Research Progress on Medicinal Active Components of Endophytes in Plants
Long CHEN Miao ZHANG
Abstract Endophytes are a type of microorganisms which live in plant tissues and have no obvious disease symptoms to plants, mainly including fungi, bacteria and actinomycetes. Endophytes can produce the same or similar active substances as host plants, so they have become a hot spot in the research of natural medicinal active products in recent years. Endophytes provide abundant resources for searching medicinal active substances because of their abundant species and huge reserves. In this paper, the species, structural characteristics, separation methods and pharmacological studies of active products of endophytic fungi were reviewed, in order to provide theoretical reference for the research of medicinal active components of endophytic fungi.
Key words Endophytes of plants; Medicinal use; Active ingredients
Received: March 5, 2021 Accepted: May 23, 2021
Supported by The Basic Ability Improvement Project of Young and Middle-aged Professors in Guangxi Universities (2017KY0294);Guangxi Key Laboratory of Zhuang and Yao Ethnic Medicines (GKJZ[2014]32); Collaborative Innovation Center of Zhuang and Yao Ethnic Medicines (GKKY[2013]20); Ethnic Medicine Resources and Application Engineering Research Center of Guangxi Zhuang Autonomous Region (GFGGJH[2020]2605); "The Eighth Batch of Guangxi Specially-employed Expert Projects" (GRCTZ[2019]13).
Long CHEN (1988-), male, P. R. China, assistant research fellow, master, devoted to research about development of endophyte resources in medicinal plants.
The authors have declared no conflict of interest.
*Corresponding author. E-mail: 6764074656@qq.com.
Plant endophytes are a type of microorganisms that all or partially live in healthy plant tissues and do not cause obvious symptoms of infection in host plants, mainly including bacteria, fungi and actinomycetes[1-2]. When endophytes grow in hosts, they coordinate and adapt to each other, forming a mutually beneficial symbiosis. In 1993, the strobel research team of Montana State University in the United States[3] isolated an endophytic fungus capable of synthesizing the anticancer substance paclitaxel from Taxus brevifolia for the first time, which opened the prelude to the research on the medicinal active ingredients of plant endophytes. After nearly 30 years of research, it has been found that plant endophytes and their metabolites can produce abundant medicinal active ingredients. These active products are widely used in all walks of life in society through industrial fermentation technology, biosynthesis technology and chemical synthesis technology, such as medicine, food, agriculture, environmental protection, etc.[4-6].
In this paper, we reviewed the research contents of the types, structural characteristics, separation methods, and application development of active products of plant endophytes in the past five years.
Types of Medicinal Active Ingredients of Endophytes and Their Metabolites
Plant endophytes coexist with their host plants, and they also interact with other endophytes and the external ecological environment. Such complex environment also has varying degrees of impact on plant endophytes, resulting in endophytes having strong biosynthesis ability, which lays a foundation for endophytes to produce active substances with novel structures and diverse skeleton types[7]. In addition, there are many kinds of endophytes and rich species, so they have high biodiversity and abundance. According to research[8], the main types of medicinal active ingredients of plant endophytes and their metabolites are: terpenoids, alkaloids, phenylpropanoids, saponins, quinones, and flavonoids, organic acid compounds, aromatic compounds, lactone compounds, phenolic compounds, polypeptides and glycosides.
Main Source of Medicinal Active Ingredients of Plant Endophytes[9-17]
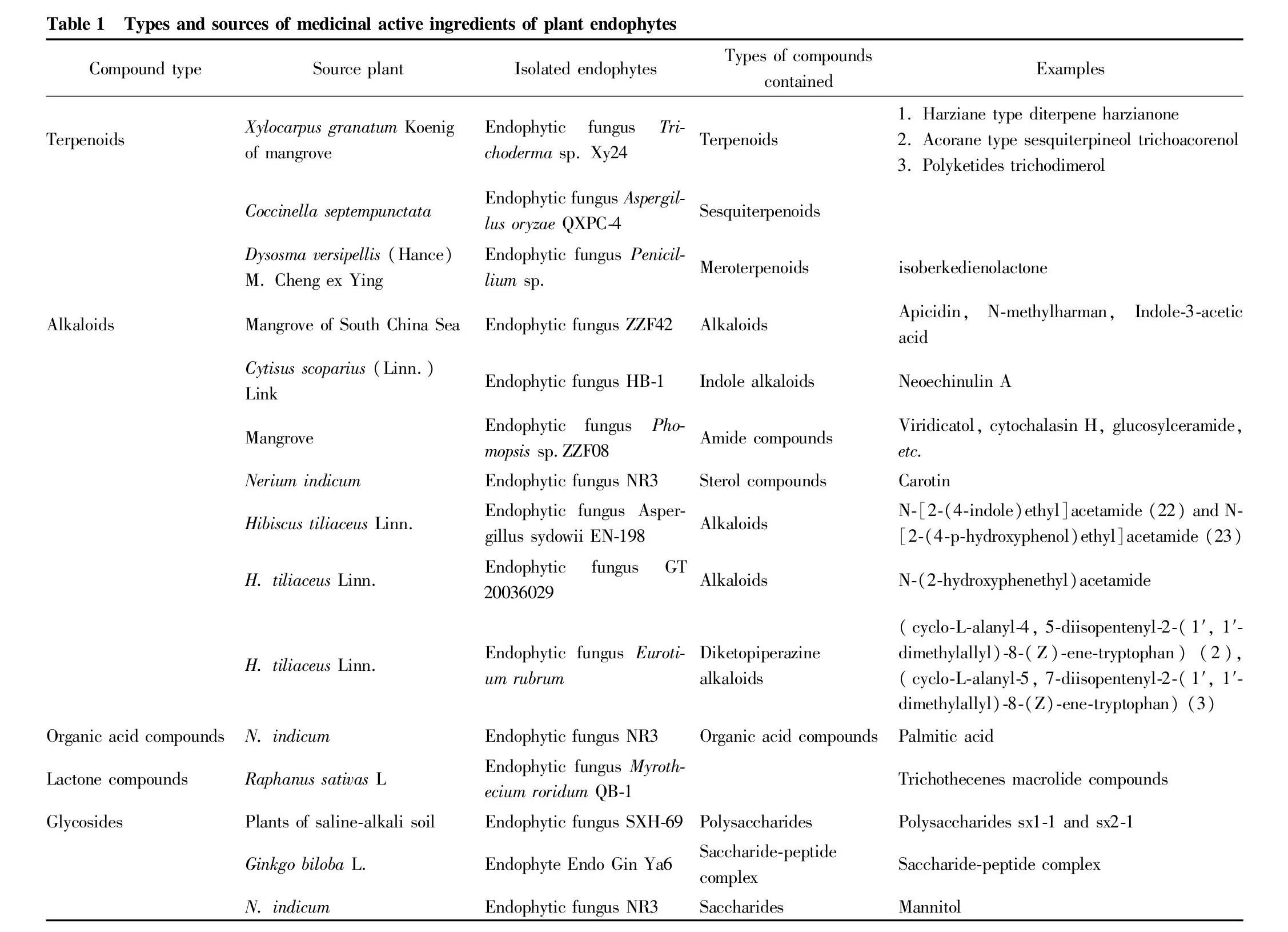
Plant endophytes can produce a variety of biologically active metabolites, which are mainly divided into terpenoids, alkaloids, organic acids, etc (Table 1).
Method for Separating Medicinal Active Ingredients from Plant Endophytes
For the isolation of medicinal active components of plant endophytes, endophytes are usually first cultured, isolated and identified, and the MTT method is used for activity screening; and the separated and purified mycelia are subjected to fermentation, and the fermentation broth is subjected to gel column chromatography, silica gel, sephadex, and analyzed by high-performance liquid chromatography, GC-MS, etc.
Du et al.[18] extracted the fermentation broth of endophytic fungus Aspergillus sydowii EN-198 used using ethyl acetate, and separated and determined the structures of six nucleoside compounds by silica gel column chromatography, sephadex column chromatography and preparative thin layer chromatography. Zhao[19] used tissue cutting method and plate streaking method to isolate and cultivate the strains, isolated and purified corresponding endophytic strains from Stemona and Dendrobium officinale,
and performed biological activity evaluation and HPLC/LC-MS chemical component analysis on the fermentation crude extract, screening out Steona sessilifolia endophyte Streptomyces (BS-1) and D. officinale endophyte Streptomyces (SH-1.2-R-15) for systematic chemical composition research. After large-scale fermentation and cultivation, the crude extract was obtained, and then separated and purified by silica gel, gel column chromatography and preparative liquid phase, etc., giving 20 compounds; combined with MS, 1D and 2D-NMR (1H-1H COSY, HSQC, HMBC, ROESY) and other spectroscopy methods, chemical correlation methods and single crystal X-ray diffraction methods, the structures of compounds were identified; and activity evaluation was performed on the obtained compounds, finding many compounds having insecticidal and cytotoxic activity. Wang[20] selected three endophytic fungi Pleosporales sp. F46, Acremonium pilosum F47 and Chaetomium nigricolor F5 of Mahonia fortunei (Lindl.) Fedde and one endophytic bacteria Bacillus wiedmannii Com1, and performed co-culture on fungus Pleosporales sp. F46 and bacterium B. wiedmannii Com1 with rice medium, fungus A. pilosum F47 and fungus Pleosporales sp. F46 with the potato dextrose medium (PDB), and fungus A. pilosum F47 and fungus C. nigricolor F5 with the PDB medium. The fermentation products were soaked in ethyl acetate, and the crude extracts were obtained by ultrasonic filtration extraction. The crude extracts were analyzed by HPLC, and the chromatographic peaks were found to be different from the chromatographic peaks of microorganisms in single culture. Normal phase silica gel chromatography, sephadex chromatography Sephadex LH-20, medium-pressure preparative chromatography, high-pressure preparative chromatography and high-performance liquid chromatography and other methods were applied to separate and purify the target products produced by co-culture. Infrared spectroscopy, ultraviolet spectroscopy, nuclear magnetic resonance and mass spectrometry were used to identify the structures of the compounds. The antimicrobial activity of the compounds were determined by the disc diffusion method, including two strains of Gram-positive bacteria Staphylococcus aureus and Bacillus subtilis, and two strains of Gram-negative bacteria Escherichia coli and Pseudomonas aeruginosa. The MTT method was used to determine the cytotoxic activity of the compounds on four kinds of tumor cells A549, MDA-MB-231, CT-26 and MCF-7.
Pharmacological Study on the Medicinal Active Components of Plant Endophytes
Antimicrobial effect
The research on plant endophytes has developed rapidly in recent years. A batch of endophytes with antimicrobial activity has been isolated from plants such as ginseng, dendrobium, Zanthoxylum nitidum (Roxb.) DC. and algae. Experiments have shown that medicinal plant endophytic fungi, bacteria and actinomycetes have certain antimicrobial activity to some extent.
Du et al.[18] isolated endophytic fungus A. sydowii EN-198 from the leaves of marine mangrove plant Hibiscus hibiscus in Dongzhai Port, Hainan, and used a variety of separation methods to isolate 14 compounds from its fermentation broth. With Staphylococcus aureus as the tested strain, the antimicrobial activity of all compounds was tested by the filter paper diffusion method. It was found that kojic acid showed good inhibitory activity, and when the addition amount was 100 μg/dish, the diameter of the inhibition zone was 10 mm, while the diameter of the inhibition zone of the positive control was 22 mm. Li[21] isolated a total of 76 endophytic bacteria, actinomycetes and fungi from the roots, stems and leaves of Artemisia annua, including 19 endophytic bacteria, 34 endophytic actinomycetes, and 23 endophytic fungi. With E. coli, B. subtilis, S. aureus, Aspergillus niger, and Saccharomyces cerevisiae as indicators, the agar block method and double-layer plate method were used to detect the antimicrobial activity of surface endophytes. It was found that the antimicrobial spectrum of endophytic bacteria was wider; and the antimicrbial spectrum of endophytic actinomycetes was narrow, but there were many high-resistant strains, especially for S. cerevisiae. Wang et al.[22] isolated and purified endophytic bacteria from mulberry leaves, and screened the antagonistic bacteria by the inhibition zone method. Meanwhile, organic solvents were used to extract the strain products, the inhibition band method was used to analyze the active substance groups, and the active substance types were qualitatively analyzed. The qualitative analysis showed that their antimicrobial active substances might be alkaloids, flavonoids, diterpenes and triterpenes. Singh et al.[23] isolated from Cyclospora lichens cyclohexenone and cyclohexanone, which have potential antifungal activity and moderate drug resistance. And at the same concentration, the inhibitory effect on S. aureus was stronger than that on E. coli. Ankit et al.[24] isolated 25 strains of endophytes from Indian laver, and the secondary metabolites of the largest isolate had good antimicrobial activity for S. aureus, Streptococcus pyogenes, E. coli, Salmonella typhimurium, and Klebsiella pneumoniae.
Anti-tumor effect
According to reports, the fermentation products of plant endophytes are extracted with ethyl acetate or methanol, and the extracts can show certain biological activity, and the monomer compounds obtained after separation and purification also show certain biological activity. Furthermore, anti-tumor activity as an important part of medical research has also attracted the attention of many scholars.
Lim et al.[25] studied the anti-cancer effect of the ethyl acetate part of marine plant endophytes, and evaluated the extract by the SRB method to have a good anti-tumor effect on the human colorectal cancer cell line HCT116. Ramalingam et al.[26] studied the anti-cancer and antimicrobial potential of marine algae endophytic fungus Penicillium aureus. After the research team cultured P. aureus with potato dextrose agar (PDB) for 21 d, the ethyl acetate (EA) extract had the highest inhibitory activity on human breast cancer cells (MCF-7). Similarly, the PDB-EA extract also had a certain inhibitory effect on human pathogens, and the extract could cause mcf-7 cell membrane damage and lead to cell apoptosis. As an epiphytic plant, D. officinale is one of the precious Chinese medicines with multiple activity. Zhao et al.[27] isolated and purified the endophytic bacteria of D. officinale, and obtained 58 endophytic bacteria of nine genera, and through an anti-cancer activity test, 3 kinds of bacteria showed strong anti-cancer effect on Hep3B2.1-7 chartreusin. Ju et al.[28] searched for endophytic bacteria with anti-tumor activity from ginseng, separated them using pseudomonas basal medium, and detected the anti-tumor activity in breast cancer cell line MDA-MB-231 through 16s rDNA molecular identification, MTT colorimetry, western blot, etc. The isolated strain G13476 was confirmed to be Pseudomonas adaceae by BLAST alignment; MTT data and western blot results showed that strain G13476 could inhibit triple-negative breast cancer by down-regulating Bcl-2 and up-regulating Bax. Gallo et al.[29] isolated an endophyte Nigrospora sphaerica that can produce extremely strong anti-tumor compounds from yacon. El-Hawary et al.[30] isolated an endophyte Aspergillus flavus SNFSt that can produce Solarargine, a compound with cytotoxic activity, from medicinal plant Solanum nigrum L. Palanichamy et al.[31] isolated the crystalline compound alternariol methyl ether (AME) with anticancer activity from the secondary metabolite of the endophytic fungus Alternaria alternata MGTMMP031. The crystalline compound (AME) had inhibitory effect on hepatocellular carcinoma (HCC) in vivo and in vitro.
Agricultural Biotechnology2021
Antioxidant activity
Antioxidant is an important way to prevent aging. For some aging-related diseases caused by free radicals, it is very important to eliminate excessive oxidative free radicals to prevent related diseases. It also makes the antioxidant activity attract much attention in the research of biological activity in the scientific community.
Feng et al.[32] studied the differences in the antioxidant activity of different endophytes (bacteria, fungi) in different seaweeds, and comprehensively evaluated their in-vitro antioxidant activity based on their ability to scavenge DPPH, hydroxyl free radicals and superoxide anions. Among the first 12 strains with strong antioxidant capacity, we further selected endophytic bacteria and endophytic fungi from Ulva lactuca L. and Undaria pinnatifida Suringar for in-depth testing, and screened out strains with good antioxidant activity. Jia et al.[33] isolated and purified endophytes from mulberry branches, and tested the different extract phases of the fermentation broth for α-glucosidase-inhibiting activity and antioxidant activity. Through preliminary activity screening, it was found that the fermentation broth of three endophytic Pseudomonas strains had better α-glucosidase-inhibiting activity and antioxidant activity.
Other pharmacological activity
Man[34] studied the phlegm-reducing and anti-tussive effects of the metabolites of wild and cultivated Glycyrrhiza uralensis endophytes in Gansu, and found that 7 strains of G. uralensis endophytes (JTYB029, JTZB005, JTZB006, JTZB043, JTZB058, JTYB029, JTZB005, JTZB006, JTZB043, JTZB058, JTZB060, JTZB063) belongs to genus Bacillus. Ali et al.[35] obtained an endophytic fungus Bipolaris sorokiniana LK12 that produces compounds BZR-cotoxin I and IV with moderate anti-lipid peroxidation and urease activity from plant Rhazya stricta. Chen et al.[36] isolated an endophyte Pseudomonas sp. Lk9 from S. nigrum, which can produce surfactants.
Conclusions
The research on the medicinal active ingredients of plant endophytes is the main content of the current endophytic bacteria research. Plant endophytes have become research hotspots in the fields of natural product chemistry, development of alternative resources for traditional Chinese medicine, research on medicinal active ingredients, and research and development of new drugs due to their advantages of wide sources, rich species, and easy cultivation. Existing research data show that plant endophytes and their fermentation metabolites can be separated into almost all kinds of main chemical components, and most of the separated chemical components have certain biological activity, showing very large development potential and application prospects. However, the research on the medicinal active ingredients of plant endophytes still faces many problems and difficulties. First of all, the isolation of endophytes is the prerequisite and guarantee for the research on the medicinal active ingredients of plant endophytes. However, there are many studies on the isolation of plant endophytes, but the research is not thorough enough, and the separation means and methods are still relatively simple. Secondly, the mechanism by which plant endophytes produce active ingredients is still unclear. The ability of endophytes to produce the same and similar chemical components as hosts may be related to the symbiosis with host plants. However, the study of symbiosis mechanism has always been a difficulty in endophyte research, and the symbiosis mechanism has not yet been understood. Finally, as a kind of microorganisms, plant endophytes are also affected by the external environment. Therefore, it is necessary to carry out research on the biological stability of endophytes in vitro.
Therefore, carrying out multi-disciplinary, multi-field, multi-means collaborative cross-over research is important to the research of medicinal active ingredients of plant endophytes and their metabolites. Similarly, for how to develop, use, and protect plant endophytes, a huge treasure house of microbial resources, is the key.
References
[1] RAMAPPA VENKATESH KUMAR, RAGHWENDRA PRATAP SINGH, PRIYAMVADA MISHRA. Endophytes as emphatic communication barriers of quorum sensing in Gram-positive and Gram-negative bacteria: A review[J]. Environmental Sustainability, 2019, 2(4): 455-468.
[2] CHEN L, LIANG ZN, ZHU H. Research advances in the studies of plant entophytic[J]. Biotechnology Bulletin, 2015, 31(8): 30-34.
[3] STIERLE ANDREA, STROBEL GARY, STIERLE DONALD. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew[J]. Science, 1993, 260(5105): 214.
[4] SEBOLA TENDANI E, UCHE-OKEREAFOR NKEMDINMA C, MEKUTO LUKHANYO, et al. Antibacterial and anticancer activity and untargeted secondary metabolite profiling of crude bacterial endophyte extracts from baker leaves[J]. International Journal of Microbiology, 2020(4). https://doi.org/10.1155/2020/8839490.
[5] POVEDA JORGE, ZABALGOGEAZCOA IrIGO, SOENGAS PILAR, et al. Brassica oleracea var. acephala (kale) improvement by biological activity of root endophytic fungi[J]. Sci Rep 10, 20224 (2020). https://doi.org/10.1038/s41598-020-77215-7.
[6] VASSALLO ALBERTO, MICELI ELISANGELA, FAGORZI CAMILLA, et al. Temporal evolution of bacterial endophytes associated to the roots of Phragmites australis exploited in phytodepuration of wastewater[J]. Frontiers in microbiology, 2020(11): 1652.
[7] WANG ZR. Study on the secondary metabolites of endophytes in medicinal plant Mahonia fortunei (Lindl.) Fedde based on co-cultivation strategy[D]. Qingdao: Qingdao University, 2020. (in Chinese)
[8] HUANG L, GAO YY, XU CW, et al. Research progress on the diversity of endophytes in medicinal plants and the functions of their active metabolites[J]. Guizhou Forestry Science and Technology, 2019, 47(3): 59-64. (in Chinese)
[9] ZHANG M, LI N, CHEN RD, et al. Two terpenoids and one polyketide compound of Trichoderma sp. Xy24, an endophytic fungus of the genus Xylocarpus Koenig from mangrove trees[J]. Journal of Chinese Pharmaceutical Sciences, 2014, 23(6): 421-424. (in Chinese)
[10] REN R. Study on the secondary metabolites of three symbiotic fungi[D]. Nanjing: Nanjing University, 2015. (in Chinese)
[11] LI JW, DUAN RG, ZOU JH, et al. Meroterpenoids and isoberkedienolactone from endophytic fungus Penicillium sp. associated with Dysosma versipellis[J]. Acta Pharmaceutica Sinica, 2014, 49(6): 913-920. (in Chinese)
[12] TAO YW, LING HP, ZHANG JY, et al. Amide metabolites of the mangrove endophytic fungus Phomopsis sp. ZZF08 from the South China Sea[J]. Acta Scientiarum Naturalium Universitatis Sunyatseni, 2017, 56(5): 73-78. (in Chinese)
[13] KONG Y, MA YM, GUAN L, et al. Study on secondary metabolites and antifungal activity from an endophytic fungi NR3 of Neriumindicum Mill. cv. Paihua[J]. Journal of Shaanxi University of Science & Technology, 2019, 37(2): 52-56, 62. (in Chinese)
[14] DU FY, LI XM, LI CS, et al. Chemical constituents of Aspergillus sydowii EN-198, an endophytic fungus derived from the marine-mangrove plant Hibiscus tiliaceus[J]. Marine Sciences, 2012, 36(12): 6-11. (in Chinese)
[15] LI LY, DENG ZW, YU SJ, et al. Metabolites of endophytic fungus GT20036029 isolated from the mangrove plant Hibiscus tiliaceus[J]. Natural Product Research and Development, 2007.
[16] GUO T, LI J, GUO TT, et al. Isolation, chemical components and structural characterization of extracellular polysaccharides produced by the saline-alkali plant endophytic fungus SXH-69[J]. Chinese Journal of Marine Drugs, 2015, 34(6): 23-27. (in Chinese)
[17] WANG S. Identification of endophytic bacteria from Ginkgo biloba L. and optimization of fermentation conditions for extracellular polysaccharides of Endo Gin Ya6[D]. Dalian: Dalian Polytechnic University, 2015. (in Chinese)
[18] DU FY, LI XM, LI CS, et al. Chemical constituents of Aspergillus sydowii EN-198, an endophytic fungus derived from the marine-mangrove plant Hibiscus tiliaceus[J]. Marine Sciences, 2012, 36(12): 6-11. (in Chinese)
[19] ZHAO HM. Study on the chemical and biological activity of the secondary metabolites of endophytes from Stemona sessilifolia (Miq.) Miq. and Dendrobium officinale Kimura et Migo[D]. Nanjing: Nanjing University of Chinese Medicine, 2020. (in Chinese)
[20] WANG ZR. Study on the secondary metabolites of endophyte in medicinal plant Mahonia fortunei (Lindl.) Fedde based on co-cultivation strategy[D]. Qingdao: Qingdao University, 2020. (in Chinese)
[21] LI LL. Analysis of antimicrobial activity of different species of endophytes from Artemisia annua[J/OL]. Guihaia: 1-12[2021-01-22].http://kns.cnki.net/kcms/detail/45.1134.Q.20210119.1610.002.html. (in Chinese)
[22] WANG B, PAN YH, HOU JL, et al. Screening, identification of an antagonistic endophyte to pathogens of mulberry bacterial diseases from mulberry leaves and its biocontrol Activity[J]. Advances in Microbiology, 2019, 8(3): 110-120. (in Chinese)
[23] BRAHMA N SINGH, DALIP K UPRETI, VIJAI K GUPTA, et al. Endolichenic fungi: A hidden reservoir of next generation biopharmaceuticals[J]. Trends Biotechnology, 2017, 35(9): 808-813.
[24] ANKIT KUMAR SINGH, RAJESH KUMAR SHARMA, VARSHA SHARMA,et al. Isolation, morphological identification and in vitro antibacterial activity of endophytic bacteria isolated from Azadirachta indica (neem) leaves[J]. Veterinary World, 2017, 10(5): 510-516.
[25] LIM SIONG MENG, AGATONOVIC-KUSTRIN SNEZANA, LIM FEI TIENG, et al. High-performance thin layer chromatography-based phytochemical and bioactivity characterisation of anticancer endophytic fungal extracts derived from marine plants[J]. Journal of Pharmaceutical and Biomedical Analysis, 2021(193): 113702.
[26] RAMALINGAM PARTHASARATHY, MANJEGOWDA CHANDRIKA, H.C. YASHAVANTHA RAO, et al. Molecular profiling of marine endophytic fungi from green algae: Assessment of antibacterial and anticancer activities[J]. Process Biochemistry, 2020(96): 11-20.
[27] ZHAO HM, CHEN XB, CHEN XL, et al. New peptidendrocins and anticancer chartreusin from an endophytic bacterium of Dendrobium officinale[J]. Annals of translational medicine, 2020, 8(7):455.
[28] JU X, QIAN W, HU XY, et al. Isolation and anti-tumor effect of endophytic pseudomonad G13476 in ginseng[J]. Journal of Anhui Agricultural Sciences, 2019, 47(23): 193-195. (in Chinese)
[29] GALLO MARGARETH BC, CHAGAS FERNANDA O, ALMEIDA MARíLIA O, et al. Endophytic fungi found in association with Smallanthus sonchifolius (Asteraceae) as resourceful producers of cytotoxic bioactive natural products[J]. Journal of basic microbiology, 2009, 49(2): 142-151.
[30] EL-HAWARY SS, MOHAMMED R, ABOUZID SF, et al. Solamargine production by a fungal endophyte of Solanum nigrum[J]. Journal of applied microbiology, 2016, 120(4): 900-911.
[31] PALANICHAMY P, KANNAN S, MURUGAN D, et al. Purification, crystallization and anticancer activity evaluation of the compound alternariol methyl ether from endophytic fungi Alternaria alternata[J]. Journal of Applied Microbiology, 2019, 127(5): 1468-1478.
[32] FENG SZ, XIE GY, LIU NY, et al. Isolation and antioxidative activities of algal endophytes[J]. Journal of Food Science and Biotechnology, 2020, 39(6): 99-105. (in Chinese)
[33] JIA YN, LU HP, YU Y, et al. The inhibition of α-glucosidase and antioxidant activity of endophyte metabolite isolated from mulberry[J]. Food and Fermentation Industries, 2017, 43(11): 132-139. (in Chinese)
[34] MAN Q. Comparative study on antitussive expectorant effects of endophytic microorganisms and different extracts from Glycyrrhizae Radix et Rhizoma[D]. Lanzhou: Gansu University of Chinese Medicine, 2017. (in Chinese)
[35] ALI LIAQAT, KHAN ABDUL LATIF, HUSSAIN JAVID, et al. Sorokiniol: A new enzymes inhibitory metabolite from fungal endophyte Bipolaris sorokiniana LK12[J]. BMC microbiology, 16, 103 (2016). https://doi.org/10.1186/s12866-016-0722-7.
[36] CHEN L, LUO SL, LI XJ, et al. Interaction of Cd-hyperaccumulator Solanum nigrum L. and functional endophyte Pseudomonas sp. Lk9 on soil heavy metals uptake[J]. Soil Biology and Biochemistry, 2014(68): 300-308.
- 农业生物技术(英文版)的其它文章
- Effects of Pruning Methods on the Growth and Development of New Shoots and Fruit Yield and Quality of Walnut
- Selection of Grape Varieties Suitable for Double Cropping a Year in Northern Greenhouse
- Effects of Uniconazole on Photosynthetic Characteristics of Dahlia pinnata Cav. under Drought Stress
- Community Structure and Value Evaluation of Local Brassicaceae Potherbs in Shiyan City
- Home Planting Techniques of Green and Healthy Rape Sprouts
- Research Progress of Polygonatum Germplasm Resources in China

