A crate of Pandora: do amyloids from bacteria promote Alzheimer’s disease?
Vu Thu Thuy Nguyen, Kristina Endres
Incidence for microbes as drivers of Alzheimer’s disease (AD) pathology:AD is the predominant neurodegenerative disease within the elderly. Over 50 million patients suffer from dementia currently world-wide and an estimated tripling of numbers within the next 30 years is expected. Only one to maximally five percent of all cases of AD are based on mutations in the amyloid precursor protein (APP) gene or within the presenilin genes (PS1/PS2) and therefore are called familial (FAD). The majority of cases has to be designated as sporadic, which frankly only means that the origin of these cases is still enigmatic. Within the last 5 to 10 years new ideas have been born in regard to underlying causes of the sporadic manifestation by the re-discovery of our microbial commensals as important factors of health and disease of human beings. A growing number of studies reports on altered oral or gut microbial communities in AD patients as compared to unaffected, cognitively normal age-matched controls. However, still only restricted consensus of findings arises from these studies: Ruminococcus and S24-7 seem to be occasionally decreased while Odoribacter,Blautia, and Alistipes have repeatedly been shown to be increased in gut microbiota of patients or rodent disease models(Endres, 2019). Altered counts of single representatives of families or genera do not tell so much about physiologically relevant communities, functional interactions,or metabolites capable of triggering or preventing pathological alterations.
Routes for delivery of pro-amyloidogenic entities from the gut towards the brain:Occurrence of biological material with bacterial origin in vicinity or even within amyloid plaques in AD brains has already been demonstrated: lipopolysaccharides,pili proteins, as well as bacterial DNA have been found. With aging, weariness of barriers such as blood-brain barrier or gut-blood barrier increases, therefore it is highly plausible that by mistake such presumably gut-derived products find their way into the otherwise privileged and protected brain. Additionally, a provenance of amyloidogenic processes in peripheraltissue can be assumed. It is of note that APP is a ubiquitously expressed protein which has been identified in a pleithora of tissues and organs outside the central nervous system. Our own investigations revealed,for example, presence of Thy1 promoterregulated human APP expression in the gut of 5xFAD model mice (Brandscheid et al., 2017). This allows speculation if gutresident proteins or peptides might serve as a trigger for aggregation of the endogenous amyloidogenic peptide, subsequently followed by transfer towards the brain.
Parkinson’s disease (PD) presents with different symptoms as AD, takes different tracts in the brain, and also is influenced by different risk factors.However, it has in common with AD that it is a neurodegenerative disease, based on proteinaceous aggregates in the central nervous system. In contrast to AD, investigation of a potential gut-brain route contributing to PD pathology is more advanced. Scenarios with a gut-origin of the amyloidogenesis have been tested in mouse models by injecting the molecular hallmark of PD-α-synuclein fibers into the gastric wall (Uemura et al., 2018). This resulted in Lewy-body-like structures in the dorsal motor nucleus and the phenomenon could be prevented by vagotomy. Epidemiological studies from human patients also indicate that total vagotomy might lower the risk of PD (Liu et al., 2017) and thereby strengthens the hypothesis of PD origin in the gut.
For AD, functional investigations regarding a potential impact of gut-derived products on pathogenesis and delivery pathways are definitely needed but still not provided in sufficient depth. The only available investigation regarding vagotomy currently known to the authors is based on the inpatient database from the Taiwan NHI program, China. This investigation included more than 150,000 patients who underwent vagotomy along with the same number of age-matched controls and concludes that risk of dementia does not correlate with vagotomy (Lin et al., 2018). The authors state that the restricted follow-up time might hamper definite conclusion since their data showed that “vagotomy had no association with dementia before 7 years, but may provide protection for dementia after 7 years”. Another limitation of this study unfortunately is that dementia was used as an outcome and no distinction was made for PD and AD, both representing the most common forms of dementia in the elderly.The route allowing intestinal amyloids to enter the host and travel towards the brain might be totally independent from vagus nerve in AD and based on other traits than in PD.
How and if a potential transfer of bacterial amyloids from the gut lumen or mucosal surface occurs and contributes to development of human disease, still is enigmatic. Nevertheless, many plausible routes exist that might explain first entry into the intestinal tissue but also further transportation within the human host (Figure 1).
That, in general, bacteria are important factors for amyloid β deposition in the brain was impressively demonstrated by investigating APP/PS1 AD model mice raised germ-free (Harach et al., 2017): this evoked a clear reduction of pathological signs. Re-colonization with the microbiota from conventionally raised AD model mice but not from wild type mice increased deposits. However, this did not unravel which components of the commensals were eliciting the observed effects:bacterial lipopolysaccharides, toxins or even microbiota-derived secondary metabolites such as derivatives of bile acids might be underlying. Also amyloids have been found in microorganisms which might be involved.
Microbial amyloids and their function:Proteins or peptides capable of forming a stable cross-beta dominated secondary structure and subsequently depositing have been identified in viruses, yeasts, and bacteria (for the role of microbial amyloids as structural and adhesive material, toxins,and defensive agents see a summary in(Endres, 2020)). They act as typical amyloids comparable to human Aβ or α-synuclein. A prominent example of bacterial amyloids is curli. At least six proteins, encoded by two operons (csgBA and csgDEFG), are dedicated to curli formation inE. coli. CsgA and B are major structural components of the extracellular built curli fibers, while CsgD is the major regulator of the synthesis pathway. When bacteria settle on surfaces such as mammalian cells, they start a distinct genetic program which goes along with loss of motility and induction of biofilm formation. Curli fibers are components of such biofilms and help to stabilize the community. Other microbial amyloids are also biofilm components (e.g., the functional amyloid in pseudomonas C) or e.g., have been attributed to enhanced virulence (Hydrogenase maturation factor).Interestingly, a strong attachment of bacteria to our gut wall seems – in the first instance –not plausible as the intestinal wall is covered by a mucus layer. In the small intestine,this loosely attached and easy to penetrate mucin network is supported by anti-microbial peptides that hamper bacterial approach to the epithelium. In the colon, two layers of mucus protect the tissue (Figure 1).However, isolates ofE. colifrom human fecal material capable of forming curli-containing biofilms have been described (Bokranz et al., 2005) and biofilms isolated from human patients (with or without colon cancer)were able to evoke carcinogenesis in murine colon rectal cancer models (Tomkovich et al., 2019). This potentially implicates that it is not sufficient to quantitatively describe the gastrointestinal community or to identify the single participants. It might be rather a question of how these microbial organisms are organized and if harmful habitants might evade the host’s immune system by hiding in biofilm niches.
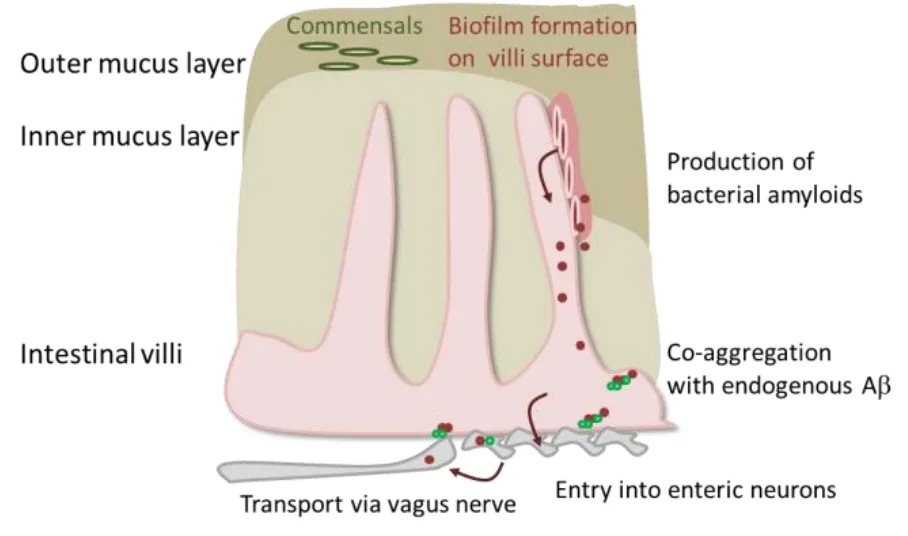
Figure 1|Putative transport of bacterial amyloids from the gut.
Impact of bacterial amyloids on amyloidogenic processes of the host:In vitroincubation of human Aβ1–42peptides with lysates ofE. coligrown under biofilm-/curli-synthesis-promoting conditions delayed start of measurable aggregation when compared to Aβ peptide alone. This shift was comparable as obtained by incubation with bacteria from a standard Lewy body-agar growth condition (Additional Figure 1A and B). However, the addition of curli-containing biofilm lysates did not induce aggregation as compared to bacterial cell lysates from normal cultivation on standard LB agar(slope quantitation in Additional Figure 1C).Such approaches certainly have to be taken with caution as the two different growth conditions will lead to various changes in expression pattern besides curli production and biofilm formation itself is highly variable and complex (Rossi et al., 2018). Additionally,in vitroexperiments lack the physiological surrounding such as pH of the gut lumen, gas atmospheric condition or other interfering molecules. Nevertheless, feeding of amyloidproducing bacteria to rats and to aggregation models for PD inC. elegansshowed the possible effect of bacterial biomolecules on neurodegenerative disorders: aged Fisher 344 rats that already possess aggregated α-synuclein in the intestinal submucosal plexus showed aggravated protein deposits- not only in the gut but also in the hippocampus (Chen et al., 2016). Moreover,they displayed elevated immune response within the brain. This was not the case when curli-deficient bacteria were used for weekly oral feeding. The aggregation-promoting effect was also observed inC. elegansstrain with muscular overexpression of YFP-tagged human α-synuclein. More recently,colonization of germ-free PD model mice with different bacterial strains confirmed these findings (Sampson et al., 2020). While mono-colonization with anE. colistrain unable to produce curli did not evoke effects on pathophysiology, curli-producing bacteria reinforced pathophysiology as demonstrated by pole descent test or adhesive removal test, both reporting on motor dysfunction of the animal model. These effects even persisted when the curli-producing strain was transferred on a complex human fecal microbiome with a low curli-producing potential.
This indicates that not only mere presence of microorganisms but also their ecological condition might impact amyloidogenic processes within the human host. Still, data for AD-centered research are scarce.
Future outlook:Including the “gut perspective” in the field of Alzheimer’s disease research opens up fascinating new ways of re-thinking the old hypothesis about origin of the disease. First evidence that the gut microbiome might impact disease development and might serve as a diagnostic or even therapeutic tool has been provided.However, many of the studies still report on observations such as changes in microbiome,while more functional investigations will be needed to allow optimal valuation of the role the microbiota plays. Functional approaches are not easy to be established:not only that a multi-organ-system has to be taken into account (brain-liver-gut) but also a multi-organismic system, providing a complex ecological network with a high dynamic due to daily diet, medication,stress experience etc. - at least in humans.The typical laboratory animals that can be better controlled for these biases might not offer optimal models as chow and digestion of rodents definitely deviate from those of human beings. Potentially, organ-on-a-chip solutions or artificial gastrointestinal tracts will help to overcome the limitations in future.
This work was supported by funding from the MWWK, Germany (research consortium NeuroDegX).
Vu Thu Thuy Nguyen, Kristina Endres*
Department of Psychiatry and Psychotherapy,University Medical Center Johannes Gutenberg-University Mainz, Mainz, Germany
*Correspondence to:Kristina Endres, PhD,kristina.endres@unimedizin-mainz.de.https://orcid.org/0000-0002-1099-8287(Kristina Endres)
Date of submission:March 26, 2020
Date of decision:April 21, 2020
Date of acceptance:June 13, 2020
Date of web publication:November 16, 2020
https://doi.org/10.4103/1673-5374.297074
How to cite this article:Nguyen VTT, Endres K(2021) A crate of Pandora: do amyloids from bacteria promote Alzheimer’s disease? Neural Regen Res 16(5):988-989.
Copyright license agreement:The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional file:
Additional Figure 1:Impact of differentially grown E. coli on Aβ aggregation in vitro.
- 中国神经再生研究(英文版)的其它文章
- Genetics provides new individualized therapeutic targets for Parkinson’s disease
- Improving brain outcomes in the growth restricted newborn: treating after birth
- Organotypic hippocampal slices,an emerging tool to model synucleinopathies
- Arteriovenous malformations: the newest Sonic hedgehog game in the postnatal brain
- Evidences of hydroxytyrosol as an anti-inflammatory agent in Parkinson’s disease: insights into the mechanisms of action
- Cannabinoid pharmacology and its therapeutic uses in Alzheimer’s disease