ω-6多不饱和脂肪酸氧化产物 4-羟基壬烯醛的研究进展
马晶晶 孙冲 张牧焓 耿志明 王道营 徐为民
摘要:4-羟基壬烯醛(HNE)是ω-6 多不饱和脂肪酸(PUFAs)的氧化产物,研究表明HNE具有生理和病理学作用,与多种疾病的发生、发展密切相关。就HNE的形成机制、形态分布、代谢、病理和生理学意义、检测方法、安全问题等进行综述,并对提升食品营养风味、减少HNE形成的新型食品加工方法进行展望。
关键词:4-羟基壬烯醛;ω-6 多不飽和脂肪酸;氧化;HNE形成机制;病理学意义;生理学意义;HNE检测
中图分类号: TS201.6文献标志码: A文章编号:1002-1302(2021)19-0057-07
生物膜磷脂的多不饱和脂肪酸(PUFAs)在酶、自由基等作用下,发生过氧化反应,产生一系列复杂产物,如氢过氧化物、醇、酮、醛等[1]。内源性4-羟基壬烯醛(HNE)来源于生物体内ω-6 PUFAs的氧化,是体内脂质氧化最具代表性的醛类产物[2]。现有研究表明,HNE具有降低或抑制酶活、修饰蛋白质结构、干扰细胞正常功能、损伤细胞组分等作用[3];内源性HNE与多种疾病的发生、发展密切相关,如炎症、动脉粥样硬化、缺血再灌注损伤、帕金森综合症和阿尔兹海默病等[4]。因此,HNE相关研究一直是病理和生理学研究的热点。
除了内源性HNE,外源性HNE也广泛存在于富含油脂的食品中[5]。研究表明,通过食物摄取进入体内的HNE,能够发挥内源性HNE同等的生理作用[6]。近年来,随着食品中HNE的发现,其形成机制、形态分布及潜在的安全危害也引起了人们广泛关注。本文就HNE的形成机制、形态分布、代谢、病理和生理学意义、检测方法等进行简要综述,并对食品中HNE的相关研究作一些展望。
1HNE的形成机制
自发现以来,科研工作者陆续提出HNE的多种形成机制和途径。研究表明,HNE是ω-6 PUFAs的氧化产物,ω-6 PUFAs首先氧化形成氢过氧化物,然后再进一步降解产生HNE[7-8]。
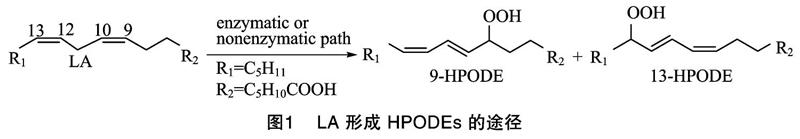
亚油酸(LA)是哺乳动物体内含量最多、也是HNE相关研究中最常用的ω-6 PUFA,本文选择LA来阐述HNE的形成机制。LA氧化形成HNE主要分为两大步骤:第1步,LA转变为氢过氧化十八碳二烯酸(HPODEs);第2步,HPODEs降解形成HNE。
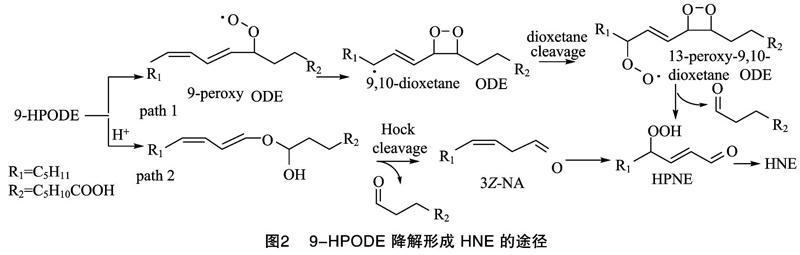
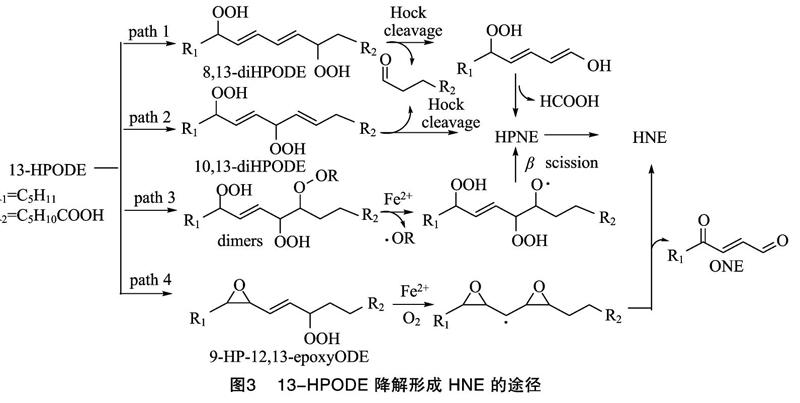
第1步,LA转化为HPODEs,可以借助酶促或自由基诱导的方式,HPODEs主要包括9-HPODE和13-HPODE这2种异构体(图1)。研究表明,大豆脂氧酶可以选择性地促进LA生成13-HPODE,而番茄脂氧酶作用时主要得到9-HPODE[9];自由基诱导氧化条件下,2种异构体生成的比例接近 1 ∶1[10]。第2步,HPODEs降解形成HNE,主要以自由基诱导为主,9-HPODE和13-HPODE这2种异构体在自由基诱导体系下降解形成HNE的机制并不相同,9-HPODE有2种途径形成HNE(图2):一种是9-HPODE发生氢氧均裂,得到的过氧自由基进攻邻位的碳碳双键,形成9,10-二氧环丁烷自由基,该自由基结合一分子氧后,裂解形成4-过氧化壬烯醛(HPNE),HPNE是HNE的前体物质,在适宜的还原体系中,可转变为HNE[11];另一种是在酸性条件下,经Hock重排,裂解形成相应中间体3Z-壬烯醛(3Z-NA),3Z-NA可经氧化、还原形成HNE[12]。与9-HPODE相比,13-HPODE降解形成HNE的途径要复杂一些,可分为4种途径(图3):第1种是13-HPODE的8号碳结合一分子氧,转变为8,13-DHPODE,经Hock重排得到5-过氧化癸二烯醇,该化合物的烯醇端碳碳双键经氧化断裂,可得到相应的甲酸和HPNE,HPNE可进一步转化为HNE[13];第2种是13-HPODE的10号碳受到氧进攻,转变为10,13-DHPODE,该中间体经Hock重排、裂解形成HPNE,再还原得到HNE[14];第3种是在形成10,13-DHPODE的基础上,继续结合一分子烷氧自由基,形成相应的二聚体,在二价铁离子的作用下,经β裂解、还原形成HNE[15];第4种是13-HPODE形成环氧中间体,在金属离子及氧气作用下,形成不对称的二环氧碳酰自由基,碳碳键断裂后,形成相应的产物4-羰基-2-壬烯醛(ONE)和HNE[16]。除了自由基途径,在植物脂氧酶(9-LOX)和过氧化氢裂解酶的共同作用下,LA也能够通过酶促途径降解形成HNE[15]。
HNE的形成受诸多因素干扰,形成过程复杂,是否还存在其他形成途径还有待进一步探究。
2HNE的形态分布
HNE分子结构中含有3种官能团,即醛基、碳碳双键和羟基,其化学性质活泼,除了游离形态外,HNE还能与多种其他化合物作用,形成结合形态。
蛋白质是HNE作用的主要对象,迈克尔(Michael)加成反应和形成席夫(Schiff)碱是HNE和蛋白质结合的2种主要方式。HNE与蛋白质上多种氨基酸(包括半胱氨酸、组氨酸、赖氨酸、精氨酸等)残基的侧链结合,以改变蛋白质的结构和功能[17]。Xu等报道了HNE通过和蛋白质上的赖氨酸结合,形成具有荧光性的共价偶联产物[18]。2001年,Gardner等报道了HNE与血凝素样氧化低密度脂蛋白受体-1(LOX-1)侧链上的组氨酸形成Michael加成产物,屏蔽了铁离子结合位点,从而降低酶活[19]。
作为巯基化合物的代表,谷胱甘肽(GSH)是细胞内一种重要的水溶活性物质。在生理条件下,HNE能够和GSH形成复合物,加剧氧化应激的程度[20]。
磷脂是细胞膜的重要组成部分,也能够和HNE结合,磷脂酰乙醇胺、磷脂酰丝氨酸等分子中含有活泼的伯胺基团,通过Michael加成反应或形成Schiff碱的方式与HNE结合[21]。
HNE和核酸类物质形成复合物,干扰DNA复制、RNA转录,对细胞产生基因毒性[22-23]。DNA中的鸟嘌呤是HNE攻击的主要对象,Chen等分别报道了HNE和鸟嘌呤的结合方式及形成的相应产物[24-25]。
除了这些结合形态,HNE还能与内源性的多肽结合,如含组氨酸的肽段、胰岛素、血管紧张素等[26-29]。此外,HNE 还能与一些小分子结合,如辅助因子、维他命、H2S等[30-32]。
3HNE的代谢
健康人体内HNE能够维持一定的水平,离不开代谢作用。体内HNE的代谢可以分为第Ⅰ相代谢和第Ⅱ相代谢2种方式,前者主要借助氧化还原酶将HNE转化为无毒化合物,后者主要通过与GSH结合来清除HNE。
3.1第Ⅰ相代谢
第Ⅰ相代谢主要包含氧化和还原2种途径。HNE氧化代谢途径主要通过醛脱氢酶(ALDH)来实现,该酶是缓解及预防产生细胞毒性的一类酶,能够选择性地将HNE分子中的醛基转化为羧基,形成无活性的烯酸(HNA)[33]。HNA经β氧化,可转变为CO2和H2O[34];在细胞色素P450(CYP450)的作用下,经ω氧化进一步转化为二醇和三醇,端位的醇羟基可继续氧化为相应的羧酸[35]。乙醇脱氢酶(ADH)、醛酮/还原酶(AKR)以及烯醛/烯酮氧化还原酶(AOR)是HNE还原代谢常用的酶。ADH和AKR能够选择性地将醛基还原成醇羟基,形成无活性的二醇产物(DHN)[36]。而AOR是一类具有还原碳碳双键能力的酶。近期的研究表明,AOR过度表达,可以将体内堆积的HNE转化4-羟基壬醛(HN),从而保护细胞免受HNE的侵害[37]。
3.2第Ⅱ相代谢
第Ⅱ相代谢主要由HNE和GSH形成无活性大分子(GS-HNE)来完成,在谷胱甘肽轉移酶(GSTs)、羰基还原酶(CBR1)等催化下,GSH与HNE通过Michael加成反应,保留的醛基可以继续与分子内的巯基形成环化产物,所得环化产物和直接加成产物的比例接近于1 ∶1[3]。在氧化或还原酶作用下,GS-HNE可进一步形成GS-HNA、GS-ONA、GS-DHN等化合物[38-40]。在γ谷氨酸转移酶以及胱氨酸S-聚合-N-乙酰转移酶的作用下,GS-HNE、GS-DHN、GS-HNA等的GS端可进一步水解、酰化,转变为硫醇尿酸(MA),形成的 HNE-MA、HNA-MA和DHN-MA可直接排出体外[41]。
4HNE的病理和生理学意义
健康人体血浆中HNE的平均含量约为 11.62 μg/L[42],体内HNE的浓度与细胞种类及代谢环境等因素有关,胞内HNE的浓度对细胞有明显影响。图4展示了HNE浓度由较低的生理水平逐渐升高后,细胞内的生化过程及状态变化:生理水平HNE在各种酶作用下,被完全代谢,不影响细胞的存活;低浓度HNE能够发挥重要的信号传导作用,如刺激基因表达、促进抗氧化能力、提高应激能力等;中等水平的HNE,能够发出蛋白质受损信号,影响蛋白质和细胞器正常功能,引发自噬、衰老及细胞周期停滞;高浓度和极高浓度的HNE,能够继续和蛋白质、DNA等形成复合物,引发细胞毒性和基因毒性,产生病理作用,引发细胞程序性凋亡[43]。
氧化应激和不断积累的脂质过氧化是多种疾病发病和病变的重要影响因素,HNE作为活泼的脂质氧化标志物,在地中海贫血、疟疾、维生素E缺乏、B16黑色素瘤、低血容量性休克和创伤患者的相关组织中均能检测到[44-49]。此外,HNE与心脏疾病、癌症、神经退行性疾病等的发生、发展密切相关。研究表明,扩张型心肌病患者体内HNE修饰蛋白的表达量比健康人群高5倍[50]。除了心脏疾病,HNE对癌症发生及发展有重要的影响。HNE不仅能够修饰DNA的结构,还能够干预DNA的修复机制,导致DNA受损,促进癌症的形成[51]。神经系统含有丰富的ω-6 PUFAs、金属离子,参与频繁的氧化还原转换,是形成HNE的重要场所。帕金森综合征是第二大神经退行性疾病,HNE不仅能够修饰大脑路易斯体中α-突触核蛋白的结构,诱导该蛋白
发生降解、聚集,还能够作用于多巴胺转运器,抑制多巴胺吸收,降低体内多巴胺水平,这二者均能加重帕金森综合征病情[52-53]。阿尔茨海默病是研究最为广泛的神经退行性紊乱病,研究表明患者体内的HNE水平显著高于健康人群,HNE通过修饰β-淀粉样蛋白结构、促进自由基形成,加剧该病的发展[54-55]。
5HNE的检测方法
HNE具有的病理生理学作用引起了研究人员广泛关注,体内HNE水平被认为是脂质代谢最重要的指标之一。HNE本身容易挥发、分解,当pH值>9或pH值<1,或者温度高于50 ℃时,HNE容易分解;HNE在氮气吹扫、真空浓缩等过程中也容易挥发,这给HNE的提取工作带来了极大挑战。此外,HNE具有紫外、荧光检测响应低,质谱离子化效率不高等特性,须借助衍生试剂来提高检测灵敏度。目前,按照HNE的形态,检测方法可以分为游离形态和结合形检测方法。
5.1游离形态HNE的检测方法
光谱分析法和质谱法是游离形态HNE常用的检测方法。这2种方法常借助衍生试剂来完成,因此衍生试剂的选择是一个关键因素,应满足衍生速度快、衍生产物稳定、容易分离和检测等要求。光谱法常用的衍生试剂有肼和酮类。2,4-二硝基苯肼是最常用的肼类衍生试剂,它能与HNE结合形成稳定的苯腙,该衍生产物具有良好的紫外吸收能力,可以通过高效液相-紫外检测器(HPLC-UV)进行检测分析,适用于油脂、肉品、水产、蔬菜等样本[56]。1,3-环己二酮是酮类常用的衍生试剂,在铵盐的作用下,它与HNE作用形成的产物具有荧光性,可通过高效液相-荧光检测器(HPLC-FLD)来进行定量分析[57]。质谱技术的成熟,使得HNE的分析更加精准,通过质荷比的特异性,可对相应物质进行定性定量分析。气相色谱-质谱联用(GC-MS)技术是HNE常用的检测手段,羟胺类衍生试剂与HNE经醛胺缩合、羟基衍生操作后,形成的产物适合采用GC-MS技术分析[58]。近年来,食品中HNE的分析方法取得了较多关注,植物油脂富含不饱和脂肪酸,是HNE形成的重要来源,Globisch等采用两步衍生法,先将HNE转化为稳定的肟醚,再对羟基进行硅烷基化,采用GC-MS法对多种植物油的HNE进行普查[59-60]。苯肼类衍生结合液相色谱串联质谱(LC-MS/MS)技术也是食品中HNE常用的检测方法,适应于多种油脂、油炸食品中HNE含量的分析[61-64]。最近,笔者所在课题组就肉制品中HNE的提取及分析方法进行研究,开发了液氮速冻制样、同位素内标添加、低温液提取的方法,提升了肉制品中HNE分析方法的灵敏度和精密度[65]。尽管游离形态HNE的分析技术研究取得了一定的进展,但仍存在一些问题:(1)游离态HNE提取方法不完善,提取过程中难以控制HNE的形成、转化以及分解;(2)样品重复性差,回收率不高。
5.2結合形态HNE的检测方法
与游离形态HNE检测相比,结合形态HNE的检测情况相对复杂一些,不仅需要找出HNE结合形态的形成部位、种类、结合方式等相关信息,还需建立合适的检测方法。免疫组织化学和免疫细胞化学是结合形态HNE检测早期的使用方法,可将免疫反应的特异性和组织化学的可见性巧妙结合,促进了部分结合形态HNE的定位、定性以及定量分析,如检测低密度脂蛋白上HNE-赖氨酸的水平、血清中HNE-多聚氨基酸的结合种类和数量、细胞裂解产物中HNE-组氨酸的含量以及尿液中DHN-HNE的含量等。尽管这些技术证实了HNE能以结合态的方式存在,但存在抗体制备专一性不强、交叉反应率高等问题。肽段质量指纹图谱、质谱序列、中性扫描损失等是质谱常用的分析手段,用来解析HNE修饰蛋白、肽段等的种类和结合位点,这些检测有效促进了结合形态HNE的分析工作,但由于结合形态HNE的丰度不高,许多工作仍集中在富集方法、HNE修饰种类、结合位点等方面,结合形态HNE的分析工作仍处于起步阶段,面临的困难和需要解决的问题还有很多[14]。
6食品中HNE的安全问题
不论是新鲜食材,还是贮藏、加工等过程中的食品,脂质氧化都不可避免。人体内的HNE包括内源性和外源性2种:内源性的HNE由细胞或组织中ω-6 PUFAs分解代谢产生;外源性的HNE主要通过膳食摄入。通过膳食获取的HNE含量与食品中HNE含量紧密相关,食品的种类、贮藏加工方式均会影响HNE的形成,研究人员对各类食品中的游离形态HNE含量进行了调查分析,发现油脂、油炸土豆及肉制品中富含HNE,过多地摄取这些食物可能会影响人类的健康[66]。此外,HNE性质比较活泼,能够与多种大分子活性物质形成复合物,在一定条件下,这些结合态的复合物与游离形态的HNE可互相转化,已有研究报道尚不能提供完整、可靠的HNE含量、形态等信息,但从中可以看出,富含不饱和脂肪酸的食物普遍存在HNE。由于结合形态HNE的存在,真实HNE的含量可能远远超过检测水平。
值得注意的是,除了膳食本身携带的HNE,膳食中HNE的前体物质进入消化器官后,能够继续形成HNE,提高体内HNE水平,并可能发挥内源性HNE相同的病理生理学作用[67-69]。迄今为止,人体内HNE水平与多种疾病相关性的研究均未能有效区分体内HNE的来源,因此食品中普遍含有的HNE对人体健康具有潜在的安全风险。食品中HNE的安全隐患不容忽视,食品尤其是肉制品中HNE的形态鉴定与分布、分析技术、形成规律及机制、暴露风险评价等值得开展进一步研究。
7研究展望
HNE是脂质氧化过程中ω-6 PUFAs的氧化产物。尽管在生理和病理学领域的研究取得了重大进展,但在食品领域的研究尚处于起步状态。HNE在食品贮藏及加工过程中的形成机制、形态分布及演变情况、定量分析方法、风险评估等将受到越来越多的关注。目前食品中减少HNE形成的方法主要包括采用多酚类阻断HNE形成、把控食物原料、控制食品加工方法等,希望开发更多的方法在提升食品营养价值、风味的同时,又能抑制HNE的形成。
参考文献:
[1]王淑慧,潘道东,曹锦轩,等. 鸭脂氧化及其挥发性香气成分气相色谱-质谱分析[J]. 食品科学,2014,35(2):205-208.
[2]Sakai T,Kuwazuru S,Yamauchi K,et al. A lipid peroxidation-derived aldehyde,4-hydroxy-2-nonenal and omega 6 fatty acids contents in meats[J]. Bioscience Biotechnology and Biochemistry,1995,59(7):1379-1380.
[3]Schaur R J. Basic aspects of the biochemical reactivity of 4-hydroxynonenal[J]. Molecular Aspects of Medicine,2003,24(4/5):149-159.
[4]陈娟,冉丕鑫. 4-羟基壬烯醛在疾病发生机制方面的研究进展[J]. 呼吸杂志,2006,26(11):821-824.
[5]Guillén M D,Goicoechea E. Toxic oxygenated alpha,beta-unsaturated aldehydes and their study in foods:a review[J]. Critical Reviews in Food Science and Nutrition,2008,48(2):119-136.
[6]Wilson R,Lyall K,Smyth L,et al. Dietary hydroxy fatty acids are absorbed in humans:implications for the measurement of ‘oxidative stress in vivo[J]. Free Radical Biology & Medicine,2002,32(2):162-168.
[7]Esterbauer H,Benedetti A,Lang J,et al. Studies on the mechanism of formation of 4-hydroxynonenal during microsomal lipid peroxidation[J]. Biochimica et Biophysica Acta,1986,876(1):154-166.
[8]Esterbauer H,Zollner H,Schaur R J. Aldehydes formed by lipid peroxidation:mechanisms of formation,occurrence and determination [M]//Vigo-Pelfrey C. Membrane Lipid Oxidation. Boca Raton,FL:CRC Press,1990:239-283.
[9]Schneider C,Tallman K A,Porter N A,et al. Two distinct pathways of formation of 4-hydroxynonenal[J]. Journal of Biological Chemistry,2001,276(24):20831-20838.
[10]Upston J M,Neuzil J,Witting P K,et al. Oxidation of free fatty acids in low density lipoprotein by 15-lipoxygenase stimulates nonenzymic,alpha-tocopherol-mediated peroxidation of cholesteryl esters[J]. The Journal of Biological Chemistry,1997,272(48):30067-30074.
[11]Kaur K,Salomon R G,Oneil J,et al. (Carboxyalkyl)pyrroles in human plasma and oxidized low-density lipoproteins[J]. Chemical Research in Toxicology,1997,10(12):1387-1396.
[12]Gardner H W,Grove M J. Soybean lipoxygenase-1 oxidizes 3Z-nonenal[J]. Plant Physiology,1998,116(4):1359-1366.
[13]Schneider C,Boeglin W E,Yin H,et al. Synthesis of dihydroperoxides of linoleic and linolenic acids and studies on their transformation to 4-hydroperoxynonenal[J]. Lipids,2005,40(11):1155-1162.
[14]Spickett C M. The lipid peroxidation product 4-hydroxy-2-nonenal:Advances in chemistry and analysis[J]. Redox Biology,2013,1(1):145-152.
[15]Schneider C,Porter N A,Brash A R. Routes to 4-hydroxynonenal:fundamental issues in the mechanisms of lipid peroxidation[J]. The Journal of Biological Chemistry,2008,283(23):15539-15543.
[16]Gu X,Salomon R G. Fragmentation of a linoleate-derived γ-hydroperoxy-α,β-unsaturated epoxide to γ-hydroxy-and γ-oxo-alkenals involves a unique pseudo-symmetrical diepoxycarbinyl radical[J]. Free Radical Biology & Medicine,2012,52(3):601-606.
[17]Castro J P,Jung T,Grune T,et al. 4-Hydroxynonenal (HNE) modified proteins in metabolic diseases[J]. Free Radical Biology & Medicine,2017,111:309-315.
[18]Xu G,Liu Y,Sayre L M. Independent synthesis,solution behavior,and studies on the mechanism of formation of a primary amine-derived fluorophore representing cross-linking of proteins by(E)-4-hydroxy-2-nonenal[J]. The Journal of Organic Chemistry,1999,64(16):5732-5745.
[19]Gardner H W,Deighton N. Effect of 4-hydroxy-2(E)-nonenal on soybean lipoxygenase-1[J]. Lipids,2001,36(6):623-628.
[20]Hartley D P,Ruth J A,Petersen D R. The hepatocellular metabolism of 4-hydroxynonenal by alcohol dehydrogenase,aldehyde dehydrogenase,and glutathione S-transferase[J]. Archives of Biochemistry and Biophysics,1995,316(1):197-205.
[21]Guichardant M,Taibi-Tronche P,Fay L B,et al. Covalent modifications of aminophospholipids by 4-hydroxynonenal[J]. Free Radical Biology & Medicine,1998,25(9):1049-1056.
[22]Minko L G,Kozekov,D L,et al. Chemistry and biology of DNA containing 1,N(2)-deoxyguanosine adducts of the α,β-unsaturated aldehydes acrolein,crotonaldehyde,and 4-hydroxynonenal[J]. Chemical Research in Toxicology,2009,22(5):759-778.
[23]Feng Z,Hu W,Amin S,et al. Mutational spectrum and genotoxicity of the major lipid peroxidation product,trans-4-hydroxy-2-nonenal,induced DNA adducts in nucleotide excision repair-proficient and-deficient human cells[J]. Biochemistry,2003,42(25):7848-7854.
[24]Chen H J,Gonzalez F J,Shou M G,et al. 2,3-Epoxy-4-hydroxynonenal,a potential lipid peroxidation product for etheno adduct formation,is not a substrate of human epoxide hydrolase[J]. Carcinogenesis,1998,19(5):939-943.
[25]Wacker M,Wanek P,Eder E. Detection of 1,N-2-propanodeoxyguanosine adducts of trans-4-hydroxy-2-nonenal after gavage of trans-4-hydroxy-2-nonenal or induction of lipid peroxidation with Carbon tetrachloride in F344 rats[J]. Chemico-Biological Interactions,2001,137(3):269-283.
[26]Boldyrev A A,Aldini G,Derave W. Physiology and pathophysiology of carnosine[J]. Physiological Reviews,2013,93(4):1803-1845.
[27]Beretta G,Artali R,Regazzoni L,et al. Glycyl-histidyl-lysine (GHK) is a quencher of alpha,beta-4-hydroxy-trans-2-nonenal:a comparison with carnosine. insights into the mechanism of reaction by electrospray ionization mass spectrometry,1H NMR,and computational techniques[J]. Chemical Research in Toxicology,2007,20(9):1309-1314.
[28]Aoi W,Naito Y,Yoshikawa T. Role of oxidative stress in impaired insulin signaling associated with exercise-induced muscle damage[J]. Free Radical Biology & Medicine,2013,65:1265-1272.
[29]Takahashi R,Goto T,Oe T,et al. Angiotensin Ⅱ modification by decomposition products of linoleic acid-derived lipid hydroperoxide[J]. Chemico-Biological Interactions,2015,239:87-99.
[30]Aldini G,Vistoli G,Stefek M,et al. Molecular strategies to prevent,inhibit,and degrade advanced glycoxidation and advanced lipoxidation end products[J]. Free Radical Research,2013,47(Suppl 1):93-137.
[31]Dwyer J P,Greco B A,Umanath K,et al. Pyridoxamine dihydrochloride in diabetic nephropathy (PIONEER-CSG-17):lessons learned from a pilot study[J]. Nephron,2015,129(1):22-28.
[32]Kuiper H C,Bruno R S,Traber M G,et al. Vitamin C supplementation lowers urinary levels of 4-hydroperoxy-2-nonenal metabolites in humans[J]. Free Radical Biology & Medicine,2011,50(7):848-853.
[33]Yoval-Sánchez B,Rodríguez-Zavala J S. Differences in susceptibility to inactivation of human aldehyde dehydrogenases by lipid peroxidation byproducts[J]. Chemical Research in Toxicology,2012,25(3):722-729.
[34]Grune T,Siems W G,Petras T. Identification of metabolic pathways of the lipid peroxidation product 4-hydroxynonenal in in situ perfused rat kidney[J]. Journal of Lipid Research,1997,38(8):1660-1665.
[35]Jin Z C,Berthiaume J M,Li Q L,et al. Catabolism of (2E)-4-hydroxy-2-nonenal via ω-and ω-1-oxidation stimulated by ketogenic diet[J]. The Journal of Biological Chemistry,2014,289(46):32327-32338.
[36]Boleda M D,Saubi N,Farrés J,et al. Physiological substrates for rat alcohol dehydrogenase classes:aldehydes of lipid peroxidation,omega-hydroxyfatty acids,and retinoids[J]. Archives of Biochemistry and Biophysics,1993,307(1):85-90.
[37]Dick R A,Kwak M K,Sutter T R,et al. Antioxidative function and substrate specificity of NAD(P)H-dependent alkenal/one oxidoreductase. A new role for leukotriene B4 12-hydroxydehydrogenase/15-oxoprostaglandin 13-reductase[J]. The Journal of Biological Chemistry,2001,276(44):40803-40810.
[38]Black W,Chen Y,Matsumoto A,et al. Molecular mechanisms of ALDH3A1-mediated cellular protection against 4-hydroxy-2-nonenal[J]. Free Radical Biology & Medicine,2012,52(9):1937-1944.
[39]Moschini R,Peroni E,Rotondo R,et al. NADP+-dependent dehydrogenase activity of carbonyl reductase on glutathionylhydroxynonanal as a new pathway for hydroxynonenal detoxification[J]. Free Radical Biology & Medicine,2015,83:66-76.
[40]Rotondo R,Moschini R,Renzone G,et al. Human carbonyl reductase 1 as efficient catalyst for the reduction of glutathionylated aldehydes derived from lipid peroxidation[J]. Free Radical Biology & Medicine,2016,99:323-332.
[41]Petras T,Siems W G,Grune T. 4-hydroxynonenal is degraded to mercapturic acid conjugate in rat kidney[J]. Free Radical Biology & Medicine,1995,19(5):685-688.
[42]Gil L,Siems W,Mazurek B,et al. Age-associated analysis of oxidative stress parameters in human plasma and erythrocytes[J]. Free Radical Research,2006,40(5):495-505.
[43]Eckl P M. Genotoxicity of HNE[J]. Molecular Aspects of Medicine,2003,24(4/5):161-165.
[44]Ramenghi U,Chiarpotto E,David O,et al. Increased production of carbonyls other than maionaldehyde in red blood cells from homocygous thalassemic subjects exposed to oxidative stress [J]. IRCS Journal of Medical Science,1985,13:273-274.
[45]Yoshino K,Sano M,Fujita M,et al. Formation of aliphatic aldehydes in rat plasma and liver due to vitamin E deficiency[J]. Chemical & Pharmaceutical Bulletin,1986,34(12):5184-5187.
[46]Buffinton G D,Hunt N H,Cowden W B,et al. Detection of short-chain carbonyl products of lipid peroxidation from malaria-parasite (Plasmodium vinckei)-infected red blood cells exposed to oxidative stress[J]. The Biochemical Journal,1988,249(1):63-68.
[47]Sano M,Suzuki M,Nakamura Y,et al. Lipid peroxidation in liver and lung of the mouse implanted B16 melanoma[M]. Amsterdam:Elsevier Science Publishers,1989:971-974.
[48]Sharaf E M,Dussing G,Egger G,et al. Detection of 4-hydroxy-nonenal,a mediator of traumatic inflammation,in a patient with surgical trauma and in the Sephadex inflammation model[J]. Progress in Clinical and Biological Research,1989,308:351-356.
[49]Lieners C,Redl H,Molnar H,et al. Lipid peroxidation in a canine model of hypovolemic-traumatic shock [J]. Progress in Clinical Biological Research,1989,308:345-350.
[50]Nakamura K,Kusano K,Nakamura Y,et al. Carvedilol decreases elevated oxidative stress in human failing myocardium[J]. Circulation,2002,105(24):2867-2871.
[51]Feng Z,Hu W,Tang M S. Trans-4-hydroxy-2-nonenal inhibits nucleotide excision repair in human cells:a possible mechanism for lipid peroxidation-induced carcinogenesis[J]. Proceedings of the National Academy of Sciences of the United States of America,2004,101(23):8598-8602.
[52]Xiang W,Schlachetzki J C,Helling S,et al. Oxidative stress-induced posttranslational modifications of alpha-synuclein:Specific modification of alpha-synuclein by 4-hydroxy-2-nonenal increases dopaminergic toxicity[J]. Molecular and Cellular Neurosciences,2013,54:71-83.
[53]Morel P,Tallineau C,Pontcharraud R,et al. Effects of 4-hydroxynonenal,a lipid peroxidation product,on dopamine transport and Na+/K+ATPase in rat striatal synaptosomes[J]. Neurochemistry International,1998,33(6):531-540.
[54]Williams T I,Lynn B C,Markesbery W R,et al. Increased levels of 4-hydroxynonenal and acrolein,neurotoxic markers of lipid peroxidation,in the brain in Mild Cognitive Impairment and early Alzheimers disease[J]. Neurobiology of Aging,2006,27(8):1094-1099.
[55]Hardas S S,Sultana R,Clark A M,et al. Oxidative modification of lipoic acid by HNE in Alzheimer disease brain[J]. Redox Biology,2013,1(1):80-85.
[56]Uchiyama S,Inaba Y,Kunugita N. Derivatization of carbonyl compounds with 2,4-dinitrophenylhydrazine and their subsequent determination by high-performance liquid chromatography[J]. Journal of Chromatography. B,Analytical Technologies in the Biomedical and Life Sciences,2011,879(17/18):1282-1289.
[57]Bailey A L,Wortley G,Southon S. Measurement of aldehydes in low density lipoprotein by high performance liquid chromatography[J]. Free Radical Biology & Medicine,1997,23(7):1078-1085.
[58]Chevolleau S,Noguer-Meireles M H,Jouanin I,et al. Development and validation of an ultra high performance liquid chromatography-electrospray tandem mass spectrometry method using selective derivatisation,for the quantification of two reactive aldehydes produced by lipid peroxidation,HNE (4-hydroxy-2(E)-nonenal) and HHE (4-hydroxy-2(E)-hexenal) in faecal water[J]. Journal of Chromatography(B,Analytical Technologies in the Biomedical and Life Sciences),2018,1083:171-179.
[59]Globisch M,Kaden D,Henle T. 4-hydroxy-2-nonenal (4-HNE) and its lipation product 2-pentylpyrrole lysine (2-PPL) in peanuts[J]. Journal of Agricultural and Food Chemistry,2015,63(21):5273-5281.
[60]Steppeler C,Haugen J E,Rdbotten R,et al. Formation of malondialdehyde,4-hydroxynonenal,and 4-hydroxyhexenal during in vitro digestion of cooked beef,pork,chicken,and salmon[J]. Journal of Agricultural and Food Chemistry,2016,64(2):487-496.
[61]Sousa B C,Pitt A R,Spickett C M. Chemistry and analysis of HNE and other prominent carbonyl-containing lipid oxidation compounds[J]. Free Radical Biology & Medicine,2017,111:294-308.
[62]Ma L,Liu G. Simultaneous analysis of malondialdehyde,4-hydroxy-2-hexenal,and 4-hydroxy-2-nonenal in vegetable oil by reversed-phase high-performance liquid chromatography[J]. Journal of Agricultural and Food Chemistry,2017,65(51):11320-11328.
[63]Tullberg C,Vegarud G,Undeland I. Oxidation of marine oils during in vitro gastrointestinal digestion with human digestive fluids-Role of oil origin,added tocopherols and lipolytic activity[J]. Food Chemistry,2019,270:527-537.
[64]Wang L,Csallany A S,Kerr B J,et al. Kinetics of forming aldehydes in frying oils and their distribution in French fries revealed by LC-MS-based chemometrics[J]. Journal of Agricultural and Food Chemistry,2016,64(19):3881-3889.
[65]Ma J J,Geng Z M,Sun C,et al. Novel sample treatment method for the determination of free (E)-4-hydroxy-2-nonenal in meat products by liquid chromatography/tandem mass spectrometry using 4-hydroxy-2-nonenal-d(3) as internal standard[J]. Rapid Communications in Mass Spectrometry,2021,35(5):e9023.
[66]Liao H X,Zhu M J,Chen Y. 4-hydroxy-2-nonenal in food products:A review of the toxicity,occurrence,mitigation strategies and analysis methods [J]. Trends in Food Science & Technology,2020,96:188-198.
[67]van Hecke T,Vossen E,Hemeryck L Y,et al. Increased oxidative and nitrosative reactions during digestion could contribute to the association between well-done red meat consumption and colorectal cancer[J]. Food Chemistry,2015,187:29-36.
[68]van Hecke T,Jakobsen L M,Vossen E,et al. Short-term beef consumption promotes systemic oxidative stress,TMAO formation and inflammation in rats,and dietary fat content modulates these effects[J]. Food & Function,2016,7(9):3760-3771.
[69]Estévez M,Luna C. Dietary protein oxidation:a silent threat to human health ?[J]. Critical Reviews in Food Science and Nutrition,2017,57(17):3781-3793.
基金項目:国家自然科学基金(编号:31901716、31671877);国家现代农业(肉鸡)产业体系建设专项(编号:CARS-41);江苏省自然科学基金(编号:BK20171324);江苏省科技计划(编号:XZ-SZ2019)。
作者简介:马晶晶(1988—),女,安徽铜陵人,博士,助理研究员,研究方向为肉品安全与质量控制。E-mail:jingjingma2017@163.com。
通信作者:耿志明,硕士,研究员,研究方向为肉品安全与质量控制。E-mail:zmgeng@163.com。

