A Review on the Isolation and Purification of D-allulose
Yanjun JIANG Junqing WANG
Abstract D-allulose has very little content in nature, and it needs to be synthesized artificially and meet the purity requirements of industrial grade. The basic physical and chemical properties of D-allulose, its preparation methods and many different ways of isolation and purification were described. In order to achieve the goal of industrial production of D-allulose as soon as possible, the research progress of D-allulose isolation and purification technologies at home and abroad in recent years was classified and discussed, so as to provide useful reference for the practical improvement of D-allulose isolation and purification process technologies.
Key words D-allulose; Isolation and purification; Research progress
Received: June 3, 2021 Accepted: August 7, 2021
Supported by Shandong Province Key R&D Program (Major Innovation Project) (No. 2020CXGC010603, No. 2019JZZY011003); National Natural Science Foundation of China (No. 31801527); Taishan Industry Leading Talent Project (No. tscy20180103).
Yanjun JIANG (1995-), female, P. R. China, master, devoted to research about microbial enzyme engineering.
*Corresponding author. E-mail: wjqtt.6082@163.com.
D-allulose (or D-psicose) has a variety of special physiological functions and is a new type of functional factor with special health care functions discovered in recent years[1]. It has broad application prospects in the fields of medicine and food. However, there are many factors that restrict the large-scale industrial production of D-allulose. The current restrictive factors mainly come from several aspects such as its complicated operation, difficult separation and purification, and low conversion efficiency. In recent years, there have been some new advances in the research on the production, separation and purification of D-allulose. In order to meet the requirements of industrial production, this paper specifically described several methods for the separation and purification of D-allulose.
Properties and Preparation of D-allulose
Physicochemical properties and physiological functions of D-allulose
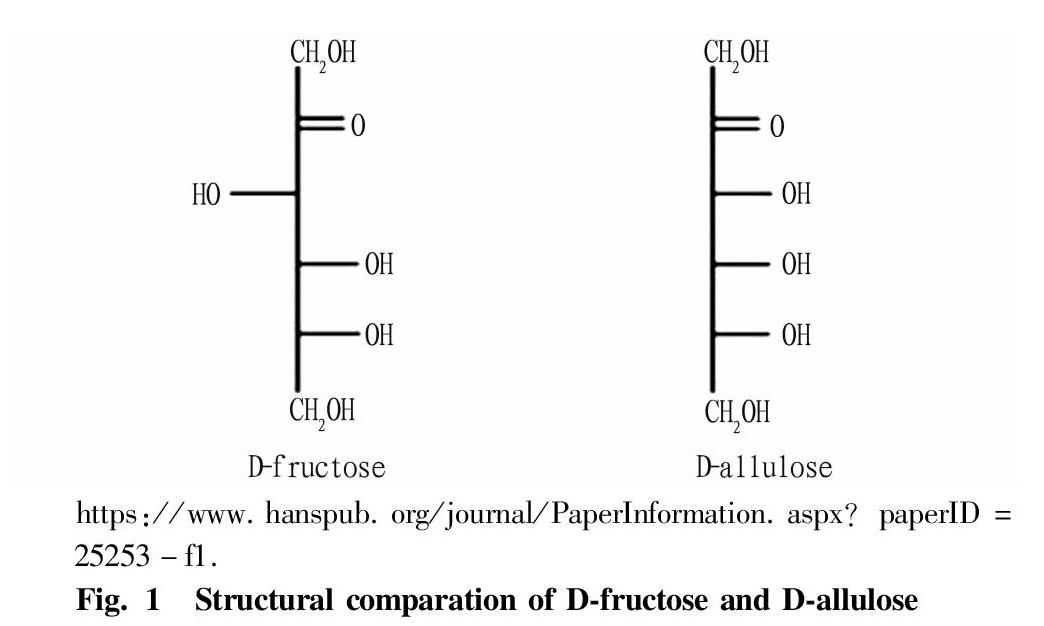
D-allulose is a rare sugar, an epimer of D-fructose at the C3 position, as shown in Fig. 1. D-allulose appears as white powdery crystals[2], and its chemical formula is C6H12O6. It is stable under normal temperature and pressure, odorless, and its molar mass is 180.156 g/mol. It is easily soluble in solvents such as methanol, water and ethanol. Specifically, 100 g of pure water can dissolve 291 g of D-allulose under normal temperature and pressure, and the obtained solution has a density reaching 1.35 kg/L and a mass percentage of 74 wt%[3]. Its sweetness is 70% of sucrose[4], but the calories are only 0.007 kcal/g, which is only 1% of the calories of sucrose, which is almost negligible[5].
D-allulose has a variety of special physiological functions and is also of great significance to human health, such as: preventing and protecting nerves[6], treating neurodegeneration[5], assisting in the treatment of atherosclerosis in diabetic patients[7], enhancing the tolerance to insulin, effectively inhibiting the production of hyperlipidemia and hyperglycemia[8], regulating blood sugar to maintain a stable state, and preventing diabetes[9]. Compared with D-glucose, D-allulose is absorbed by the body more slowly, and can compete with the proteins that transport D-glucose and D-fructose on the cell membrane surface[10], thereby reducing the human bodys absorption of D-fructose and D-glucose in daily diet, which has a very obvious effect on reducing abdominal fat accumulation[11]. D-allulose can inhibit pro-inflammatory cytokines, thereby having an anti-inflammatory effect[12], and it also has the ability to scavenge reactive oxygen species (ROS) free radicals[13]. These characteristics of D-allulose make it play an important role in the field of medicine and health food. In terms of food safety of D-allulose, it has passed the US FDA safety certification in 2014 and obtained GRAS certification in 2015[14], allowing D-allulose to be used as a dietary additive and an ingredient for some foods[5].
Preparation of D-allulose
The content of D-allulose in nature is very small. Through the artificial synthesis method, synthesizing D-allulose in microbes using D-allulose 3-epimerase (DPE for short) under the most suitable conditions can be put into mass production. There are two main kinds of synthesis methods: chemical methods and biological methods. Early D-allulose synthesis methods include selective aldol condensation synthesis method[15], ring-closing conversion synthesis method[16], etc., and subsequent development methods include addition reaction method, catalytic hydrogenation method, etc. Chemical synthesis has complex and uncontrollable steps, low conversion efficiency, poor atom economy, environmental pollution and other problems, and are thus not enough to become the mainstream method for preparing D-allulose. In contrast, the bioconversion method has many advantages: simple reaction steps, mild conditions, high specificity, and good environmental compatibility. Gao et al.[17] used DL-3-phosphoglycerol as a substrate, and obtained D-sorbose and D-allulose by a "one-pot four-enzyme" one-step reaction method based on phosphatase and L-rhamnulose-1-phosphate aldolase (RhaD) after optimization, and the total yield of the two monosaccharides reached 36%. YOSHIHARA et al.[18] used immobilization technology to continuously prepare D-allulose. They obtained D-allulose by immobilizing DPE enzymes on an ion exchange resin. Song et al.[19] successfully realized the production of D-allulose by using the residues of cabbage from the genus Brassica. The Oh team of Sejong University in South Korea[20] found that the addition of borate could gradually shift the balance toward the direction of synthesis of D-allulose. The affinity of borate to different sugars is different, and the affinity to D-allulose is higher than that of D-fructose, so it combines with D-allulose to form a D-allulose-borate complex, which does not participate in the reaction[2].
Purification Method of D-allulose
Difficulties in purification and separation of D-allulose
In recent years, Chinas research and development results on the synthesis of D-allulose have been excellent, but compared with the maturity of the development of D-allulose abroad, China still has many shortcomings in terms of technology research and development, property rights protection of production equipment, D-allulose compounding, market development and application[21].
In view of the reversible reaction of D-fructose isomerization catalyzed by DPE enzymes, the highest conversion rate of D-fructose can reach 28%[22]. Under the effect of Co2+, the conversion rate can reach about 32%[23]. D-fructose and D-allulose are two monosaccharides that are epimers of each other. The chemical properties of the two substances are almost the same, and purifying D-allulose from mixed sugar liquids of D-fructose and D-allulose has always been a difficult problem to solve.
Based on the reported literatures, Chens team from the COFCO Nutrition and Health Research Institute[24-25], Jiangs team from the State Key Laboratory of Food Science and Technology of Jiangnan University[26-28], Suns team from the State Key Laboratory of Industrial Enzymes of Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences[29-31] and other teams have invested a lot of manpower and material resources in the development of strains and gene discovery. There has also been a phenomenon of too many similar and repeated tasks, which also reflects the lack of information and experience exchanges between various research and development units to a certain extent, and also causes a waste of national R&D resources. At present, the separation and purification of D-allulose is mainly divided into direct separation methods (such as chromatographic separation technology, simulated moving bed) and indirect separation methods (such as microbial fermentation, biological enzyme conversion).
Chromatographic separation technology
Chromatographic separation technology is a method that can effectively separate different components in a complex mixture. Its principle is that when a stationary phase and a mobile phase composed of substances with different distribution coefficients move against each other, the substances move together with the mobile phase, and are separated after multiple distributions. Xing et al.[32] prepared D-allulose by biological method using D-fructose as a substrate. For the separation and purification of the product, regenerated anion and cation exchange resins were used for decolorization and desalination, and then DTF-Ca2+ ion exchange resin was used for separation and purification, the separated samples from which were eluted with a fraction collector. When the flow rate was 1 ml/min, the injection volume was 10 ml, and the column temperature was 60 ℃, the purity of the D-allulose separated was determined by high performance liquid chromatography (referred to as HPLC) to be 98.3%. However, because the properties of D-fructose and D-allulose are very close, it is more difficult to directly separate D-fructose and D-allulose by chromatographic separation technology.
Simulated moving bed
Simulated moving beds (SMB) are a kind of device that uses the adsorption principle of a chromatographic column to separate liquids. They depend on a countercurrent continuous operation mode, which constantly changes the positions of the material inlet and outlet of the suction equipment, producing an effect equivalent to the continuous upward movement of the material and the continuous downward movement of the adsorbent. SMB have the characteristics of low operating cost, good separation effect, and simple operation. They are suitable for large-scale continuous production and have been widely used in the separation of mixed sugars[33]. Compared with traditional batch chromatography, SMB process can make the yield of a purified material higher, while reducing the amounts of eluent and filler used. In the SMB process, entire column beds can be effectively used. The adsorption particles of SMB are fixed after being filled, and the movement of the stationary phase in the system is simulated through the relative movement of the liquid flow and the adsorption particles by constantly switching the positions of the inlet and outlet of the eluent[34]. When all volume currents are optimized, a static state is formed. At this time, the current of the medium-concentration purified extract and raffinate does not change anymore. In this case, the system can run continuously. Long et al.[35] used a four-column simulated moving bed packed with DOWEX 50WX4 calcium ion exchange resin to separate DPE-catalyzed products, and the purity of the D-allulose product was 99.36%. Xing et al.[36] measured the separation effect of D-allulose using ion exchange resins under different conditions and found that the purity of D-allulose was 98.3%. Recently, Li[37] successfully achieved high-level expression in the food-grade microorganism Bacillus pumilus. After the immobilized enzyme-catalyzed reaction, the mixed sugar solution was separated by an SMB, and the purity of D-allose separated and purified by a simulated moving bed could reach 98.5%. For the industrialization of D-allulose, the advantages of simulated moving beds are obvious. It has faster work efficiency and can save more time. Even a small SMB system can achieve higher production efficiency and higher purity than a batch system; it reduces operating costs, saves 90% of solvents, and reduces 80% of solid phase costs; it keeps the sample in a concentrated state, and can get almost pure products and reduce concentration work; and such purification process allows large-scale continuous production, and has great potential. However, its shortcomings are also very prominent, such as high capital investment in equipment, difficult device maintenance, large water consumption, low separation efficiency, high energy consumption in the subsequent product concentration process, and greatly increased costs, which hinder its large-scale application in industry.
Microbial fermentation
Microbial fermentation refers to the process of using microorganisms to convert raw materials or substrates into products required by humans through specific metabolic pathways under suitable conditions. Dry yeast powder or yeast mud can be used to separate mixed liquids mutually using substances in the mixed liquids as raw materials under aerobic or anaerobic conditions. The use of microbial fermentation can shorten the production cycle, increase efficiency, increase production, reduce fermentation losses, increase yield, save equipment investment, labor and workshop area, and reduce energy consumption and maintenance costs. In order to improve the purity of xylose and arabinose in a mother liquor of xylose, Wang et al.[38] used high-active dry yeast for brewing to remove glucose in the mother liquor, and the fermentation was carried out at 30 ℃ for 6 h. The amount of yeast added was 2%, and the pH of the mother liquor was 4.5. When the mother liquor concentration was 12%, the removal rate of glucose was at least 96%. Cui et al.[39] used the fermentation method of Saccharomyces cerevisiae to ferment for 8 h at 34 ℃ and 200 r/min. The maximum glucose concentration was 0.2%, and the concentrations of arabinose and xylose were increased while removing glucose in the mother liquor of xylose. The bakers yeast fermentation method was used to desugar egg white, that is, the amount of dry yeast added was 0.2%, the fermentation was selected at 30 ℃, and the pH was 5.5, and the desugaring rate was 95% when the fermentation time was 3.5-4.5 h[40]. In 2000, the IZUMORI team from Kagawa University used bakers yeast when fermenting D-fructose. The researchers treated the reaction product with bakers yeast in a 40 L jar under aerobic conditions for 24 h. The HPLC images showed that D-fructose had been completely degraded, and the supernatant was concentrated by vacuum evaporation at 35 ℃ into a syrup, which was precipitated and purified to crystals using ethanol, obtaining about 20 kg of D-allulose crystals within 60 d. The simple operation and low cost can save a lot of labor, so the method has the potential to be put into mass production[41]. Song et al.[19] used the fermentation method to effectively hydrolyze D-fructose in cabbage residues by DPE enzymes, and then consumed D-fructose in the mixed liquid through anaerobic fermentation of S. cerevisiae, obtaining D-allulose, as well as bioethanol at the same time, which has far-reaching significance for biomass economy and environmental protection. Although the method of using yeast separation has certain advantages, in actual situations, problems such as long-term bacterial contamination affect the separation and purification of D-allulose, so that the scope of its application has not been popularized[2].
Biological enzyme conversion method
The biological enzyme conversion method for removing D-fructose is to a technology that uses enzymes to convert D-fructose in mixed liquids into another substance, reduces the difficulty of separating D-allulose through different adsorption capacities of resins for different substances and thus separates different substances in mixed sugar liquids. Chen et al.[42] used immobilized glucose isomerase (GI) and immobilized glucose oxidase (GOD) alternately to gradually convert D-fructose into D-glucose and then to gluconic acid in a mixed sugar liquid until all D-glucose in the mixed liquid was converted into gluconic acid, then used the adsorption of ion exchange resin D309 to remove gluconic acid in the mixed liquid, and finally obtained D-allulose with a purity of 91.2%. The production method has a simple process, does not use chemical agents and does not waste raw materials, but has a complicated operation process and a long production cycle. Such method is a pure and green method for preparing D-allulose, has greater industrial production and application value, and provides a brand-new idea for the separation and purification of D-allulose.
Agricultural Biotechnology2021
Summary and Prospect
In recent years, D-allulose has been attracting attention. Taking green environmental protection as the criterion, using fruit and vegetable residues as raw materials to prepare D-allulose is also a hot direction in recent years. Chen[43] used bagasse and microalgae hydrolysate as raw materials to produce D-allulose. After the co-expression and synergistic effect of xylose isomerase and DPE enzyme, under the conditions of a temperature of 50 ℃, a pH of 7.5, and the addition of Co2+ and Mg2+, the yields of D-allulose in bagasse and microalgae hydrolysate were 1.42 and 1.69 g/L, respectively. Patel et al.[44] used recombinant Bacillus subtilis to perform a whole-cell catalytic reaction with a system of 1 L of apple juice, mixed fruit juice and honey, and only obtained about 196 g/L of D-allulose from 700 g/L D-fructose, but providing a new research direction for the economic production of D-allulose.
The safety of D-allulose has been approved by the US Food and Drug Administration (FDA)[33]. However, traditionally, Escherichia coli is basically used as the expression host of D-allulose 3-epimerase, which does not meet food safety requirements. In recent years, more and more researchers have expressed D-allulose in food-grade[45] safe strains, such as using Corynebacterium glutamicum or B. subtilis[46] as host cells for D-allulose 3-epimerase. For example, CJ CheilJedang Corporation mainly expressed D-allulose 3-epimerase gene from Agrobacterium tumefaciens in C. glutamicum, fixed enzymes using sodium alginate and supplied a D-fructose liquid to packed bed columns to prepare D-allulose[47]; He[48] used B. subtilis as the host bacteria and used recombinant spores circularly to produce D-allulose; Park et al.[49] used C. glutamicum as a host for DPE expression and D-allulose production, and C. glutamicum was permeabilized and reacted under optimized conditions, showing that metal ion Co2+ improved enzyme activity most obviously. After analysis, the conversion rate reached 31% (w/w), which was 1.4 times that of non-permeable cells, and its volumetric productivity was 353 g/(L·h).
Finding new DPE enzymes with good thermal stability, good pH stability and high conversion efficiency is an important task for realizing the industrial production of D-allulose. Conducting in-depth research on the catalytic mechanism of existing DPE enzymes, deeply exploring their functional groups and conformations and screening out mutant DPE enzymes with good thermal stability and high enzymatic activity through artificial site-directed or random mutations is also an important means to improve the yield of D-allulose. In addition, new separation methods with convenient operation and low cost also play a decisive role in realizing the industrial production of D-allulose.
References
[1] LI QX, LIN CF, MU WM, et al. Research of the immobilization of microbial cells in sodium alginate for D-allulose conversion[J]. Science and Technology of Food Industry, 2015, 36(7): 172-176. (in Chinese)
[2] HUANG QY, XU Z, XIONG Q, et al. Progress in research and development of calorie-free sweetener D-allulose production[J]. Industrial Microbiology, 2020, 50(3): 57-63. (in Chinese)
[3] ZHANG WL. High-level expression, enzymatic properties and molecular modification of D-allulose 3-epimerase[D]. Wuxi: Jiangnan University, 2017. (in Chinese)
[4] CHUNG MY, OH DK, LEE KW. Hypoglycemic health benefits of D-allulose[J]. J Agric Food Chem, 2012, 60(4): 863-869.
[5] HUANG WL. Effects of D-psicose on the regulation of blood glucose and lipid metabolism in rats[D]. Wuxi: Jiangnan University, 2016. (in Chinese)
[6] LYU YR. Screening of D-psicose-producing bacteria and optimization of fermentation conditions[D]. Nanning: Guangxi University, 2012. (in Chinese)
[7] MURAO K, YU X, CAO WM, et al. D-psicose inhibits the expression of MCP-1 induced by high-glucose stimulation in HUVECs[J]. Life Sci, 2007, 81(7):592-599.
[8] WEN YW, ZHANG T, MU WM, et al. Heterologous expression and enzymatic characterization of D-psicose 3-epimerase[J]. Journal of Food Science and Biotechnology, 2018, 37(3):289-296. (in Chinese)
[9] SHEN XM, WANG J, ZHANG Y, et al. Research progress of D-psicose: Function and its biosynthesis[J]. Chinese journal of biotechnology, 2018, 34(9):1419-1431. (in Chinese)
[10] HOSSAIN A, YAMAGUCHI F, MATSUO T, et al. Rare sugar D-allulose: Potential role and therapeutic monitoring in maintaining obesity and type 2 diabetes mellitus[J]. Pharmacology & Therapeutics, 2015(155): 49-59.
[11] MATSUO T, BABA Y, HASHIGUCHI M, et al. Dietary D-psicose, a C-3 epimer of D-fructose, suppresses the activity of hepatic lipogenic enzymes in rats[J]. Asia Pac J Clin Nutr, 2001, 10(3): 233-237.
[12] MOLLER DE, BERGER JP. Role of PPARs in the regulation of obesity-related insulin sensitivity and inflammation[J]. International Journal of Obesity, 2003, 27(Suppl 3): 17-21.
[13] SUNA S, YAMAGUCHI F, KIMURA S, et al. Preventive effect of D-psicose, one of rare ketohexoses, on di-(2-ethylhexyl) phthalate (DEHP)-induced testicular injury in rat[J]. Toxicology Letters, 2007, 173(2): 107-117.
[14] HOSSAIN A, YAMAGUCHI F, HIROSE K, et al. Rare sugar D-psicose prevents progression and development of diabetes in T2DM model Otsuka Long-Evans Tokushima fatty rats[J]. Drug Des Devel Ther, 2015(9): 525-535.
[15] NORTHRUP A B, MACMILLAN D W C. Two-step synthesis of carbohydrates by selective aldol reactions[J]. Cheminform, 2004(5691): 1752-1754.
[16] ANDREANA P R, MCLELLAN J S, CHEN Y, et al. Synthesis of 2,6-dideoxysugars via ring-closing olefinic metathesis[J]. Organic letters, 2002, 4(22): 3875-3878.
[17] GAO YH, JIN ZC, QIAN C, et al. One-pot four-enzyme synthesis of D-sorbose and D-psicose based on aldolase RhaD[J]. Science and Technology of Food Industry, 2013, 34(21): 210-214. (in Chinese)
[18] YOSHIHARA A, KOZAKAI T, SHINTANI T, et al. Purification and characterization of D-allulose 3-epimerase derived from Arthrobacter globiformis M30, a GRAS microorganism[J]. Journal of Bioscience and Bioengineering, 2017, 123(2): 170-176.
[19] SONG Y, NGUYEN QA, WI, et al. Strategy for dual production of bioethanol and d-psicose as value-added products from cruciferous vegetable residue[J]. Bioresource Technology, 2017, 223, 34-39.
[20] KIM NH, KIM HJ, KANG DI, et al. Conversion shift of D-fructose to D-psicose for enzyme-catalyzed epimerization by addition of borate[J]. Appl Environ Microbiol, 2008, 74(10): 3008-3013.
[21] GUO YH, WANG J, WANG XY, et al. Research and industrialization progress of D-psicose in China[J]. Modern Food, 2020(6): 34-40. (in Chinese)
[22] SUN F, SU LQ, ZHANG K, et al. D-psicose 3-epimerase gene overexpression in Bacillus subtilis and immobilization of cells[J]. China Biotechnology, 2018, 38(7): 83-88. (in Chinese)
[23] LI XB. Study on the expression of D-psicose 3-epimerase and its immobilized conversion to D-psicose[D]. Tianjin: Tianjin University of Science and Technology, 2013. (in Chinese)
[24] SHEN XM, WANG J, ZHANG Y, et al. Research progress of D-psicose: Function and its biosynthesis[J]. Chinese Journal of Biotechnology, 2018, 34(9): 1419-1431. (in Chinese)
[25] DENG LC, DING ZY, WANG XY, et al. Functional properties and application progress of D-psicose[J]. Contemporary Chemical Industry, 2018, 47(5): 995-998. (in Chinese)
[26] HUANG WL, JIANG B, ZHANG T. D-psicose, favorably alters lipid metabolism in Wistar rats[J]. Journal of Food Science and Biotechnology, 2018, 37(4): 344-349. (in Chinese)
[27] WEN YW, ZHANG T, MU WM, et al. Heterologous expression and enzymatic characterization of D-psicose 3-epimerase[J]. Journal of Food Science and Biotechnology, 2018, 37(3): 289-296. (in Chinese)
[28] ZHANG WL, JIA M, YU SH, et al. Improving the thermostability and catalytic efficiency of the D-psicose 3-epimerase from Clostridium bolteae ATCC BAA-613 using site-directed mutagenesis[J]. Journal of Agricultural and Food Chemistry, 2016, 64(17): 3386-3393.
[29] ZENG Y, PEI WW, ZHU YM, et al. Research progress on the flavor, physiological functions and application of 3 kinds of natural sweeteners[J]. Journal of Food Safety & Quality, 2019, 10(15): 4840-4847. (in Chinese)
[30] ZHANG LL, ZHU YM, MEN Y, et al. Substrate-binding site of D-psicose 3-epimerase from Ruminococcus sp.[J]. Microbiology, 2014, 41(5): 811-817. (in Chinese)
[31] LI XB, ZHU NM, BAI W, et al. Immobilization of D-psicose 3-epimerase on chitosan for D-psicose conversion[J]. Science and Technology of Food Industry, 2013, 34(17): 158-162. (in Chinese)
[32] XING QC, MU WM, JIANG B, et al. Separation and purification of D-psicose[J]. Science and Technology of Food Industry, 2011, 32(9): 236-242. (in Chinese)
[33] HAN SL, CAI JZ, LIAO JH. Synthesis and development of the new functional sweetener D-psicose[J]. Guangdong Chemical Industry, 2016, 43(13): 142-143. (in Chinese)
[34] LEE JW, KIENLE A, SEIDELMORGENSTERN A. On-line optimization of four-zone simulated moving bed chromatography using an equilibrium-dispersion model: II. Experimental validation[J]. Chemical Engineering Science, 2020, 225(2): 115808.
[35] LONG NVD, LE TH, KIM JI, et al. Separation of D-psicose and D-fructose using simulated moving bed chromatography[J]. Journal of Separation Science, 2009, 32(11): 1987-1995.
[36] XING Q C, MU W M, JIANG B, et al. Separation and purification of D-psicose[J]. Science and Technology of Food Industry, 2011, 32(9): 236-235.
[37] LI C. Production of rare sugars D-psicose and D-allose by biotransformation[D]. Jinan: Shandong University, 2018. (in Chinese)
[38] WANG XJ, WANG CF, QIN QY, et al. Study on removal of glucose from xylose mother liquid by yeast fermentation[J]. Food Research and Development, 2010, 31(3): 154-156. (in Chinese)
[39] CUI SF, HUANG WH, SUN L, et al. Optimization of fermentation conditions to remove glucose in xylose mother liquid by Saccharomyces cerevisiae[J]. China Food Additives, 2012(1): 82-86. (in Chinese)
[40] CAO RY, YANG YJ, XU YQ. Yeast fermentation to remove glucose in egg white[J]. Sichuan Food and Fermentation, 2005(1): 23-26. (in Chinese)
[41] TAKESHITA K, SUGA A, TAKADA G, et al. Mass production of D-psicose from D-fructose by a continuous bioreactor system using immobilized D-tagatose 3-epimerase[J]. J Biosci Bioeng, 2000, 90(4): 453-455.
[42] CHEN XY, WANG W, XU JL, et al. Production of D-psicose from D-glucose by co-expression of D-psicose 3-epimerase and xylose isomerase[J]. Enzyme Microb Technol, 2017(105): 18-23.
[43] CHEN X. Summary of patented technology of D-psicose[J]. Technology Innovation and Application, 2020(22): 20-22. (in Chinese)
[44] PATEL SN, KAUSHAL G, SINGH SP. A novel D-Allulose 3-epimerase gene from the metagenome of a thermal aquatic habitat and D-Allulose production by Bacillus subtilis whole-cell catalysis[J]. Applied and Environmental Microbiology, 2020, 86(5).
[45] HUANG Q, LI WF. Advance in food-grade selection markers[J]. Food and Fermentation Industries, 2007(7): 112-118. (in Chinese)
[46] JIA M, MU WM, ZHANG T, et al. Expression of d-psicose 3-epimerase in Bacillus subtilis[J]. Journal of Food Science and Biotechnology, 2014, 33(11): 1129-1135. (in Chinese)
[47] CHO, YOUNG MOON, KIM, et al. Psicose epimerase mutant and method for preparing psicose by using same[P]. CA2910625, 2018-11-06.
[48] HE WW. Efficient expression of D-psicose 3-epimerase in Bacillus subtilis and its application[D]. Wuxi: Jiangnan University, 2017. (in Chinese)
[49] PARK CHUL-SOON, KIM TAEYONG, HONG SEUNG-HYE, et al. D-allulose production from d-fructose by permeabilized recombinant cells of Corynebacterium glutamicum cells expressing D-allulose 3-epimerase Flavonifractor plautii[J]. PloS one, 2016, 11(7): 1-22.
- 农业生物技术(英文版)的其它文章
- Effects of Pruning Methods on the Growth and Development of New Shoots and Fruit Yield and Quality of Walnut
- Selection of Grape Varieties Suitable for Double Cropping a Year in Northern Greenhouse
- Effects of Uniconazole on Photosynthetic Characteristics of Dahlia pinnata Cav. under Drought Stress
- Community Structure and Value Evaluation of Local Brassicaceae Potherbs in Shiyan City
- Home Planting Techniques of Green and Healthy Rape Sprouts
- Research Progress of Polygonatum Germplasm Resources in China

