Species and Functions of Rhizobia Growing on Trees and Colonization Mechanisms
Weixin CHEN Xiaolin SHEN Chaoyi YE Yihan SU Chengxiang XU
Abstract The current research progress in China shows that the tree species of rhizobia that have been found are mainly trees of 11 genera in Papilionaceae, Elaeagnaceae and Mimosaceae, and the rhizobia are mainly α-rhizobia, which come from multiple genera. Temperature, pH value, NaCl content and antibiotics all affect the growth of tree rhizobia; and different species of rhizobia have different stress resistance, which is related to their living environment. Rhizobium inoculation can promote plant height, biomass, root-to-shoot ratio, and root architecture of trees. We discussed and analyzed the development history and application status of rhizobia research, and prospected their future development directions. In the future, tree rhizobia will surely be used as a means of production to promote efficient agricultural production and sustainable agricultural systems.
Key words Rhizobium; Leguminosae; Elaeagnaceae; Biological characteristics; Mechanism
Received: July 29, 2021 Accepted: September 30, 2021
Supported by National Natural Science Foundation of China (31270674); National Project of Special Fund for Undergraduate Science and Technology Innovation Cultivation in Guangdong Province (202010580009); Provincial Project of Special Fund for Undergraduate Science and Technology Innovation Cultivation in Guangdong Province (S202010580042); "Climbing Program" of Special Fund for Undergraduate Science and Technology Innovation Cultivation in Guangdong Province (pdjh2020b0640).
Weixin CHEN (2000-), female, P. R. China, major: bioscience.
*Corresponding author. E-mail:xucx2013@163.com.
Rhizobia can form nodules or stem nodules with plants, and can convert nitrogen in the air into nitrogen nutrients that plants can use. They are an important component of biological nitrogen fixation, and the fixed nitrogen will remain in the nodules and increase soil fertility. At present, 98 species of rhizobia in 13 genera have been discovered and named. In the world, German scientists discovered in 1888 that the symbiosis of legumes and rhizobia can convert nitrogen into nitrogen nutrients needed by plants. The non-leguminous nitrogen fixation system has a wider range of infection, and more than 220 species of woody dicotyledons of 24 genera in 8 families that can nodulate and fix nitrogen with rhizobia have been known currently[1]. Therefore, in the research and development of biological nitrogen fixation, the non-leguminous symbiotic nitrogen fixation system has more advantages and potential than the leguminous rhizobium nitrogen fixation system. Trees are a very important plant group, but Chinas research in this field is still at the preliminary stage and has not yet reached the stage of practical application. In view of this, based on our accumulation in the past, combined with China Knowledge Network (CNKI) database resources, the research progress on species, functions and mechanisms of tree rhizobia were reviewed.
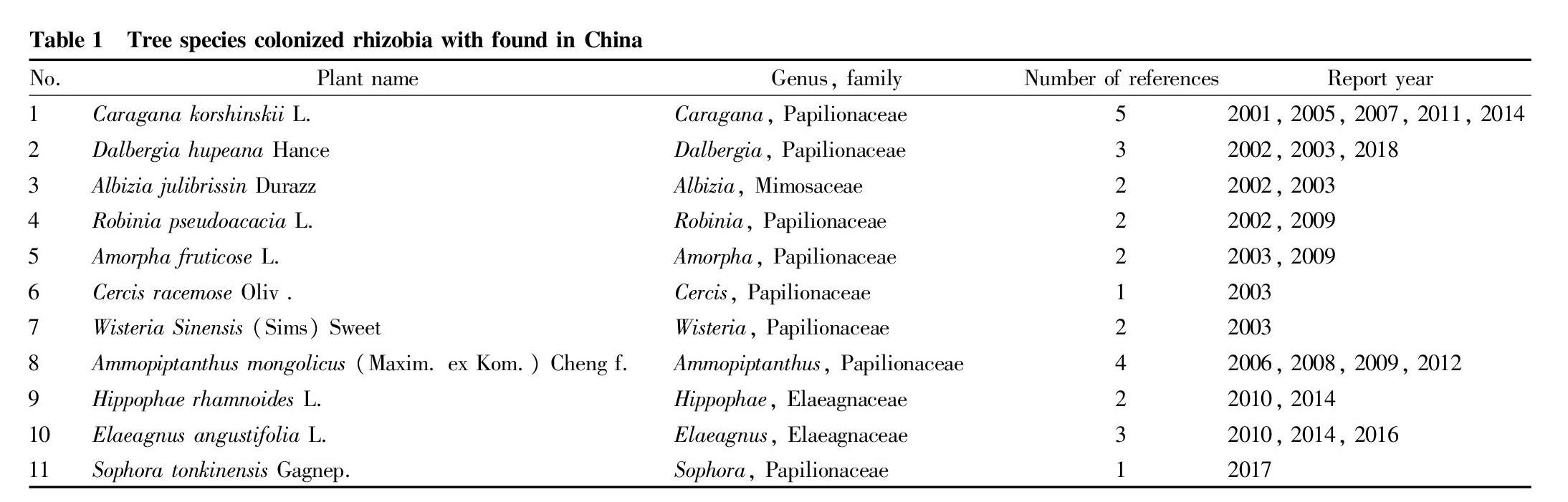
Tree Species Colonized with Rhizobia Found in China
Rhizobia are a type of widely distributed gram-negative rod-shaped bacteria, mainly belonging to α-rhizobia, which can be divided into Azorhizobium, Bradyrhizobium, Mesorhizobium, Rhizbium and Sinorhizobium. There are other α-rhizobium genera, such as Mycobacterium, Ochrobactrum, Aminobacter, Microvirga and Phyllobacterium[2-3]. In addition, β-rhizobia such as Burkholderia and Cupriavidus can also form a symbiotic relationship with plants[4]. The nodules of Ammopiptanthus mongolicus are columnar, 3-5 mm long, brown or light brown. The internal structure is divided into two parts: the inner and outer parts. The outer part is composed of parenchyma cells, and the central part is composed of meristem and infected tissue from front to back. In old nodules, there are decaying tissues at the base, and the decaying tissues increase with the aging degree of nodules. The nodules of seabuckthorn are of the alder type and coral-like, and the most basic unit of the nodules is nodule petal. Most of the nodule petals are nearly spherical or spherical, and a few are elliptical to cylindrical. One-year-old Caragana korshinskii plants nodulate early, and the root system can form at about 40 d after emergence nodules, which are initially white and transparent and gradually develop into an irregular short rod shape. With plant dormancy and overwintering, they will continue to develop next year and have active apical meristem, and the nodules elongate and branch to form rod-shaped and Y-shaped nodules, and the 3 and 4-year-old nodules, in addition to rod-shaped and Y-shaped, continue to develop into palmate, multi-branched and coral-shaped.
Biological Characteristics of Rhizobia Growing on Trees
Growth characteristics of rhizobia
After Gram staining, rhizobia show a negative short rod-like shape without spores, and there may be a transparent nodular part that is not stained red in the middle. When Rhizobium strains are cultured on YMA (yeast mannitol agar) medium at 28 ℃, single colonies visible to the naked eye will appear within 3-5 d, and the colony diameter is generally 3-8 mm. The colonies are translucent, milky white, round, neat at edges, slightly raised in the middle, mucous, and viscous. When they are cultured on Congo red YMA medium at 28 ℃ for 3-7 d, the colonies do not absorb color. When they are cultured on PKO (Pikovaskaias) medium at 28 ℃ for 3-7 d, single colonies will appear.
Effects of temperature on the growth of rhizobia
Rhizobia can grow at 4-37 ℃, and grow best at 28 ℃. Rhizobia on different species of trees have different tolerance to high temperature or low temperature, and the temperature ranges within which they can grow is also different. Euchresta japonica rhizobia responded more consistently to different temperatures, had poor tolerance to low temperatures, and grew well around 30 ℃[5]. When R. caragana rhizobia are cultured purely, the optimum growth temperature was 25-30 ℃[6]. Holly rhizobia had a wide adaptive range of temperature. Specifically, 94.1% of the strains could grow at 12 and 37 ℃, 41.2% of the strains were still viable at 4 ℃, and 23.5% of the strains could still grow at 45 ℃. After being treated at 60 ℃ for 10 min and then cultured at 28 ℃, all strains were viable and show strong heat resistance[7]. C. korshinskii rhizobia could not grow at 4 ℃, but all strains could grow at 10 and 40 ℃. After 10 min of heat shock treatment at 60 ℃ and being cultured at 28 ℃, 93% of the strains could continue to grow, showing strong high temperature tolerance[8]. Robinia pseudoacacia, Amorpha fruticosa, and Dalbergia hupeana rhizobia hac a wide adaptive range of temperature. 17.6% of the strains could grow at 4-60 ℃, and the tolerance of A. fruticosa, and D. hupeana rhizobia to 60 ℃ high temperature and 4-10 ℃ low temperature was stronger than that of R. pseudoacacia rhizobia. The resistance of the strains to temperature is mainly affected by host plants, and to a certain extent by the altitude and soil type[9]. The size, shape, color, and nodulation rate of Hippophae rhizobia were different in different habitats, seasons and types. The higher the altitude of the distribution area, the smaller the nodules and nodule petals[10]. In general, the rhizobia of E. japonica and forage grass are more affected by temperature, while the rhizobia of A. mongolicus, R. pseudoacacia, and A. fruticosa are less affected by temperature fluctuations, and can still grow and develop normally at high temperature or low temperature even after heat shock treatment.
Effects of pH on the growth of rhizobia
Generally, over-acid and over-alkaline environmental conditions are not conducive to the growth of rhizobia, and will affect the nodulation of plant roots, and the quantity and quality of rhizobia will also be affected. Plant growth pH is 6.0-10.0, the best growth pH is 7.0. Liu et al.[6] studied the rhizobia of legumes in the central and eastern regions of Inner Mongolia, and showed that when the rhizobia of Caragana was cultivated purely, the optimum pH was 6.8-7.2, within which Caragana grew well. The rhizobia of R. pseudoacacia, D. hupeana, and A. fruticosa in Liangshan area of Sichuan had strong acid and alkali tolerance. All strains could tolerate the environment of pH 5-12, and 76.7% of the strains were still vigorous in the environment of pH 4-12[9]. In addition, Tang et al.[5] found that E. japonica rhizobia had a relatively consistent response to acid and alkali, and could grow at a pH of 6.0-10.0, and some strains could still grow at a pH of 11, so the alkali-tolerant germplasm resources are abundant. However, E. japonica rhizobia was more sensitive to acidic environment, and the strains could not grow again when the pH value was lower than 6.0. He et al.[7] also believed that A. mongolicus rhizobia had strong acid and alkali tolerance and a wide range of acid and alkali tolerance, and 94.1% of A. mongolicus rhizobia could grow in the range of pH 5-11.
Effects of NaCl content on the growth of rhizobia
The rhizobia of different types of trees have different salt tolerance, and their ability to withstand salt stress and their effects are also different. Strains extracted from R. pseudoacacia, A. fruticosa, and D. hupeana plants in Liangshan, Sichuan were generally poor in salt tolerance. 86.7% of the strains could not grow on 1% NaCl plates, and only 13.3% of the strains could grow on 4% NaCl plates. The salt tolerance of D. hupeana rhizobia was stronger than that of R. pseudoacacia and A. fruticosa rhizobia. Tang et al.[5] pointed out through research that E. japonica rhizobia could grow under salt-free conditions. As the content of NaCl increased, the growth of the strains was inhibited. When the content of NaCl reached 1% and above, the strains could not grow. Research on the stress resistance of rhizobia on the desert plant C. korshinskii and their phylogenetic analysis showed that C. korshinskii rhizobia generally had strong salt tolerance, acid and alkali tolerance. They could grew in a medium containing 1% NaCl. 92% of the strains could tolerate 2% NaCl, 80% of the strains could tolerate 3% NaCl, 70.8% of the strains could tolerate 4% NaCl, 36.1 % of the strains could tolerate 5% NaCl, and even 12.9% of the strains could tolerate 6% NaCl. With the continuous increase of NaCl concentration, the salt tolerance of the strains gradually decreased, and the viability gradually decreased[8]. In addition, Yao et al.[11] found that when the growth range of NaCl concentration was 0-7.0% (w/v), rhizobia grew best at 1.5%-2.0%. The above research results all indicate that the salt tolerance of rhizobia in different types of trees is different, and their salt tolerance is also affected by host plants and the soil environment.
Stress resistance of rhizobia
Sensitivity to antibiotics is an important physiological and biochemical characteristic of rhizobia. Leguminous plants form a symbiotic system with rhizobia through nodulation to promote the growth of host plants, and enhance the stress resistance of host plants because effective root nodules contain a large number of antioxidant molecules and have higher antioxidant enzyme activity[12]. Wang et al.[13] pointed out that rhizobia were more sensitive to antibiotics and were inhibited by antibiotics. Meanwhile, the synthesis of rhizobium extracellular polysaccharides were closely related to osmotic regulation. The habitat lacking water and nitrogen in the distribution area of A. mongolicus affects the symbiotic nature of hosts and rhizobia. Both the hosts and bacteria are constantly adapted to the arid environment through natural selection, showing the characteristics of salt tolerance, acid and alkali tolerance, and high temperature tolerance[14]. The vast majority of C. korshinskii rhizobia are extremely resistant to penicillin and could grow normally in a medium containing 100 μg/ml penicillin. 62% of the strains could tolerate 300 μg/ml penicillin. More than 90% of the strains could tolerate 5 μg/ml of chloramphenicol, kanamycin, streptomycin and gentamicin, while 50-100 μg/ml of kanamycin, streptomycin and gentamicin had a strong inhibitory effect on the strains, and most strains could not grow at this concentration[8]. The strains of E. japonica rhizobia had the worst tolerance to Km and Gm, and could not grow at a low content (5 μg/ml); they were resistant to Cb and could still grow at a higher content (300 μg/ml); and they could grow in 100 μg/ml Cm and 10 μg/ml Nm, but the resistance to different antibiotics or different concentrations of the same antibiotic varied greatly between strains[5].
Utilization of carbon and nitrogen sources by rhizobia
Rhizobia in root nodules have a special ability to fix free nitrogen in the air. Caragana rhizobia can use inositol, threonine, glutamic acid, etc. as the sole carbon and nitrogen source, but cannot use sodium formate[6]. The research on the genetic diversity and nitrogen-fixing activity of C. korshinskii has found that the indigenous Caragana rhizobia can be divided into three types, namely high-efficiency, medium-efficiency and low-efficiency strains. The nodules formed by the high-efficiency root nodule strains had brown-red color, high leghemoglobin content, strong strain vigor, and high nitrogen-fixing activity. The nodules formed by the low-efficiency rhizobium strains were small, slender, dark green in section, and had poor bacteroid tissue development. The high-efficiency rhizobia could increase the dry weight of Caragana plants by 28.9%-79.5%, and the efficiency was very significant[15]. All the tested strains of A. mongolicus rhizobia could utilize sodium pyruvate, maltose, glucose, sodium acetate, sodium citrate, L-arabinose, sucrose, L-arabinose, sodium succinate, rhamnose, galactose, D-xylose, lactose, and fructose, which account for 66.7% of the total carbon source types, and only 94.6% of the tested strains could not use all carbon sources. Almost all strains could use carbon sources other than sorbose, sodium tartrate, sodium malonate, starch, D-sorbitol, erythritol, inositol, and maltose, but 88.8% of the tested strains could not use starch[16].
Weixin CHEN et al. Species and Functions of Rhizobia Growing on Trees and Colonization Mechanisms
Effect of Rhizobia on the Growth and Development of Trees
Effect of rhizobia on plant height
Rhizobia can have a significant impact on the height of trees. Ten months after the treatment of Dalbergia odorifera, the plant height of the trees inoculated with rhizobia (BJ) was 6.81% higher than that of no inoculation (CK), and the difference was significant (P<0.05)[17]. Isolation, identification and research of seabuckthorn rhizobia found that Frankia actinomycetes in seabuckthorn nodules had the characteristics of directly promoting seabuckthorn nodulation, and could promote the growth of host plant seabuckthorn. It was also found that non-Frankia in nodules actinomycetes all could promote the growth and development of plants. Compared with the plants not inoculated with rhizobia, seabuckthorn plants inoculated with rhizobia had a significant increase in plant height. It was concluded that in promoting nodulation and nitrogen fixation of seabuckthorn plants and promoting growth and development, both Frankia and non-Frankia actinomycetes played a huge role[18]. The above research results also show that different inoculation methods, different growth environments, and the selection of rhizobia strains have certain effects on plant height.
Effects of rhizobia on plant biomass and root-to-shoot ratio
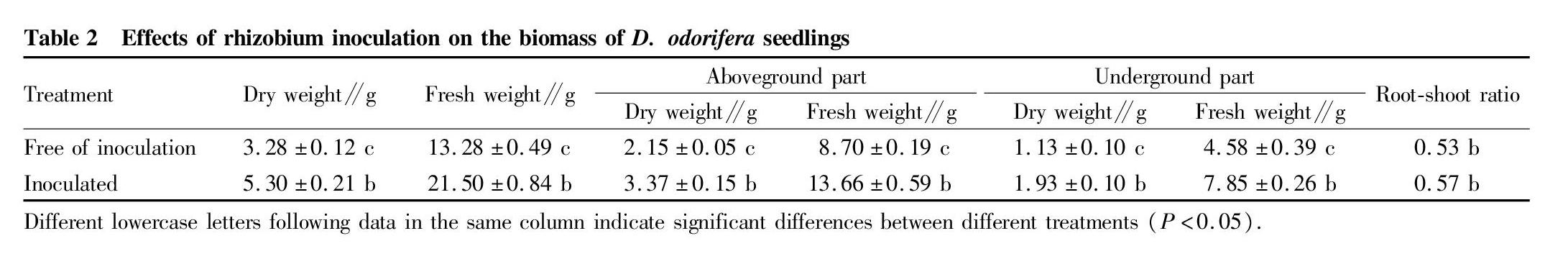
The biomass of seedlings and the root-shoot ratio are important indexes of seedling growth. Rhizobium inoculation can increase the biomass and the root-shoot ratio of D. odorifera seedlings. Compared with the control, the inoculation treatment had significant difference (P<0.05). The total dry weight, total fresh weight, dry weight of aboveground part, fresh weight of aboveground part, dry weight of underground part, and fresh weight of underground part increased by 61.9%, 61.9%, 56.7%, 57.0%, 70.8%, and 71.4% respectively, compared with the control (Table 2). Ormosia henryi Prain trees were inoculated with different rhizobia, and the results showed that the root surface area, average root diameter, biomass of O. henryi trees increased significantly[19]. Liu et al.[20] inoculated Medicago sativa WL319 with Sinorhizobium SD101, and the results showed that the aboveground biomass of M. sativa and the chlorophyll content in the leaves significantly increased.
Effects of rhizobia on plant root architecture
Plant root architecture is an important botanical trait and ecological index of plants. In addition to being regulated by plant genetic factors, it is also regulated by environmental factors, and environmental factors often affect the root architecture by affecting the occurrence of lateral roots[20]. The study on D. odorifera showed that the total root length of plants treated with rhizobia increased by 13.80% compared with the CK treatment, and the main root length difference between the two treatments was significant. The primary lateral root length increased by 13.62% compared with the CK treatment, the secondary lateral root length increased by 22.23% compared with the CK treatment, and the ternary lateral root lengths of the two treatments also differed significantly. The study showed that rhizobia as a special environmental factor affected the root architecture of plants[17]. Wang et al.[17] also believed that the root surface area and root volume of plants inoculated with rhizobia were significantly different from those free of inoculation (P<0.05). Comparing the two, the root surface area increased by 42.17%; the root volume increased by 10.74%; the average root system diameter increased by 25.42%; the number of root tips increased by 12.48%; and the number of root branches increased by 28.73%. Root surface area and root volume are important indexes of the distribution range of roots in the soil, while the average root diameter, number of root tips, and number of root branches reflect the absorptive capacity of plant roots. The inoculation of rhizobia obviously increased the absorption capacity of the root system and the distribution range of the root system of D. odorifera seedlings.
Mechanism of Action of Tree Nodules and Rhizobia
Under suitable conditions, rhizobia can infect plant roots or stems and form symbiotic nodules for nitrogen fixation, and convert free nitrogen in the air into compound nitrogen that can be absorbed and utilized by plants in the form of symbiosis, which is of great significance to the development of Chinas agriculture. After plants are inoculated with rhizobia, crop yield will be significantly increased. Rhizobium inoculants are one of the good substitutes for chemical fertilizers, and have no impact on the surrounding ecological environment, which is conducive to the sustainable development of agriculture. Studies have found that the mechanism of action of rhizobia is not simple. Rhizobia invade host roots and stimulate certain cells in the root cortex and pericycle, causing these cells to grow intensively and local parts of roots to expand and form nodules. After rhizobia settle in the roots, plants supply rhizobia with mineral nutrients and energy, rhizobia fix free nitrogen in the air and provide nitrogen nutrients for plants. The two in a balanced state are in an antagonistic parasitic relationship and exhibit symbiosis.
Conclusions
Trees are a very important plant group. The symbiotic nitrogen fixation system of terrestrial plants is mainly rhizobia of leguminous and non-leguminous plants. The host plants of the two systems are different, and the infecting bacteria are different, but the nodulation characteristics, action functions, and symbiotic appearance characteristics are similar or the same. Both of the two transform nitrogen in the air into plants and soil in the form of organic nitrogen, which is the main action of maintaining the balance of nitrogen in the atmosphere and terrestrial ecosystems. The nitrogen-fixing capacity of non-leguminous rhizobia is comparable to that of leguminous rhizobia, and the nitrogen-fixing capacity of rhizobia in some families is greater than that of legumes. Meanwhile, non-leguminous plant rhizobia can infect host trees across different families, genera, and species. They have unique nitrogen-fixing mechanisms and long-lasting nitrogen-fixing time, and are more meaningful in the research and development of biological nitrogen-fixing systems, but the research on nitrogen fixation of trees is still very preliminary at present.
The industrialization of rhizobia is mature and widely used. In the United States, the worlds largest soybean producer, the use rate of rhizobia fertilizer alone is more than 50%, which is equivalent to applying 619 t of nitrogen fertilizer. In Argentina, the use rate of rhizobium fertilizer is more than 90%, saving an average of 3 billion US dollars of fertilize every year. In a soybean planting experimental area in Brazil, inoculation of a single rhizobium inoculant can increase soybean yield by 30%. With the rapid development of life sciences, Chinas research on rhizobia has also entered the fast lane. The future development direction of rhizobium biology will be to screen and collect strains with excellent phenotypes, and to screen and improve nitrogen-fixing rhizobia by linking these new or known phenotypes with genomic information under the guidance of systems biology while comprehensively considering various environmental variables and crop growth to provide genetic tools for growth and excellent resistance of trees, so as to develop efficient and sustainable agricultural production systems.
References
[1] TERPOLILLI JJ, HOOD GA, POOLE PS. What determines the efficiency of N2-fixing rhizobium-legume symbioses[J]. Advances in Microbiol Physiology, 2012(60): 325-389.
[2] PRASAD G, ANN MH, LIONEL M, et al. Lehume-nodulating betaproteobacteria: Diversity, host range, and future prospects[J]. Molecylar Plants-Microbe interactions, 2011(24): 1276-1288.
[3] MARCO AR, ERNESTO OO, ESPERANZA MR. Symbiovars in rhizobia reflect bacterial adaptation to legumes[J]. Systematic and Applied Microbiology, 2011(34): 95-104.
[4] ERNESTO OO, MARIANGELA H, ESPERANZA MR. Dinitrogen-fixing prokaryotes[J].The Prokaryotes-Prokaryotic Physiology and Biochemistry, 2013(11): 427-451.
[5] TANG MQ, MIN DD, LI G. The biological characteristics of Euchresta japonica rhizobia and the effects of different strains on the medicinal active ingredients of E. japonica seedlings[J]. Jiangsu Agricultural Sciences, 2017, 45(1): 131-134. (in Chinese)
[6] LIU MH, RUAN WB. Analysis of diversities among strains of rhizobia in middle and eastern Inner Mongolia and effect of soil water gradient on their distribution[J]. Chinese Journal of Applied & Environmental Biology, 2003, 9(4): 391-394. (in Chinese)
[7] HE HB, JIA KF, JIA GX. A preliminary study on the stress resistance of rhizobia isolated from Ammopiptanthus mongolicus[J]. Journal of Plant Ecology, 2006, 30(1): 140-146. (in Chinese)
[8] DAI JX, WANG YJ, GUO JJ. Analysis of stress resistance and phylogenesis of rhizobia isolated from Caragsana spp.[J]. Agricultural Research in the Arid Areas, 2011, 29(4): 223-227. (in Chinese)
[9] XU KF, ZHANG XP, CHEN YX. Preliminary study on the stress tolerance of rhizobia isolated from Robinia pseudoacacia, Amorpha fruitcosa and Dalbergia hupeana[J]. , 2009, 48(2): 321-328. (in Chinese)
[10] ZHANG AM, NIU SQ, ZHANG K. Hippophae and Frankia isolation[J]. Grassland and Turf, 2010, 30(2): 43-46. (in Chinese)
[11] YAO YX, JIE WG, DU Y. Taxonomy, identification and application of Rhizobium[J]. Chinese Agricultural Science Bulletin, 2020, 36(2):100-105. (in Chinese)
[12] SHRIVASTAVA P, KUMAR R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation[J]. Saudi J Biol Sci, 2015, 22(2): 123-131.
[13] WANG J, MA YZ, SHI QL, et al. Study on diversity and particularity of rhizobia resources in Shanxi[J]. Chinese Journal of Applied&Environmental Biology, 1999(5): 79-84.
[14] YANG JK, ZHOU Q, ZHOU JC. Effect of pH on nodulation of soybean rhizobia from Weifang and Huayuankou soils[J]. Chinese Journal of Applied Ecology, 2001(12): 639-640.
[15] tao l. Studies on genotypic diversity of the rhizobia isolated from Caragana, and the dynamics of nitrogen fixation ability to root nodule of Caragana microphylla Lam.[D]. Beijing: China Agricultural University, 2005. (in Chinese)
[16] BI JT, XIE RM, WEI XL. Analysis of phenotypic diversity of rhizobia isolated from Ammopiptanthus mongolicus and 16S rDNA PCR-RFLP[J]. Journal of Agricultural Sciences, 2009, 30(3): 14-20. (in Chinese)
[17] WANG HX, LENG N, JU CH, et al. The combined effect of mycorrhizal fungi and rhizobia on the root architecture and nutrient absorption of Dalbergia odorifera seedlings[J]. Jiangsu Agricultural Sciences, 2018, 46(13): 120-124. (in Chinese) (in Chinese)
[18] LI LK. Isolation and identification of Rhizobium from seabuckthorn and effects of Rhizobium on the growth and development of plants[D]. Changchun: Jilin Agricultural University, 2018. (in Chinese)
[19] DUAN RY, WEI XL, AN CR. Growth of Ormosia henryi seedlings and photosynthetic and physiological characteristics with rhizobia inoculation[J]. Journal of Zhejiang A&F University, 2018, 35(6): 1098-1106. (in Chinese)
[20] LIU SX, WEI GJ, JING RY. Effects of Sinorhizobium SD101 inoculation and shading on nitrogen fixation and photosynthesis of Medicago sativa L.[J]. Crops, 2018(5): 156-161. (in Chinese)
- 农业生物技术(英文版)的其它文章
- Effects of Pruning Methods on the Growth and Development of New Shoots and Fruit Yield and Quality of Walnut
- Selection of Grape Varieties Suitable for Double Cropping a Year in Northern Greenhouse
- Effects of Uniconazole on Photosynthetic Characteristics of Dahlia pinnata Cav. under Drought Stress
- Community Structure and Value Evaluation of Local Brassicaceae Potherbs in Shiyan City
- Home Planting Techniques of Green and Healthy Rape Sprouts
- Research Progress of Polygonatum Germplasm Resources in China

