Translational products of adipose tissue-derived mesenchymal stem cells: Bench to bedside applications
Shilpa Sharma, Sathish Muthu, Madhan Jeyaraman, Rajni Ranjan, Saurabh Kumar Jha
Shilpa Sharma, Department of Pediatric Surgery, All India Institute of Medical Sciences, New Delhi 110029, India
Shilpa Sharma, Sathish Muthu, Madhan Jeyaraman, Indian Stem Cell Study Group, Lucknow,Uttar Pradesh 226010, India
Sathish Muthu, Department of Orthopaedics, Government Medical College and Hospital,Dindigul, Tamil Nadu 624304, India
Sathish Muthu, Madhan Jeyaraman, Research Scholar, Department of Biotechnology, School of Engineering and Technology, Greater Noida, Sharda University, Uttar Pradesh 201306, India
Madhan Jeyaraman, Rajni Ranjan, Department of Orthopaedics, School of Medical Sciences and Research, Sharda University, Greater Noida, Uttar Pradesh 201306, India
Saurabh Kumar Jha, Department of Biotechnology, School of Engineering and Technology,Sharda University, Greater Noida, Uttar Pradesh 201306, India
Abstract With developments in the field of tissue engineering and regenerative medicine,the use of biological products for the treatment of various disorders has come into the limelight among researchers and clinicians. Among all the available biological tissues, research and exploration of adipose tissue have become more robust.Adipose tissue engineering aims to develop by-products and their substitutes for their regenerative and immunomodulatory potential. The use of biodegradable scaffolds along with adipose tissue products has a major role in cellular growth,proliferation, and differentiation. Adipose tissue, apart from being the powerhouse of energy storage, also functions as the largest endocrine organ, with the release of various adipokines. The progenitor cells among the heterogeneous population in the adipose tissue are of paramount importance as they determine the capacity of regeneration of these tissues. The results of adipose-derived stemcell assisted fat grafting to provide numerous growth factors and adipokines that improve vasculogenesis, fat graft integration, and survival within the recipient tissue and promote the regeneration of tissue are promising. Adipose tissue gives rise to various by-products upon processing. This article highlights the significance and the usage of various adipose tissue by-products, their individual characteristics, and their clinical applications.
Key Words: Adipose tissue; Stem cells; Fat graft; Clinical applications; Mesenchymal stem cells
INTRODUCTION
With developments in the field of tissue engineering and regenerative medicine, the use of biological products for the treatment of various disorders has come into the limelight among researchers and clinicians. Among all the biological products, keen interest has been shown in adipose tissue and its by-products for translation from bench to clinical applications, with due consideration to its various unique properties.Because of the heterogeneous cellular population of adipose tissue, adipose tissuederived products possess a greater advantage of regeneration compared with bone marrow-derived products[1,2].
Traditionally adipose tissue or fat graft transfer was practiced for elective cosmetic procedures and plastic and reconstructive surgery[3]. Because of the regeneration potential possessed by adipose tissue, use has become widespread in cosmeticdermatological procedures, facial rejuvenation, breast and buttocks augmentation, and genital aesthetics[4-6]. Cellular viability and survival at the transplanted site depends on various factors involving the recipient site, donor site, laboratory processing, and manipulation[7,8].
Adipose tissue engineering aims to develop by-products and their substitutes for regeneration and restoring function[9]. That further eliminates the need for organ transplants and mechanical device placement. The use of biodegradable scaffolds along with adipose tissue products has a major role in cellular growth, proliferation,and differentiation[10,11]. Once implanted at an appropriate site, scaffolds degrade,progenitor cells proliferate with the help of growth factors and cytokines to form new tissue. Preclinical studies have investigated the ability of biomolecules and biodegradable three-dimensional scaffolds to interact with adipose tissue products to promote the adipogenesis of stem cells in vitro[12-15]. In this review, we discuss the differential characteristics of adipose tissue derivatives and elucidate their applications in clinical scenarios.
ADIPOSE TISSUE BIOLOGY
An adipocyte is usually 50-150 mm in diameter, it dies from ischemia if it grows larger,and its life expectancy varies from few months to 10 years in humans[16]. Adipose tissue, a specialized connective tissue with lipid-rich adipocytes, it contains a heterogeneous population of cells but adipocytes represents only 20% or less of the cellular mixture[17]. Based on adipocyte morphology, the two types of adipose tissue are white adipose tissue found in adults and brown adipose tissue found in newborns[18]. Adipose tissue, apart from being the powerhouse of energy storage, also functions as the largest endocrine organ, with the release of various adipokines.Adipose tissue-derived adipokines include leptin, adiponectin, apelin, chemerin,interleukin (IL)-6, 8, and 10; monocyte chemoattractant protein (MCP)-1, plasminogen activator inhibitor (PAI)-1, retinol binding protein (RBP)-4, tumor necrosis factor(TNF)-α, progranulin, complement C1q tumor necrosis factor-related protein (CTRP)-4, interferon (IFN)-γ, and interferon-γ-inducible protein (IP)-10, which are readily available sources to induce stem cells[19-22]. The adipokines work in a paracrine fashion when transplanted as a cellular therapeutic tool[23,24]. The components of adipose tissue are lipid-laden adipocytes, fibroblasts, neural and vascular progenitor cells, multipotent progenitor cells, pericytes, extracellular matrices, cytokines, growth factors, and immune cells such as CD4+ T cells, as shown in Figure 1. The progenitor cells in adipose tissue are of paramount importance, as they have the capacity for regeneration of these tissues. The progenitor cells differentiate into mesodermal,ectodermal, and endodermal lineages[25-27]. Tissue engineering experts focus on adipose tissue and its products for their plasticity, relative ease of harvest, and potential autologous usage. Stem cells and progenitor cells from freshly prepared SVFs constitute up to 3%, which represents 2500-fold more than the stem cells isolated from bone marrow source (0.002%)[28].
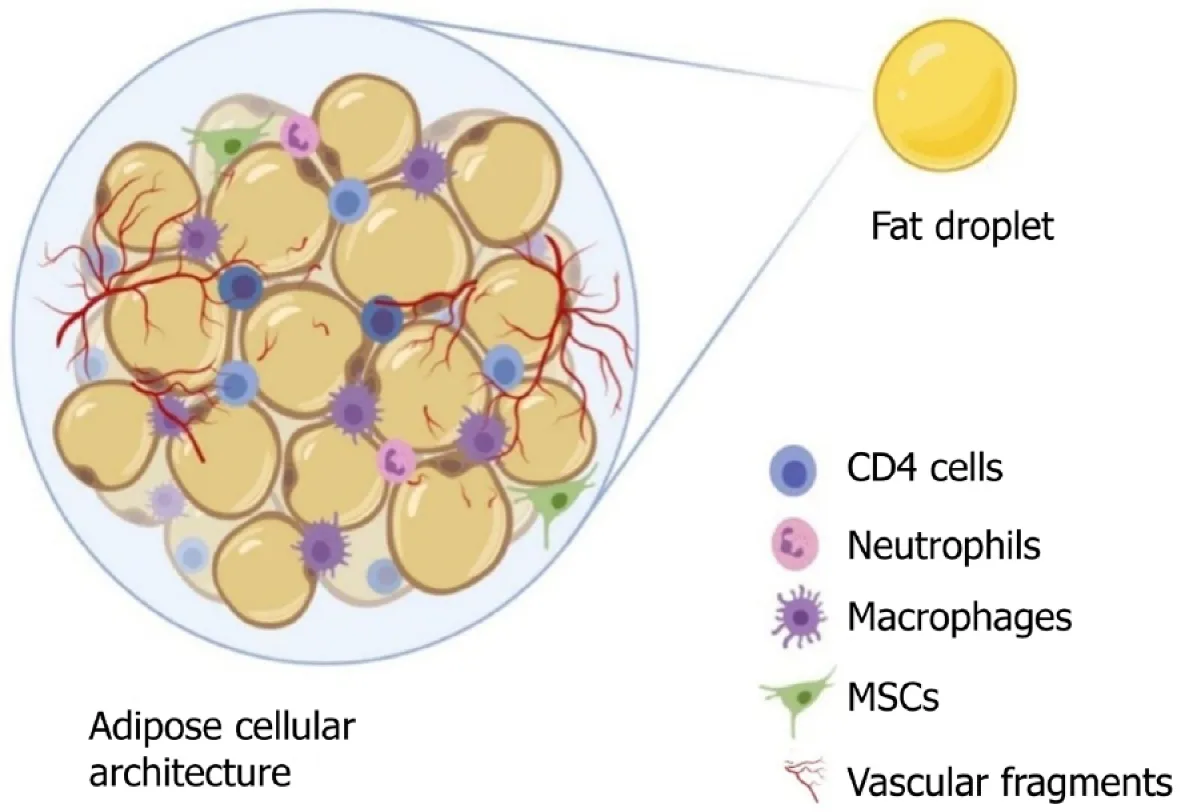
Figure 1 Organizational structure of adipose tissue. MSCs: Mesenchymal stem cells.
The literature on adipose tissue survival and regeneration depicts “cell survival theory” and “cell replacement theory”[29-32]. Various studies have proven the promising results of adipose-derived stem cell (ADSC)-assisted fat grafting, which provides numerous growth factors and adipokines for improved vasculogenesis, fat graft integration, and survival within the recipient tissue[8,33]. Adipocytes contain three zones namely, (1) the outer surviving layer (100-300 microns), (2) middle regenerating layer (600-1200 microns), and (3) inner necrotic layer, as shown in Figure 2[30-32,34].
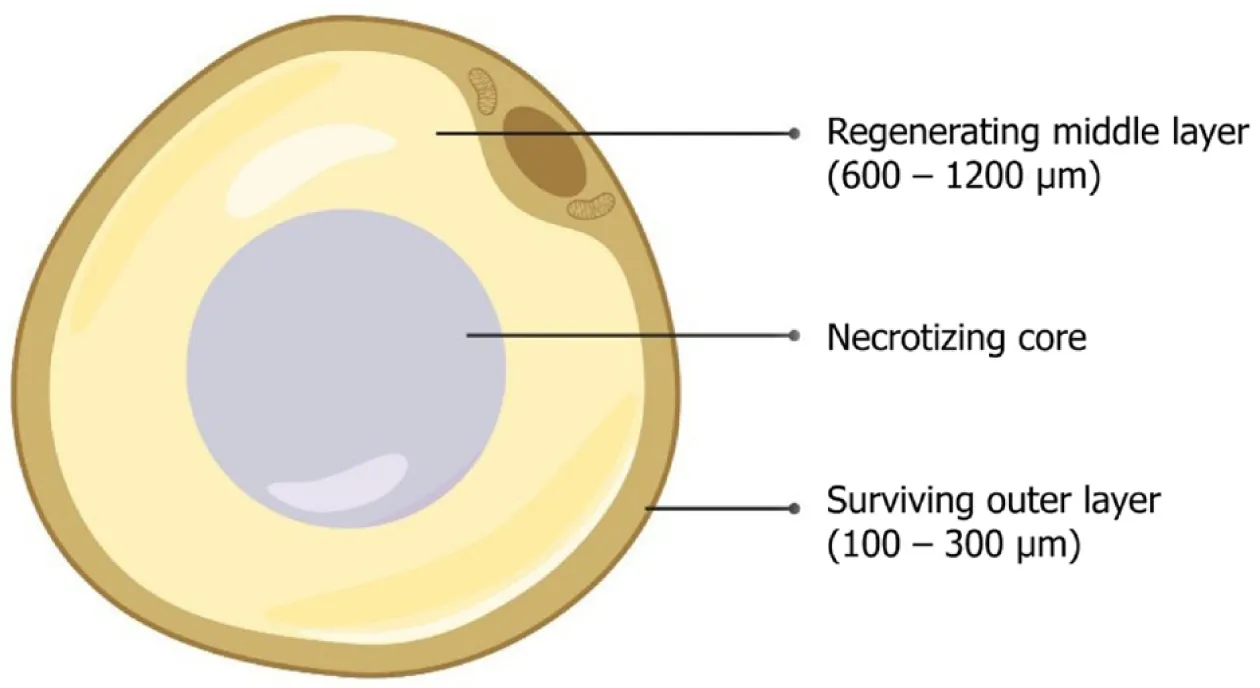
Figure 2 Zones of an adipocyte.
Yoshimuraet al[35] demonstrated the sequence of changes that happen after grafting or transplantation of adipose tissue. In preclinical studies, it is shown that all adipocytes undergo apoptosis in the initial few days of grafting. Activation of adipogenesis was by adipose-derived progenitor cells, which were augmented by the adipokines at 3 mo after grafting. By the end of 9 mo, lipid droplets were absorbed by macrophages. The final fat graft retention at the recipient site was determined by the rate of successful replacement of the adipocytes[35]. ADSCs possess various advantages over bone marrow-derived mesenchymal stem cells (BM-MSCs)[36-38].Harvesting adipose tissue by liposuction is less painful than bone marrow aspiration[39,40]. The quantity of stem/stromal cells obtained from adipose tissue than that obtained from bone marrow[41]. In long-term culture, ADSCs are more genetically stable[42,43].
Characterization of ADSCs
Researchers demonstrated that ADSCs have a consistent phenotyp and reproductive capacity, based on cellular yield, cellular viability, adipocyte differentiation, and cell surface markers[44-46]. During initial culture, ADSCs are polygonal cells that adhere to the flask surface. They exhibit fibroblastic plastic morphology and expand in in vitro cultures. within 2 d of primary culture, 90% of cells become confluent when subcultured within 2 d of primary culture with a demonstrated ADSC yield of 87%and viability of 94%[47]. Lunaet al[47] recovered 1 × 106adipocytes, 1 × 106ADSCs, 1× 106vascular endothelial cells, and 1 × 106other cells from 1 g of adipose tissue.
During in vitro culture, the ADSCs immunophenotype changes from CD34+, major histocompatibility complex (MHC) I and II molecules, CD80+, CD86+, CD45+, CD11a+,CD14+, CD117+, human lymphocyte antigen (HLA)-DR+, NOG+, undifferentiated embryonic cell transcription factor (UTF)1+, WNT6+, and WNT8A+to increased expression of CD9+, CD13+, CD29+, CD44+, CD63+, CD73+, CD90+, CD105+, CD166+, bone mor-phogenetic protein receptor (BMPR)2+, collagen type VI alpha 2 chain (COL6A2)+,transforming growth factor (TGF)-βR1+, and vascular endothelial growth factor(VEGF)-A+[48-52]. The lack of expression of HLA class I and II molecules in coculture and serial passages, confers ADSCs the property of immunosuppression and make them suitable for allogenic transplantation[53-55].
MSCs from adipose tissue have strong positive expression of STRO-1+, CD29+,CD73+, CD90+, CD105+, CD166+& CD44+and weak positive expression for CD34+and CD45+[56-57]. MSCs possess enhanced angiogenesis associated with the increased expression of CD105+and CD34+. Proliferation and differentiation of MSCs is enhanced by CD9+, CD29+, CD44+, CD49d+, and CD106+[58-61]. Gronthoset al[62]demon-strated that adipose tissue-derived stroma-vascular fraction (SVF) support hematopoiesis in vitro. After about 8-12 cellular doublings in culture, ADSCs express CD34+[63]. Various theories are available for the attribution of stem cell properties to pericytes. Szökeet al[64] stated that pericytes are present in both MSCs and ADSCs whereas Traktuevet al[65] and Crisanet al[66] stated that CD34+and CD34-pericytes are identical to adipose-derived stem cells.
Immunomodulation of ADSCs
The cellular components of ADSC induce and activate quiescent native MSCs to secrete biological micromolecules at the site of injury to establish local homeostasis by increasing the permeability of cells at the injury site, downregulating inflammatory processes, and recognition of host-tissue progenitor cells for final differentiation into the cells of interest in the injured tissue[67]. Therefore, ADSCs activate adaptive cellular responses and secrete IL-1Ra, IL-1β, PGE2, IDO, IL-4 & -10, and TGF-β that modulate and prime the native immune cells. The micromolecular interactions lead to a cascade of events responsible for immunotolerance of engraftment to a foreign site.
The immunotolerant/immunosuppressive/immunomodulatory activity of ADSCs is caused by the interplay of regulatory T cells, cytotoxic T cells, and B cells as shown in Figure 3. Immunomodulatory activity includes the inhibition of INF-γ and TNF-α production by effector T lymphocytic cells, upregulation of IL-4 and IL-10 by native immune cells, and inhibition of proliferation, migration, and differentiation of B cells that produce immunoglobulins[68]. The activity of natural killer cells is suppressed by the production of indolamine 2,3-dioxygenase by MSC-like cells[69,70]. Dendritic cells enhance the expression of IL-4 IL-10 and suppressed INF-γ and TNF-α[71,72]. The cascade of events directly suppresses tissue inhibitors of matrix metalloproteinases(TIMPs) and matrix metalloproteinases (MMPs), resulting in the conversion of the proinflammatory environment to an anti-inflammatory environment. Adipose-derived MSCs secrete extracellular vesicles such as exosomes as a channel of communication with neighboring cells[73]. They act not onlyviadirect cell-to-cell interaction but alsoviaparacrine mechanisms[74] and are key mediators of signaling molecules such as TGF-β1[75], IL-10[76], PGE2[77], NO[78] and IDO[79,80]. The bioactive factors in-turn help to promote tissue regeneration and repair at the site of action.
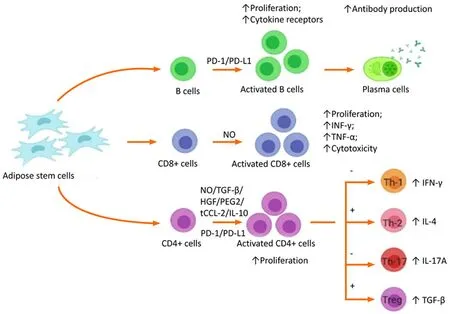
Figure 3 Immunomodulatory effects of mesenchymal stem cells via T and B lymphocyte system.
Derivatives and applications of adipose tissue
The various derivatives of adipose tissue that are of practical utility, with therapeutic potential are shown in Figure 4 and are discussed below:

Figure 4 Derivatives of the adipose tissue complex.
Adipose stem cells:ADSCs are one of the forms of MSCs of adipose origin with an inherent property of self-renewal and multipotent differentiation[73,81]. Advantages of easy accessibility of the source, abundant availability, and fewer ethical concerns than embryonic stem cells, render ADSCs more suitable for use in regenerative medicine and tissue engineering. Tissue-engineered 3D scaffolds with ADSCs and biomolecules such as growth factors and extracellular matrix materials play a robust role in treating various disorders by cellular proliferation and differentiation[4].Studies found that ADSCs have more grounded immunomodulatory impact than BMMSCs[82-84]. Adipose tissue contains stem and progenitor cells in amounts of up to 3% of the uncultured SVF, which is 2500 times more than the stem cells obtained from bone marrow[85,86]. Depending on the donor and tissue harvesting site, lipoaspirates yield 1% to 10% stem cells, which is approximately 0.5 × 104to 2 × 105MSC/g adipose tissue[87]. ADSCs act in paracrine fashion by releasing adipocytokines, cytokines, and growth factors that form a secretome[88].
Following liposuction, adipose tissue is washed in phosphate-buffered saline containing 5% antibiotic, followed by tissue digestion by collagenase-1[89]. After the disintegration of adipose tissue, the resultant sample is transferred to tubes and centrifuged at 2000 rpm for 5 min to obtain a SVF that contains the ADSCs. The resultant cellular pellet is resuspended in 1 mL lysis buffer, recentrifuged at 2000 rpm for 5 min and the resulting cellular suspension is passed through a 70 μm cell strainer.The cellular mixture is transferred to lysine-coated culture plates and incubate at 37 °C with 5% CO2. To obtain ADSCs, about 500 mg of the adipose tissue cell suspension is inoculated in the wells[87,90]. According to International Fat Applied Technology Society, uncultured ADSCs express CD34+, CD45-, CD235a-and CD31-[63,91], whereas cultured ADSCs express CD73+, CD90+, CD105+, CD44+, CD45-and CD31-[91,93].
The short- and long-term storage of ADSCs have been investigated. The cellular proliferative capacity of ADSCs decreases with the length of storage[43]. Hence, they must be supplemented with 10% human serum or platelet-rich plasma (PRP) in 0.9%saline at 4 °C for the first 2 h and not more than 4 h[1,94]. For long-term storage,ADSCs can be stored at −80 °C in liquid nitrogen for up to 6 mo[95,96]. Certain strategies can be followed to enhance and optimize the potential of the ADSC, such as culturing them in hypoxic conditions, which not only enhances the immunomodulatory effect but also reduces the risk of chromosomal aberration and tumorigenesis[97]. Further, the cells can be cryopreserved in the early passages for future usage thereby making them available for future use[98].
ADSCs are used in cardiac tissue engineering where they enhance regeneration of myocardial tissues, improve left ventricular ejection fraction, and reduce the scar volume in ventricular wall in rodent models[99]. ADSCs are seeded as bioscaffold in the healing of cutaneous ulcers and soft tissue injuries[100,101]. Several studies have proven the beneficial role of ADSCs in multiple sclerosis, diabetes mellitus, and rheumatoid arthritis. The anti-inflammatory and immunomodulatory effects of ASCs have been demonstrated in various preclinical models of autoimmune diseases[102].ADSCs have regenerative potential for bone and cartilage healing in addition to electrical stimulation of cells and tissues[103]. Various researchers across the globe have been working on the therapeutic efficacy of ADSCs in osteoarthritis knees and hips[104-106]. Agostiniet al[107] developed a protocol for ADSC application in osteoarthritis in terms of isolation, dose, frequency, analysis, and follow-up. There is no consensus on the use of culturedvsuncultured cells in the management of osteoarthritis[106,108]. ADSCs have been used in sports injuries of ligaments and tendons[109,110], but evidence to support the safety and efficacy is lacking.
The potency of the stem cells to differentiate into various lineages including nerve cells and nervous tissue makes them a good candidate for use in neurological disorders. Cultured and uncultured cells have been used in the treatment of various neurological diseases such as Alzheimer’s disease, Parkinson’s disease, intervertebral disc, amyotrophic lateral sclerosis, multiple system atrophy, post-polio residual paralysis and traumatic brain injury[111-114]. The cells enhanced neurovasculogenesis, counterfeit fibrosis, oxidative stress, anti-inflammation, and neuromodulation.In neurological diseases, the research toward the use of ADSCs is ongoing in animal models.
Microfat:Compared with nanofat, microfat appears deep yellow, with a fine granular structure and intact three-dimensional adipose tissue architecture[115]. Microfat is composed of mature adipocytes, SVF cells, pericytes, capillary fragments, and fibrous scaffolds are well preserved[116-118]. Hence, they are a natural recipient site for survival of grafts, and provide a niche for SVF cellular mixtures for tissue regeneration and rejuvenation. Yanget al[117] harvested microfat from adipose tissue with cannulas that had side holes of less than 1 mm. Examination of the harvests revealed intact fat lobular structures without need of any inter-syringe passages of emulsification procedures. Side holes of < 0.8 mm allowed the processed microfat to easily pass through the needles without any blockage. Such harvests, used for skin and facial rejuvenation through 27 gauge needles, revealed the preservation of micro-functional units of fat tissue that retained the regenerative properties of ADSCs[117]. Caggiatiet al[119] demonstrated a higher yield of ADSCs from lipoaspirates harvested by barbed compared with blunt cannulas, but they found that barbed cannulas cut the fibrous septum and produced higher quantities of coarse fibers that increased the probability of molecular blockage in the needle. The cellular components in the microfat induced tissue regeneration through the recruitment of monocyte/macrophage differentiation at the recipient site[35,120]. The cellular yield in microfat (2.28 ± 1.90) × 105cells/mL is higher than the yield in nanofat (4.12 ± 1.37) × 104cells/mL, indicating that nanofat involves mechanical emulsification[117]. Microfat components integrate into the local environment because of the intact microintegrity of cellular transplant, with inherent resistance to attack by host immune-mediated cells[121].
Microfat possess various advantages. (1) No mechanical emulsification or centrifugation is involved in preparation, other than the shearing forces during harvesting. (2)Intact vascular fragments with viable cellular components are present in the mixture.(3) There is less ischemic exposure time during adipose tissue harvest and while preparing the microfat graft. (4) Preservation of viable adipocytes that restore the degenerated and atrophied skin and subcutaneous tissues[117]. Compared with other adipose derivatives, microfat retains the three-dimensional architecture of the native tissue essential for the survival of mature adipocytes, thereby providing a natural niche for ADSC cells to ensure optimal tissue regeneration. Microfat is used in esthetic procedures. Because the three-dimensional architecture is retained in microfat, it is used as a lipofiller in breast reconstruction and facial and gluteal augmentation[122-125]. Microfat is also being tried in scar revision, burn injuries, lipodystrophies,rhinoplasty and wrinkles[3,40,117,126,127].
Nanofat:In 2013, Tonnardet al[128] developed nanofat, which is an ultrapurified adipose tissue-derived product that is devoid of mature adipocytes but contains CD34+ ADSCs, microvascular fragments, growth factors, biological peptides, and cytokines[93,94]. It is a liquefied, autologous injectable product with the property of biological integration with adjacent cells and tissues because of its homogenous consistency[129]. The size of nanofat components is approximately 400 to 600 μm[130]. Nanofat behaves much like adipose tissue-derived mesenchymal stromal cells.At the site of injury, stromal cells initiate a site-specific reparative response comprised of remodeling the extracellular matrix (ECM), enhanced and sustained angiogenesis,and immune system modulation. Because of the multi- and pluripotent nature of the cellular components in nanofat, it possesses the ability to differentiate into multiple lineages. Hence, nanofat can be used in preclinical and translational research in tissue engineering. Various preclinical and clinical studies have demonstrated antifibrotic,proangiogenic, neuroregenerative properties, and enhanced collagen deposition potential of adipose tissue-derived nanofat[131-134]. Apart from being an adipogenic derivative with ADSC, the proportion of ADSCs in nanofat is higher than that in microfat. The differences might be attributable to the method of preparation of the microfat, where the fibers and their accompanying capillaries that were the location of ADSCs are removed. In contrast, the ADSCs were mechanically separated from the native site and concentrated in nanofat, thereby making it more effective in terms of the number of ADSCs delivered to the target site. Seséet al[135] estimated the total cellular load in mechanically prepared nanofat as 6.63 million cells/g of lipoaspirate whereas in enzymatically disintegrated SVF it was 0.68 million cells/g of lipoaspirate.The nucleated cellular count was 70% in nanofat and 7.3% in SVF. The cellular burden in nanofat contains predominantly the stromal cellular population[136].
Nanofat grafting enhances neoangiogenesis without producing any visible scars,and provides a favorable outcome in esthetic medicine for breast, buttocks, and genital augmentation, facial rejuvenation, and facial volume augmentation[137,138]. Nanofat injections retract the atrophic scars because of the presence of adipose tissue-derived stromal cells, and avoids the need for surgical procedures. Nanofat components can regenerate dermis and subcutaneous fatty tissues and enhance the dermo-epidermal junction[139]. They regenerate by laying down adipose tissue-derived ECM, collagen deposition, and neoangiogenesis. Klingeret al[140] reported that autologous fat grafting allowed the regeneration of skin that was soft, flexible, and matched the color of neighboring skin. This concept of skin rejuvenation can be extrapolated to scars present in joints, eyelids, the face, and mouth.
Nanofat grafting beneath and within the substance of the scar improves the quality,integrity, and texture of burn scars[141]. Improved skin texture, elasticity, skin moisture, facial rejuvenation, and anti-aging properties can be achieved by combining nanofat (autocrine and paracrine effects) with platelet-rich fibrin (PRF)[142]. In a preclinical trial, nanofat injection improved the thickness of the dermal layer and promoted angiogenesis in the photoaged skin of nude mice[143]. A wide range of improvements in wrinkles, discolorations, and burn scars have been seen with nanofat application[141]. Esthetically, nanofat grafting is used for the correction of dark circles[144,145], malar bags[3], hollow eyes[145], and blepharoplasty[146]. Because of fat atrophy in the aging process, nanofat has emerged as a plausible technique for facial rejuvenation[126,147,148]. Nanofat is also being increasingly used in primary rhinoplasty procedures[149]. Nanofat is being used to correct slight skin irregularities that do not require cartilage grafting[127,150]. Segretoet al[151] evaluated the role of a combination of nanofat grafting with autologous PRP in chronic nonhealing infected wounds, where it enhanced the regeneration of soft tissue. The multi-differentiation potential of adipose tissue, which is a component in nanofat grafting, allows evaluation in avascular necrosis of femoral head, mild to moderate grades of osteoarthritis of the knees, tendinopathies, and nonunion of fractures. Preclinical and clinical studies of nanofat use have proven its regenerative capacity in various clinical settings.
Microvascular fragments:Microvascular fragments (MVFs), the byproduct of adipose tissue, range from 40-180 mm, and are composed of arteriolar, capillary, and vein segments[152-154]. MVFs release VEGF and basic fibroblast growth factor (bFGF)under culture conditions[155]. They are the richest source of proangiogenic factors that induce vasculogenesis in a paracrine fashion[154,156,157]. MVF contains Sca 1/VEGFR-2+ endothelial progenitor cells and MSCs expressing CD44+, CD73+, CD90+ and CD117+[158]. The components of MVF exhibit the morphology of intact lumen,endothelium, and perivascular stabilizing cells. MVF are the building blocks for therapeutic vasculogenesis[159].
It was initially speculated that the high vascularization potential of MVFs is mainly derived from stem cell populations. McDanielet al[160] compared the regenerative properties of conventionally isolated adipose-derived stem cells and multipotent cells derived from an explant culture of microvascular fragments. They found that the latter source exhibited a higher proliferation rate, an increased expression of genes involved in differentiation, and an improved ability to form capillary-like structures. In line with the concept of the “stem cell niche,” the findings indicated that compared with single-cell isolates, microvascular fragments including stem cell components provides a more physiological environment that maximizes the regenerative activity.Transplanted or injected MVFs rapidly integrate with native tissues to promote neoangiogenesis in the physiological environment[161]. The three phases of MVFinduced neoangiogenesis are (1) immature vascular segments with high proliferation capacity; (2) vascular remodeling with a high rate of apoptosis; and (3) vascular maturation with microvascular network organization[162,163]. The complex pattern of neoangiogenesis is associated with the upregulation of angiogenic genes.
Researchers have observed the regenerative potential in cartilage defects and skeletal muscle injury[164-167], myocardial infarction[152,163,168], partial- or complete-thickness skin defects[169,170] and diabetes mellitus[171]. MVF loaded scaffolds can reverse lymphatic network disorders by reducing edema formation and promoting vasculogenesis in the area of repair[154,172-174]. With the available,diverse potentials of MVF, the application of MVF in the clinical setting is plausible with the imperative question of technical and regulatory compliance from bench to bedside.
SVF:SVF is an ultra-byproduct of adipose tissue through various processing methods[175-177]. The development of biocellular regenerative medicine and cellular biology,has increased the use of SVF by regenerative surgeons and researchers. The components of SVF are MSCs, HSCs, T-regulatory cells, pericyte endothelial cells, mast cells, complex microvascular beds with fibroblasts, WBC, dendritic cells, intraadventitial smooth muscular-like cells, and others, and extracellular matrix[44,177-179]. A sufficient number of SVF cells can be obtained without culture. Current restrictions on the use of cultured cells in humans cite the possibilities of contamination, tumorigenesis, unexpected cell differentiation, and limited cell sources. SVFs behave much like BM-MSCs. SVF mixtures contain 30% MSCs, 3% endothelial cells,and 14% endothelial precursor cells[180]. BM-MSCs contain 0.001% MSCs, 0.1%endothelial cells, and 2% endothelial precursor cells[181]. About 2% of isolated SVF express the hematopoietic markers CD34+and CD 45+and 7% express the MSC markers CD105+, and CD146+[92,182,183]. SVF cells express cell surface markers similar to those of BM-MSCs, such as CD105+/SH2+, CD90+, CD29+, CD44+, CD71+, and SH3+, along with low expression of CD31+, CD45+, and CD24+[184-186].
Various methods of isolation of SVF have been described in the literature[90,187-190]. SVF isolation needs fat obtained by liposuction and transportation to a cGMP certified laboratory for further processing[191-193]. The global researchers and clinicians widely use the enzymatic method for isolating and harvesting SVF by collagenase enzymes. After the addition of collagenase enzyme to the lipoaspirate,digestion and disintegration of adipose cells take place and result in the formation of an aqueous mixture including two phases, a floating adipocyte fraction and precipitated cellular components[194,195]. The separation can be enhanced by density gradient centrifugation and filtration[191]. The lower portion of the tube contains a yellowish red pellet which is the SVF mixture that can be used for in vitro expansion[196]. Because of the ethical issues associated with SVF isolation by the enzymatic method, researchers opt for alternative methods for SVF isolation[197,198].Researchers proposed mechanical agitation and disruption of adipose tissue, which releases a mixture of cellular components and stromal cells. The proportion of cells in the SVF obtained by mechanical disruption is lower that obtained by enzymatic degradation, but the regenerative potential of SVF mixtures obtained by mechanical disruption and enzymatic degradation are the same[198]. The anti-inflammatory effects of SVF result from the presence of higher amounts of IL-1 and 10 receptor antagonists, which are expressed by TNF-α and leptin released by monocytes and macrophages in the SVF mixture[199]. SVF mixtures provoke adaptive immunity by producing T-reg cells by suppressing the activation of dendritic cells[200,201].
Krawiecet al[202] demonstrated that SVF components seeded with biodegradable porous scaffoldsin vivofor 8 wk resulted in the generation of tissue-engineered vascular grafts when populated with primary vascular components such as smooth muscle cells, endothelial cells, collagen, and elastin. ADSCs or SVF combined with PRP enhanced the regenerative potential in the form of neoangiogenesis, cellular proliferation, and differentiation[203]. The combination of SVF with PRP enhanced the stemness of MSCs, and the growth factors present in PRP induced locally available stem cells and prolonged the survival time and survival rates of cells present in the PRP[204]. Such combination treatments have been evaluated in preclinical and clinical trials of wound healing, osteoarthritis of the knees, bone and tendon regeneration,periodontal engineering, fat grafting procedures, and vascular diseases[203,205-208].Osteochondral defects of the knees in goats were managed by the application of acellular type 1 and 3 collagen scaffolds along with SVF cells[209]. In rat models of acute kidney injury, the transplantation of autologous SVF cells induced antiapoptotic effects and the release of growth factors like VEGF and HGF to regenerate kidney cells[210]. In preclinical studies, the therapeutic effect, safety and functional outcome of autologous SVF uncultured or culture-expanded cells were observed in acute myocardial infarction, skin flap necrosis, and erectile dysfunction with cavernous nerve injury[211-213]. In human clinical studies, the use of SVF cells was extensively studied in conditions like breast reconstruction surgery, traumatic calvaria defects,types 1 and 2 diabetes mellitus, Crohn’s disease with enterocutaneous fistula, and burn injuries[214-218]. Zhaoet al[219] evaluated the efficacy of SVF in diabetic feet in terms of cellular survival, proliferation rate and differentiation and they determined the characteristics of transplanted cells. Various researchers demonstrated endobronchial administration of autologous SVF cells for treating idiopathic pulmonary fibrosis[220]. Autologous SVF is also being evaluated in COVID-19 individuals[221-223]. When cocultured with 5% or 20% PRP, the viability, motility, proliferation rate,and differentiation of ADSCs were increased[224]. In the case of fat grafting, clinicians are challenged by the fat graft rejection, with a reported resorption rate ranging from 25%-80%, which is mostly the result of apoptosis of mature adipocytes[225]. When the lipoaspirate was given along with SVF, a 35% greater graft retention was noted[194].Further, more prominent microvasculature was noted compared with normal graft tissue, suggesting its clinical potential as a nourishing medium that enhanced the efficacy of the native therapy. Moreover, in situations such as osteoarthritis, an ultrafiltrate fraction of an adipose derivative such as SVF would be the ideal medium of choice to tap the benefits of ADSCs in cartilage regeneration[226].
ADSC exosomes:Exosomes are the cell-free regenerative tool in the field of tissue engineering. Exosomes are extracellular vehicles that are endosome-derived lipid bilayer spherical vesicles of 40 to 150 nm in size. They are found in all cell types and body fluids and are comprised of cytokines, proteins, lipids, DNA, and RNA from the parent cell. Exosomes are an integral part of both the diagnostic and therapeutic methods used in various diseases. ADSC exosomes (EXOs) differ from other MSC EXOs in proliferation and differentiation abilities and immunosuppressive pathways.Compared with ADSCs, ADSC-derived exosomes possess have a high biosafety profile with low immunogenicity[227]. They protect cargoes from degradation, have tissue and target specificity, good tissue permeability, intercellular signaling and communication, immune function, tissue homeostasis, and development of cell fate[227].
Ogawaet al[228] observed that mRNAs of adipose tissue-derived exosomes have a significant role in metabolic, immunological, and cellular responses. Various studies demonstrated that adipose tissue-derived exosomes possess the capability of regeneration of muscles and bones, promotion of wound healing, and enhancement of cellular proliferation, and neoangiogenesis. ADSCs-EXOs enhance the proliferation and migration of vascular endothelial cells and promote vasculogenesis. They retain the fat graft volume by the stimulation of angiogenesis and regulating inflammatory responses[229,230]. ADSC EXOs were tested by Wanget al[231] for the promotion of wound healing in diabetic mice by enhancing angiogenesis, proliferation of fibroblasts,and collagen synthesis in the later stages. The evidence also supports the use of ADSC EXOs for treating diabetic foot patients[232]. To stabilize the ADSC EXOs concentration when used for local application, bioscaffolds like hydrogels or fibrin are augmented with exosomes to delay release at the therapeutic site[233,234]. ADSC EXOs are used for scarless cutaneous repair by retracting the size of scars, increasing the collagen 3 to collagen 1 ratio, and by regulating the migration, proliferation, differentiation, and gene expression of fibroblasts[235]. Kimet al[236] demonstrated that key cytokines and growth factors in ADSC EXOs facilitated tissue regeneration and repair through anti-oxidation, anti-wrinkle and skin-whitening activity. ADSC EXOs with multiple bioactive molecules for the management of aging warrants further extensive research. Zhaoet al[237] demonstrated crosstalk that facilitated immune and metabolic homeostasis, providing a vital therapy for obesity and diabetes mellitus. ADSC EXOs combined with poly(lactide-co-glycolide) scaffolds improved the osteoinductive effects, MSC migration, and homing abilities in bone regeneration[238]. Chenet al[239]demonstrated that exosomes derived from miR-375-overexpressing ADSCs incorporated with hydrogel enhanced bone regeneration in a rat model of calvarial defect. Direct stem cell transplantation with ADSC EXOs primed by TNF-α, promoted the proliferation and differentiation of human osteoblasts promoted through the Wnt signaling pathway, which further widened the application of exosomes in bone regeneration[240]. Perset al[241,242] reported that ADSC EXOs were a safe, effective,and inexpensive therapy for osteoarthritic knees. ADSC EXOs downregulated inflammation and oxidative stress in osteoarthritic knees[243]. Zhanget al[244] confirmed that intra-articular injection of ADSC EXOs inhibited cartilage and subchondral bone degradation and osteophyte formation and slowed the progression of the disease process in osteoarthritis. ADSC EXOs participated cellular communications and applications in plastic and cosmetic surgery. Although applications in clinical practice are lacking, ADSC EXOs have an increasing role in maximizing the therapeutic effectiveness for dermopathies and for tissue reconstruction[245]. There is a future for exosomes as a diagnostic tool for early identification of pathological processes involved in disease and as a therapeutic, targeted acellular conduit to optimize the pathological milieu at the site of interest.
Ethical concerns
An early therapy and drug development are dependent on translational phases, the best strategy is to standardize the process, and regularly acquire data to validate and certify the technologies being developed. The safety and efficacy of the developed therapy must be proven from various perspectives, including the donor, recipient,product, manufacturing, clinical application, and biovigilance. The procedures involved in making adipose-derived products involve the collection and preparation of fat tissue. The procedures require that the cells are obtained by safe methods without any adverse events. Clinicians who use the cells in their practice are obliged to abide by existing laws that control the use in clinical practice. The European Medicines Agency (EMA), United States Food and Drug Administration (FDA), and others consider adult human cells as biological products of two classes. The first involves cells processed with minimal manipulation techniques such as filtration, centrifugation, and mechanical disruption done in a single surgical window within a sterile operating environment. The second class of products undergoes significant manipulation such as expansion in cell culture, characterization, cultivation, and other manipulation techniques used outside of a single surgical window or the operating room. As per FDA and EMA regulations, adipose products are considered medicinal products that have to be harvested using validated procedures adhering to stringent regulatory protocols. The clinical quality attributes of therapeutic products ensure safety along with the maintenance of identity, purity, and potency as per the FDA Guidance for industry [Pharmaceutical development. Q8(R2). Current Step 4 version dated August 2009.Q8 (R2)]. In agreement with the reflection paper EMA/CAT/-600280/2010 Rev 1, 20 June 2014, it is presumed that for autologous use under a single therapeutic window with minimal manipulation in aseptic conditions, adiposederived products would not require ethical committee underwriting for clinical use.However, there are no standardized methods or means to isolate ADSCs with predetermined critical quality attribute, making isolation of ADSC for targeted therapies difficult[246]. Hence, a modular system configured as a single-use kit that contained all the essential components in the exact proportions needed would be ideal and is recommended. Such kits would allow following a specific protocol to standardize isolation of adipose derivatives with targeted action of ADSCs and comply with specific critical quality attributes.
Having discussed the practical constraints of the use of ADSCs for practical applications, the use of ADSC-derived exosomes as a therapeutic conduit is being explored[247]. Exosomes do not carry the risk of genetic instability and immune activation following their administration in the host environment. The differential advantage of using themvstheir parent cell of origin include immune privilege in the host environment, which helps them to evade the native phagocytosis, their size in nanoscale enabling them to move in and out of cells with ease, their homing molecules on their surface enabling them to migrate to their site of interest[248]. Hence, with the above-conferred advantages in using ADSC-derived exosomes, they are the principal focus of research in advancing regenerative therapy in the future.
CONCLUSION
Of late, interest in adipose derivatives for regenerative therapies and constructive tissue engineering is on the rise. Having elaborated the biology, characteristics,immunology, and clinical applications of adipose-derived products, it is imperative that evidence to strengthen the clinical applicability of these therapeutic products is needed to warrant an official recommendation from regulatory authorities. Unraveling the utility of various adipose derivatives helps in the improvisation of the existing regenerative therapies and their associated biomedical applications.
 World Journal of Stem Cells2021年10期
World Journal of Stem Cells2021年10期
- World Journal of Stem Cells的其它文章
- Impact of senescence on the transdifferentiation process of human hepatic progenitor-like cells
- Effect of glycyrrhizic acid and 18β-glycyrrhetinic acid on the differentiation of human umbilical cord-mesenchymal stem cells into hepatocytes
- Current knowledge on the multiform reconstitution of intestinal stem cell niche
- Stem cell therapy and diabetic erectile dysfunction: A critical review
- Overview of nutritional approach in hematopoietic stem cell transplantation:COVID-19 update
- Age and genotype dependent erythropoietin protection in COVID-19
