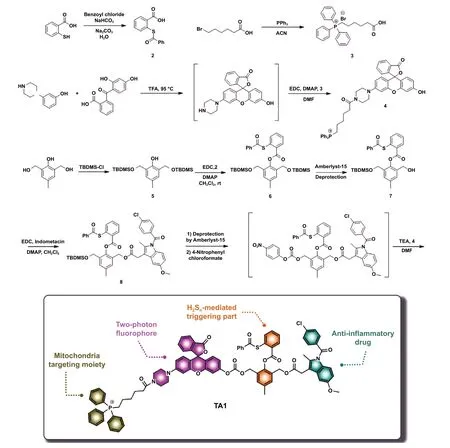
Scheme 1 Synthetic routes of TA1 and schematic illustration of H2Sn-mediated anti-inflammatory theranostics system
As H2Snis capable of triggering the selective delivery of anti-inflammatory drugs to inflammatory cells over normal cells, we for the first time designed H2Sn-triggered antiinflammatory theranostic agent 1 (TA1). “Theranostics”refers to the all-in-one system that integrates diagnostic and therapy, enabling precision and effective treatment. As seen in Scheme 1, the TA1 consists of four crucial functional parts: aryl thioester as an exclusive H2Snreactive site over other biological species [19]; triphenylphosphonium (TPP)salt unit as a mitochondrion targeting part, considering that H2Snis mostly produced and stored in mitochondria;indomethacin as an anti-inflammatory drug able to inhibit COX enzyme that inhibits a synthesis of prostaglandins followed by reducing inflammation; and Rhodol dye as a fluorescent off—on reporter for the two-photon microscopy imaging. Such a theranostic system is a useful tool to visualize the interaction of prodrug with certain biomarkers and provides useful information to assess the therapeutic efficacy,such as localization and release kinetics of the drug in situ.[20—27]. Two-photon dye utilizes a long-wavelength light as an excitation source to offer several advantages, such as minimized tissue damage and deep tissue penetration, suitable for in vivo real-time bio-imaging [28—31].
Thus, in this system, the TA1 can specifically localize in the inflammatory region, where the reaction of thioester moiety of the TA1 with H2Snselectively takes place in mitochondria, which subsequently triggers the self-immolation reaction to concomitantly release both indomethacin and Rhodol-TPP. The self-immolation reaction can promote the ring-opening of the Rhodol compartment, which emits a strong fluorescence (off—on) in the inflammatory site.Simultaneously, the released indomethacin can lessen the corresponding inflammation without exhibiting adverse effects on normal cells. Therefore, it is notable that the novel H2Sn-mediated TA1 could provide a promising dual function of anti-inflammatory treatment and precise diagnosis,simultaneously, at the inflammation site.
2 Experimental
2.1 Materials
Unless otherwise noted, all the materials for the synthesis were purchased from commercial suppliers (Sigma-Aldrich,Alfa, Samchun) and were used without further purification.All the reactions were carried out under the nitrogen. All procedures for work-up and purification were carried out with reagent-grade solvents under ambient atmosphere. Column chromatography was performed with silica gel 60 (Merck,0.063 ~ 0.2 mm) as a stationary phase. Analytical thin layer chromatography was performed using Merk 60 F254 silica gel (pre-coated sheets, 0.25 mm thick).1H and13C NMR spectra were collected in NMR solvent (CDCl3) on a Bruker 500 MHz spectrometer. All chemical shifts are reported in ppm values using the peak of TMS as an internal reference.NMR data are reported as follows: chemical shifts, multiplicity (s: singlet, d: doublet, dd: doublet of doublets, t: triplet,q: quartet, m: multiplet, br: broad signal), and the coupling constant (Hz). The ESI-MS spectra were recorded using a Shimadzu LC/MS-2020 Series instrument and TSQ-LC-MS(Korea Basic Science Institute, Seoul).
2.2 Spectroscopic Measurements
UV/Vis spectra were recorded on a Scinco S-3100 spectrometer, and fluorescence spectra were obtained using a Shimadzu RF-5301PC instrument. Stock solutions of TA1 were prepared in DMSO. All excitation and emission slit widths were set at 5 nm/3 nm. The concentration of each of the samples was fixed at 10 μM in a total volume of 3 mL.
2.3 HPLC Analysis
The aqueous solution of TA1, indomethacin, and Rhodol-TPP was prepared by mixing each compound (20 μM) in 10 mM PBS (pH 7.4). The reaction mixture of TA1 with Na2S3was prepared by mixing TA1 (20 μM) and Na2S3(200 μM) in 10 mM PBS (pH 7.4) containing cetrimonium bromide (200 μM; CTAB). Each sample was eluted at a flow rate of 1 mL/min using the mobile phase (Solvent A:deionized water containing 0.1% TFA, Solvent B: Methanol)with binary gradient (45—0% of Solvent A for 1 min, then 0% of Solvent A for 19 min, then 0—45% of Solvent A for 5 min). HPLC chromatogram was monitored using a 254 nm detector.
2.4 Cell Culture
Murine macrophage cell lines (RAW 264.7) were purchased from the Korean Cell Line Bank. Cells were cultured in DMEM containing high glucose (Hyclone) with 10% fetal bovine serum (GIBCO) and 1% antibiotics (penicillin—streptomycin) (GIBCO). Cells were maintained in a humidified atmosphere with 5% CO2at 37 °C.
2.5 Cell Viability Assay
RAW 264.7 cells (2 × 104cells mL-1) were seeded on a 96 well microplate (SPL Life Science) and cells to obtain approximately 80% confluence in 24 h. After incubation,the cells were treated with TA1 and control containing 1% DMSO for 24 h. To investigate the cell viability of the RAW 264.7 cells treated with probes, we conducted a Cyto-Tox96® Non-Radioactive Cytotoxicity Assay Kit (Promega)according to the protocol. The Absorbance was measured at 490 nm by a SPECTRA MAX microplate reader (Molecular Devices). Cell viability assays were performed 3 times in triplicate, and the cell viability (%) was calculated as a percentage of measured absorbance compared to the control cells with 1% DMSO.
2.6 One-photon Fluorescence Microscopy
RAW 264.7 Cells (5 × 105cells) were seeded on confocal glass-bottom dishes (SPL Life Science) and cells to obtain approximately 80% confluence in 24 h. After stabilization,the cells were treated with TA1 (10 μM with 1% DMSO)for 2 h at 37 °C. To monitor the effect of the exogenous increase of H2Sn, cells were pre-incubated with 5 μM Na2S3for 2 h before TA1 treatment. To monitor the effect of the endogenous increase of H2Sn, cells were pre-incubated with 10 μg/mL LPS (Escherichia coli 055:B5, 10 μg kg-1,Sigma-Aldrich) for 16 h before TA1 treatment. To monitor the effect of endogenous H2Sninhibition, cells were preincubated with 1.0 mM PAG (DL-propargyl-glycine, Sigma-Aldrich, P7888) for 8 h before TA1 treatment. After cells were washed with PBS, mitochondria was stained using a MitoTracker RedFM(Invitrogen, USA) for 30 min. After incubation, cells were washed with PBS 3 times. One-photon fluorescence images were obtained by the probes with a confocal laser scanning microscope (Carl-Zeiss LSM 700). The fluorescence channel of TA1 was excited at 488 nm, and the emission was collected by 500—600 nm.
2.7 Two-photon Fluorescence Microscopy
RAW 264.7 Cells (5 × 105cells) were seeded on confocal glass-bottom dishes (SPL Life Science) and cells to obtain approximately 80% confluence in 24 h. After stabilization,the cells were treated with TA1 (10 μM with 1% DMSO)for 2 h at 37 °C. The two-photon fluorescence microscopy images were obtained by exciting the probes with a spectral confocal microscope (Leica TCS SP2) with a modelocked titanium-sapphire laser source (Coherent Chameleon)set at wavelength 800 nm in the focal plane in × 100 oil(NA = 1.30) objective lens. To obtain images at 500—600 nm range, internal PMTs were used to obtain the signals in an 8 bit unsigned 512 × 512 pixels at 400 Hz scan speed.
2.8 Fluorescence Imaging of Acute Liver Injury in Mice Models
C57Bl/6 male mice (8 weeks old) were obtained from Orientbio (Seoul, Korea) and were established for the acute liver injury model using LPS. Animal care and experiments were conducted following guidelines by Korea University Institutional Animal Care Use Committee (KUIACUC No.201900090). Mice werei.v.injected with a freshly prepared TA1 solution (10 mg kg-1, in 5% DMSO) for 30 min. Then,5% DMSO or LPS was intraperitoneally injected. At indicated time point, fluorescence images were collected at the indicated time point in vivo imaging machine (Maestro, CRi Inc., Woburn, MA, USA) with an emission filter from 550 to 750 nm (orange channel).
2.9 ELISA Assay
Blood plasma was collected at the indicated time point, to conduct plasma enzymes and cytokines analysis in mice.Plasma activity of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), as markers of hepatic damage; TNF-α and IL-1β, as markers of pro-inflammatory factors; PGE2, as a marker of COX-2 product at inflammatory sites were analyzed by an Enzyme-Linked Immunosorbent Assay Kit (Invitrogen.). Each enzyme activity signal was measured using a Multi-Detection Microplate Reader system(HIDEX), following the manufacturer’s procedure.
2.9.1 Statistical Analysis
The data of statistical significance represent the mean ± S.E.The data were analyzed by SAS 9.4 ver. Statistical significance was determined by a two-way ANOVA test with a Bonferroni test and Student’s t-test. Different letters statistically distinct signify data sets. (p< 0.05).
3 Results and Discussion
3.1 Synthesis of TA1
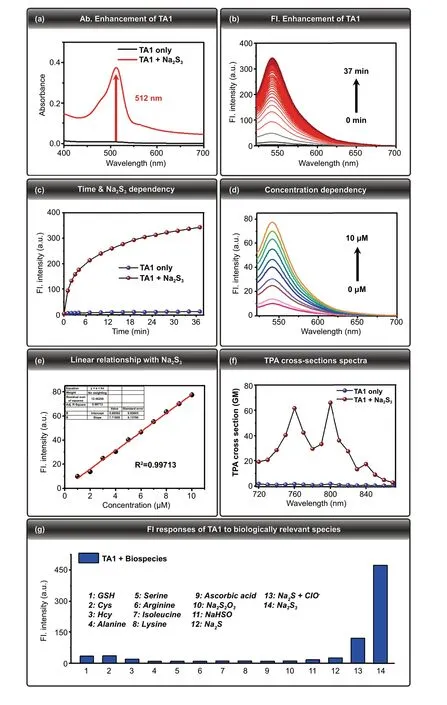
Fig. 1 a Absorbance spectra of TA1 (10 μM) in the absence of Na2S3 (black line), upon addition of Na2S3 (red line) (100 μM,10 mM PBS buffer, 0.2% DMSO, 100 μM CTAB). b Time-dependent fluorescence spectral changes of TA1 (10 μM) λex = 512 nm,λem = 542 nm, upon addition of Na2S3 (100 μM, 10 mM PBS buffer,0.2% DMSO, 100 μM CTAB). c Fluorescence intensity changes of TA1 (10 μM) in the absence of Na2S3 (blue sphere), upon addition of Na2S3 (red sphere). d Fluorescence responses of TA1 (10 μM)toward addition of a range of concentrations of Na2S3 (0 to 10 μM). e Linear relationship between the fluorescence intensity and Na2S3 concentrations. Data were acquired in 10 mM PBS buffer (0.2% DMSO,100 μM CTAB) after incubation with Na2S3 for 2 min at 37 °C.λex = 512 nm, λem = 542 nm. f Two-photon absorption (TPA) crosssections spectra of TA1 (10 μM) upon addition of Na2S3 (100 μM,10 mM PBS buffer, 0.5% DMSO, 100 μM CTAB). g Fluorescence responses of TA1 (10 μM) to biologically relevant species. Each bar represents fluorescence increases of TA1 at 542 nm to 100 μM Na2S3 or other species. 1) 1 mM GSH; 2) 500 μM Cys; 3) 500 μM Hcy; 4)100 μM Alanine; 5) 100 μM Serine; 6) 100 μM Arginine; 7) 100 μM Isoleucine; 8) 100 μM Lysine; 9) 100 μM Ascorbic acid; 10) 100 μM Na2S2O3; 11) 100 μM NaHSO3; 12) 100 μM Na2S; 13) 100 μM Na2S + 50 μM ClO—; 14) 100 μM Na2S3. The data were acquired in 10 mM PBS buffer (0.2% DMSO, 100 μM CTAB) after incubation for 30 min. λex = 512 nm. (Color figure online)
As shown in Scheme 1, the structure of target molecule TA1 comprises three parts: 1) H2Sn-reactive triggering part, 2)Rhodol-based two-photon fluorophore bearing mitochondria targeting TPP, and 3) anti-inflammatory drug, indomethacin. The TA1 was synthesized by the following procedure.First, H2Sn-reactive site 2 was prepared by s-acetylation of thiosalicylic acid using benzoyl chloride. The triggering unit, 6, was synthesized viatert-butyldimethyl silyl(TBDMS) protection at both sides of aliphatic hydroxyl group of 2,6-bis(hydroxymethyl)-p-cresol, followed by 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC)coupling reaction with 2. Then, the mono-deprotection of the TBDMS group in 6 was conducted in the presence of amberlyst-15 to yield 7. Compound 7 was then conjugated with indomethacin through EDC coupling to produce 8.Finally, the other TBDMS group of 8 was further deprotected using amberlyst-15 and subsequently reacted with 4-nitrophenyl chloroformate for activation. The resulting intermediate was reacted with TPP-conjugated two-photon Rhodol fluorophore 4 (Rhodol-TPP) which was synthesized by EDC coupling of Rhodol and 3, successfully yielding the desired product, TA1 (Scheme 1). The detailed procedures of the synthesis and structurally characterized evidence for the compounds are provided in supporting information.
3.2 Characterization of TA1
To determine whether TA1 could react with H2Snto give an off—on fluorescence change, the photophysical change of TA1 (10 μM) was investigated using UV—Vis absorption and fluorescence spectroscopy under simulated physiological conditions (10 mM PBS buffer, pH 7.4), in the presence and absence of Na2S3(100 μM), H2Sndonor. UV—Vis absorption intensity of TA1 (10 μM) markedly enhanced at 512 nm upon addition of Na2S3(100 μM, 10 mM PBS buffer, 0.2%DMSO, 100 μM CTAB) (Fig. 1a). Time-dependent fluorescence intensity changes of TA1 (10 μM) were observed, and they indicated a marginal fast (30 min) and gradual increase of its fluorescence intensity at 542 nm, upon treatment with Na2S3(Fig. 1b, c). These results confirm that the TA1 reacts with H2Snand induces cleavage of H2Sn-reactive triggering part to release the corresponding fluorophore, where the fluorescence off—on change is attributed to the ring-opening of Rhodol fluorophore upon self-immolation reaction(Fig. 2a). The fluorescence responses of TA1 (10 μM) to H2Snwere also evaluated to investigate the sensitivity to different concentrations of Na2S3(0 to 10 μM). As a function of Na2S3concentration, gradually increasing fluorescence intensity of TA1 (λex= 512 nm) was observed, with a center at 542 nm (Fig. 1d). A linear correlation of various concentrations of Na2S3with fluorescence intensities at 542 nm was also observed (Fig. 1e). Taken together, we conclude that TA1 can effectively respond to H2Snwith reliable sensitivity under physiological conditions.
For two-photon confocal-microscopic imaging of the probe, TPA cross sections of TA1 and TA1 + H2Snwere initially investigated with rhodamine 6G as the reference molecule. Upon addition of Na2S3(100 μM), TA1 exhibited 66 GM with maximum TPA cross-section value at 800 nm under physiological conditions (PBS buffer, 0.5% DMSO,100 μM CTAB), which validates that TA1 can be sufficiently sensitized by two-photon absorption (Fig. 1f).
To determine the selectivity of TA1 toward H2Snover other biological species, fluorescence experiments with a series of other biologically relevant species were also performed. The TA1 showed a high selectivity for Na2S3over amino acids and other nucleophilic sulfur species such as glutathione (GSH), cysteine (Cys), homocysteine (Hcy),S2O32-, HSO3-, and Na2S (Fig. 1g). Moreover, TA1 was also found to be inert to other reductive species such as ascorbic acid. Upon treatment of a mixed solution of Na2S(200 μM) and ClO-(50 μM) to generate H2Snin situ, TA1 showed a strongly enhanced fluorescence at 542 nm as well.These results conclude that TA1 can selectively respond to H2Snin the biological media containing various potential interferences.
3.3 Proposed Mechanism of H2Sn-responsive Activation of TA1
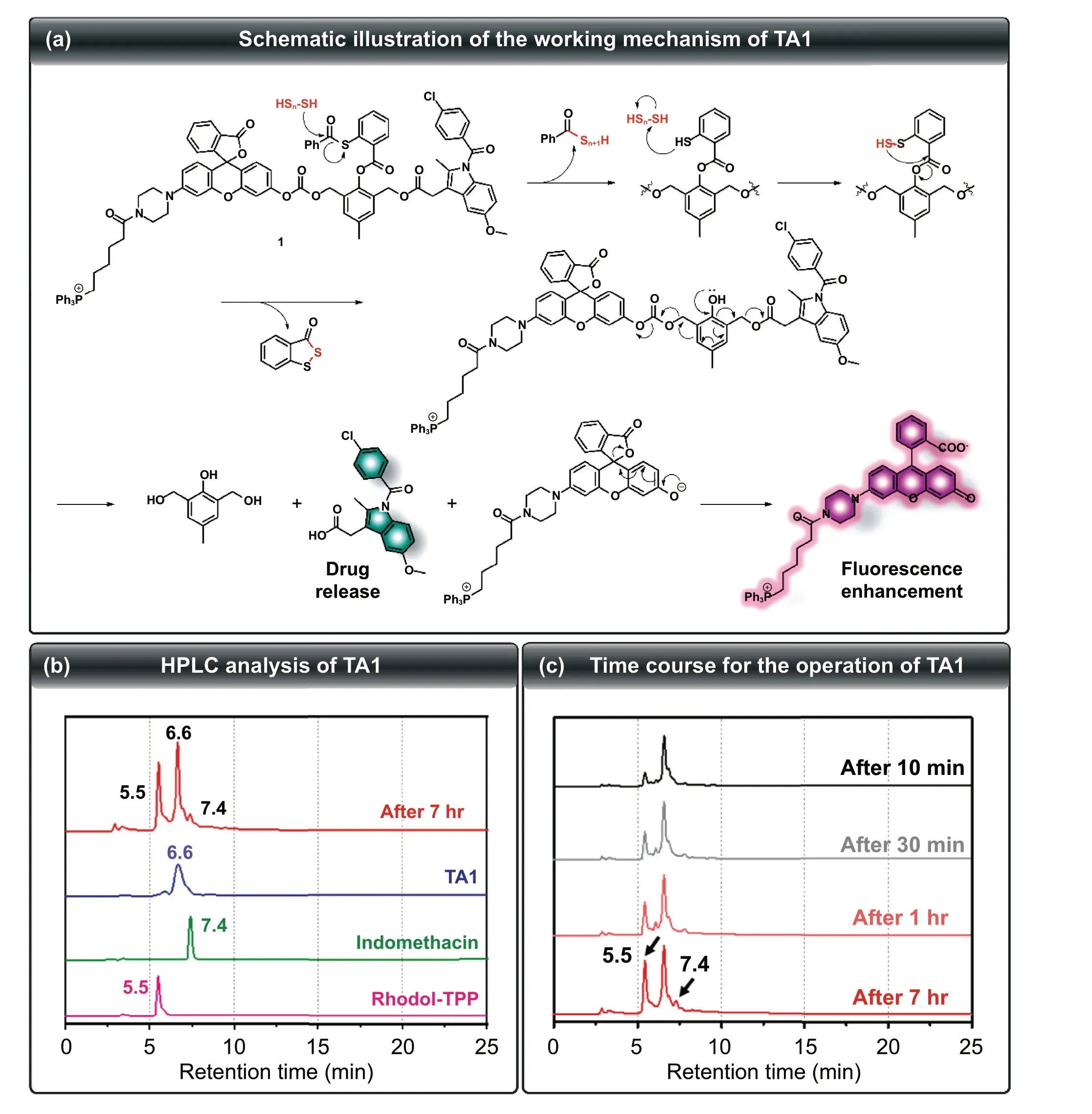
Fig. 2 a Schematic illustration of the working mechanism of TA1. b HPLC chromatogram of TA1 (blue line), indomethacin (green line), Rhodol-TPP (pink line), and the reaction mixture of TA1 with Na2S3 (red line). c Time-dependent change of HPLC chromatogram of the reaction mixture of TA1 with Na2S3. (COlor figure online)
To verify that the proposed self-immolation cleavage mechanism of the theranostics system shown in Fig. 2a is reasonably operated, HPLC analysis of TA1 in the presence of Na2S3was undertaken. As shown in Fig. 2b, the retention time for TA1, Rhodol-TPP, and indomethacin was 6.6, 5.5, and 7.4 min, respectively. Time-course experiment upon reaction with H2Sngave a Rhodol-TPP peak, cleaved from TA1, which gradually increased. Indomethacin release began after 1 h, indicating that TA1 can release both Rhodol-TPP and indomethacin, simultaneously, upon reaction with H2Sn(Fig. 2c). Moreover, the ESI—MS spectrum of TA1 in the presence of Na2S3showed two peaks ofm/z380.12 and 759.30, corresponding to indomethacin and Rhodol-TPP,respectively (Fig. S13). This result supports the mode of action that TA1 simultaneously releases both indomethacin and Rhodol-TPP in the presence of H2Sn.
3.4 Selective Activation of TA1 in vitro
The abovementioned results suggest that TA1 selectively reacts with H2Snand could be suitable for precise drug delivery as a potential theranostic agent. The TA1 was then applied to mouse macrophage cell line, RAW264.7, as a bioassay model. First, from the LDH cytotoxicity assay,we found the low cytotoxicity of TA1 in RAW264.7 cells,at various concentrations after 24 h-incubation (Fig. S14),thus suggesting that it could be further applied to an antiinflammatory therapeutic system. Subsequently, confocalmicroscopy images of the live RAW264.7 cells having endogenous H2Snwere obtained in the presence of TA1(10 μM) at 37 °C. The group treated with TA1 exhibited brighter fluorescence as it responded to H2Sncompared to that exhibited by the control group (Fig. 3a, b and S15).The group further treated with exogenous Na2S2(5 μM) displayed stronger fluorescence (Fig. 3c and S15). To further examine the responsiveness to endogenously produced H2Snby perturbing the pool, RAW264.7 cells were pre-incubated with LPS (1 μg mL-1, 16 h), which can induce an inflammatory environment to trigger the overexpression of CSE mRNA, and thus promote the production of endogenous H2Sn. Upon subsequent treatment of TA1 (10 μM, 2 h), the cells displayed a remarkable increase in fluorescence intensity (Fig. 3d and S15).
On the contrary, the pretreatment of DL-propargylglycine(PAG, 1 mM; CSE inhibitor) significantly attenuated the fluorescence intensity of TA1, thus confirming that CSE contributed to the endogenous generation of H2Sn(Fig. 3e and S15). Moreover, mitochondrial localization of TA1 was demonstrated via co-localization assays with Mito-tracker Red (Fig. 3b-e and S16), thereby proving the accessibility of TA1 to mitochondrial H2Sn. In addition, two-photon fluorescence microscopy images were collected from RAW264.7 cells for investigating the responsiveness of TA1 toward H2Snupon excitation at 800 nm (Fig. 3f-j and S15). These results are consistent with those from the one-photon fluorescence microscopy experiment. Collectively, the results thus indicate that TA1 can react with endogenous cellular H2Snin living cells, which can be directly visualized by fluorescence off—on changes with both one-photon and twophoton fluorescence microscopy.
3.5 Anti-inhibitory Effects of TA1 in vivo
Following the results that TA1 selectively responds to endogenous and exogenous H2Snin living cells and shows diagnostic abilities, the therapeutic effect of TA1 against inflammation was subsequently investigated by various biological tests. First, the western blot analysis of inflammationinduced RAW264.7 cells treated by LPS was implemented to explore COX-2 levels. The group treated with LPS exhibited high expression of COX-2 levels and another group treated withN-Acetyl cysteine (NAC, 1 mM for 12 h), a quencher of LPS-mediated inflammation, displayed decreased COX-2 levels (Fig. 4a). However, cells incubated with further treatment of TA1 exhibited a significant reduction of COX-2 expression compared to that in the control group. This result signifies that TA1 selectively releases indomethacin (IMC)upon reaction with H2Sn, existing in the inflammatory environment, to reduce COX-2 levels. We also observed that PGE2production increased in LPS-induced inflammatory response, whereas the levels were markedly reduced in TA1 treated RAW264.7 cells (Fig. 4b). Moreover, an inflammation-induced mouse model was established to confirm the in vivo theranostic potential of TA1. Ahead of investigation,LPS was intraperitoneally injected in mice to cause hepatotoxicity and inflammation. Upon the injection of TA1 into the LPS-induced acute liver injury (ALI) mouse model, the fluorescence expressions were observed to verify whether TA1 is triggered at the inflammatory site. As seen in Fig. 4c and S17, the marked fluorescence enhancement of in vivo and ex vivo imaging was observed in the liver because TA1 released both Rhodol-TPP and IMC upon reaction with H2Snat the inflammatory site. Besides, the blood serum of the ALI mouse model was isolated to examine PGE2level,which is representative of the level of inflammation. As shown in Fig. 4d, the PGE2level in the ALI mouse model was significantly high, whereas it was reduced in the serum of the mice treated with either TA1 or IMC. To investigate anti-inflammatory effects in the ALI mouse model, we further analyzed the production of pro-inflammatory cytokines,such as TNF-α and IL-1β in serum. The levels of TNF- α and IL-1β increased for 24 h in the ALI mouse model; however,the levels were significantly decreased in the group treated with TA1 or IMC (Fig. 4e, f and S18). We also confirmed that TA1 overcomes inflammatory responses, suppressing plasma levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in ALI mouse models (Fig. 4g,h). These results strongly suggest that the theranostic agent TA1, developed for the first time in this study, can selectively treat inflammation-related diseases by releasing the corresponding drug to the inflammatory site exclusively upon reaction with H2Snand, simultaneously, aid in the diagnosis of the inflammation by fluorescence imaging in vivo.
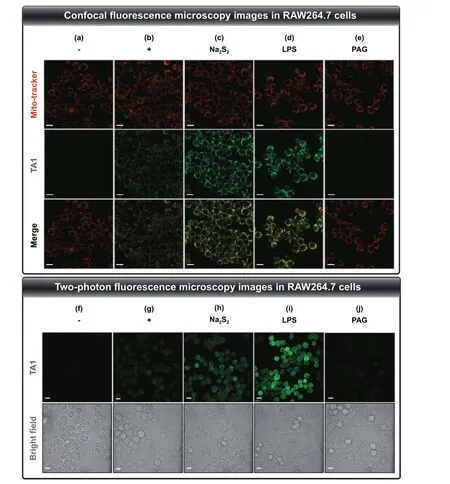
Fig. 3 Confocal fluorescence microscopy images in RAW264.7 cells incubated with TA1 (10 μM) for 2 h. The cells in each group were subjected to different treatments. a Control (1% DMSO), b TA1, c TA1 + Na2S2 (5 μM), d TA1 + LPS (1 μg mL-1), e TA1 + PAG (1 mM). Fluorescence images of cells were collected at λex = 488 nm, λem = 500—560 nm of TA1 and at λex = 579 nm, λem = 580—620 nm of Mito-tracker.Two-photon fluorescence microscopy images in RAW264.7 cells incubated with TA1 (10 μM) for 2 h. The cells in each group were subjected to different treatments. f Control (1% DMSO), g TA1, h TA1 + Na2S2 (5 μM), i TA1 + LPS (1 μg mL-1), j TA1 + PAG (1 mM). Fluorescence images of cells were collected at λex = 800 nm, λem = 500—600 nm. Scale bar = 100 μm
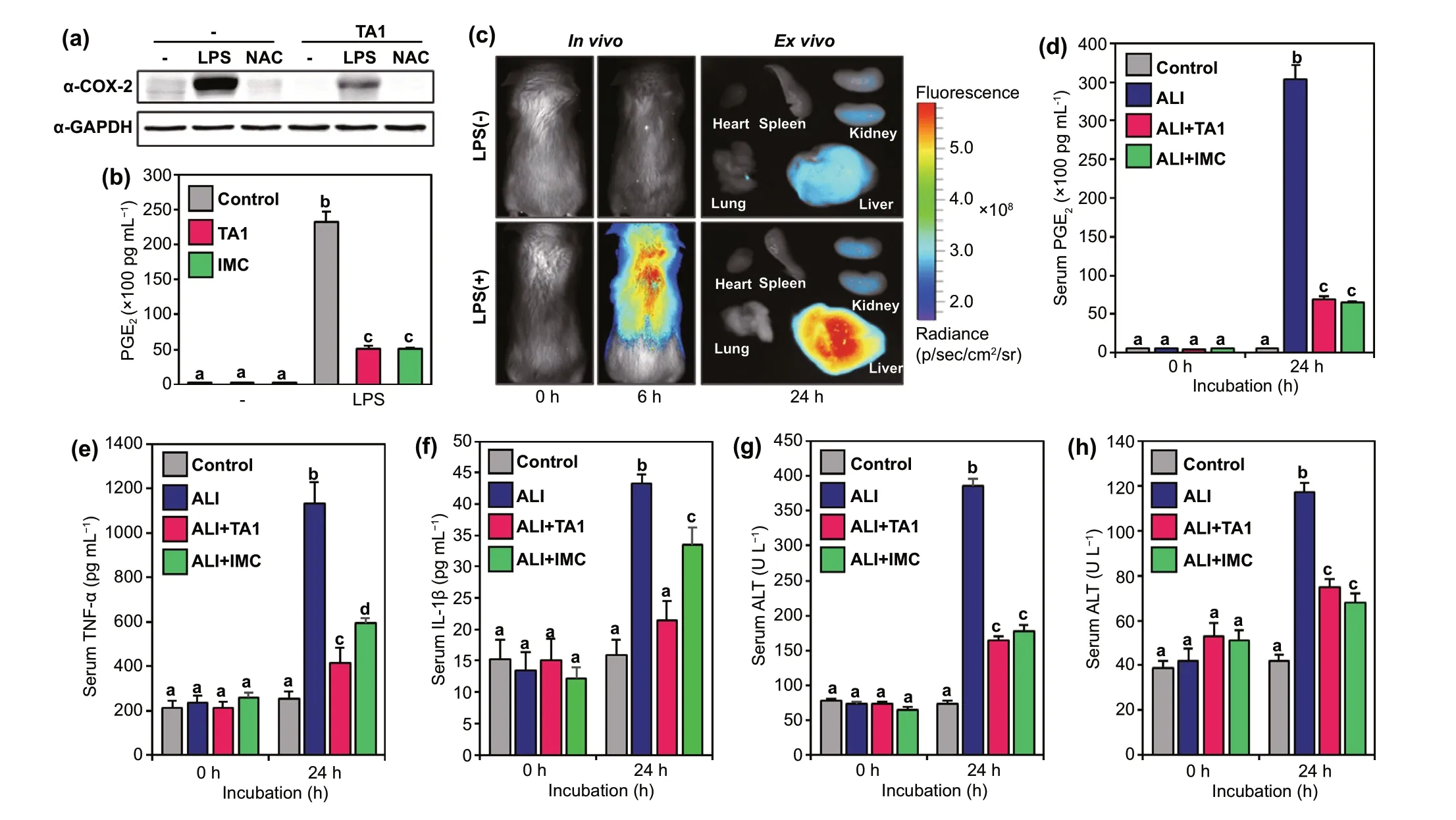
Fig. 4 Anti-inflammatory effects of TA1. a Western blotting analysis of COX-2 protein in RAW264.7 cells. Cells were pre-incubated with LPS or NAC for 16 h before TA1 treatment. b PGE2 levels of inflammation-induced RAW264.7 cells by LPS. c In vivo and ex vivo imaging of ALI mouse model. Mice were i.v. injected with TA1 for 30 min and then treated with either 5% DMSO or LPS (10 μg kg-1). d Serum PGE2 levels of ALI mouse model. Levels of pro-inflammatory cytokines e TNF-α and f IL-1β on blood collected at indicated time points (0 and 24 h) after LPS (i.p.) administration. Levels of hepatic toxicity markers g AST and h ALT on blood collected at indicated time points (0 and 24 h) after LPS(i.p.) administration. Control (5% DMSO), ALI (LPS 10 μg kg-1), ALI + TA1 (10 μg kg-1 LPS, 10 mg kg-1 TA1) and ALI + IMC (10 μg kg-1 LPS, 10 mg kg-1 IMC). Statistical significance was determined by a two-way ANOVA test with a post-hoc Bonferroni test. Different letters (e.g.,a-d) signify data sets that are statistically distinct (p < 0.05). ALI: LPS-induced acute liver injury, IMC: indomethacin, AST: aspartate transaminase, ALT: alanine aminotransferase
4 Conclusions
In summary, a novel H2Snmediated anti-inflammatory theranostic agent, TA1, was developed to selectively deliver the NSAID, indomethacin, to the inflammatory region and to visualize the inflammation site using fluorescence off—on imaging of Rhodol-TPP by triggering H2Sn. In this study,we found that TA1 exerts a selectivity to H2Snover other biological species such as amino acids and other reactive oxygen species, and the fluorescence signal of TA1 is markedly enhanced not only in the endogenous and exogenous H2Snenvironments but also in the inflammation-induced RAW264.7 cells by LPS. In addition, TA1 exhibited the ability of two-photon excited fluorescence imaging, which is highly applicable to in vitro and in vivo biological experiments. Furthermore, we found that TA1 could suppress both COX-2 level in the live cells and PGE2level in blood serum, which are factors associated with inflammation-induced mouse models where H2Snis overexpressed.Therefore, these results strongly suggest that TA1, we have first discovered has potential as a new theranostic agent as it is highly applicable to in vivo model therapeutics for inflammatory diseases.
AcknowledgementsThis work was supported by the National Research Foundation of Korea (CRI project no.2018R1A3B1052702 and 2019M3E5D1A01068998, J.S.K.), Basic Science Research Program (2020R1A6A3A01100551, M.W. and 2020R1A6A3A01100558, S.K.) funded by the Ministry of Education, and Korea University Grant.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing,adaptation, distribution and reproduction in any medium or format,as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http:// creat iveco mmons. org/ licen ses/ by/4. 0/.
Supplementary InformationThe online version contains supplementary material available at https:// doi. org/ 10. 1007/s40820- 021- 00689-1.
- Nano-Micro Letters的其它文章
- Hybrid Triboelectric-Electromagnetic Nanogenerators for Mechanical Energy Harvesting:A Review
- Nanozymes in Point-of-Care Diagnosis:An Emerging Futuristic Approach for Biosensing
- High-Efficiency Wastewater Purification System Based on Coupled Photoelectric-Catalytic Action Provided by Triboelectric Nanogenerator
- Reversible Magnesium Metal Anode Enabled by Cooperative Solvation/Surface Engineering in Carbonate Electrolytes
- Reversible Zn2+ Insertion in Tungsten Ion-Activated Titanium Dioxide Nanocrystals for Electrochromic Windows
- Magnetized Micropillar-Enabled Wearable Sensors for Touchless and Intelligent Information Communication

