Effects of Ni substitution on multiferroic properties in Bi5FeTi3O15 ceramics
Hui Sun(孙慧) Jiaying Niu(钮佳颖) Haiying Cheng(成海英)
Yuxi Lu(卢玉溪)1, Zirou Xu(徐紫柔)1, Lei Zhang(张磊)1, and Xiaobing Chen(陈小兵)1
1College of Physics Science and Technology,Yangzhou University,Yangzhou 225002,China
2School of Sino-German Engineering,Shanghai Technical Institute of Electronics&Information,Shanghai 201411,China
3National Laboratory of Solid State Microstructures,Nanjing University,Nanjing 210093,China
Keywords: layer-perovskited oxides,ferroelectricity,weak ferromagnetism,phase transition
1. Introduction
Single-phase multiferroics refer to those oxides simultaneously exhibiting ferroelectric (FE), ferromagnetic (FM),and/or ferroelastic properties,which have been widely investigated owing to their potential application in transducers,digital memories, and data storages.[1–4]Nevertheless, it is still a challenge to pursue room-temperature (RT) multiferroics because there is rare multiferroics in nature due to the exclusion between ferroelectricity and ferromagnetism.[5–7]
By combining magnetic units with ferroelectric matrices at atomic scale,the single-phase multiferroics can be obtained.Bismuth-based layered perovskite Aurivillius phase is such a kind of compounds with formula (BiFeO3)m-Bi4Ti3O12,which have been regarded as a promising candidate for RT multiferroics.[8–11]In this structure, the perovskite-type slabs (Bi2+mTi3FemO3m+10)2−are sandwiched with fluoritetype layers (Bi2O2)2+alongcaxis in a half unit cell.The magnetic transition temperatures of these oxides were reported to be far below RT, i.e., Bi5FeTi3O15(80 K),Bi6Fe2Ti3O18(160 K), and Bi7Fe3Ti3O18(190 K).[8]Then substitution with magnetic ions for Fe or raising the number of magnetic layers were employed to enhance the magnetic properties.[12–24]For instance,four-layered Bi5FeTi3O15(BFTO) showed good ferroelectricity and a high ferroelectric Curie temperature of 1023 K accompanied by a space group transition fromA21amtoI4/mmm.[6]As was reported, substitution with Co for half content of Fe in BFTO can dramatically enhance the magnetic transition temperature far above RT(~618 K),realizing the coexistence of FE and FM at RT.[12,13]And the Co-substituted BFTO showed strong magnetoelectric and magnetodielectric effects.[14]Similarly, obvious ferromagnetism can also be achieved in fivelayered Bi6FeCoTi3O18[15]and Bi6Fe1−xNixTi3O18;[18]sixlayered Bi7Fe1.5Co1.5Ti3O21[19]and Bi7Fe3−xNixTi3O21;[23]and seven-layered Bi9Fe4.7Me0.3Ti3O27(Me=Ni and Co).[22]These results confirmed that Ni substitution can not only induce ferromagnetism but significantly decrease the leakage current. Moreover, it is worth noting that the content of doped magnetic ions has a great influence on the ferroelectric and magnetic properties. Although the magnetic and dielectric properties of four-layered Bi4NdTi3FeO15were investigated,[25,26]their leaky ferroelectric hysteresis loops exhibited poor ferroelectric property. There is still a lack of detailed research on Ni-doped BFTO, such as ferroelectric and magnetic transitions.
In this paper, we prepared Bi5Fe1−xNixTi3O15(BFNTx,x=0, 0.1, 0.2, 0.3, 0.4, 0.5) ceramics by a sol-gel autocombustion method, and their microstructures, ferroelectric,dielectric,and magnetic properties were investigated in detail.As observed, the Ni content has a major influence on the microstructures and properties of BFTO,and their corresponding mechanisms were discussed. The work may be beneficial to our designing and exploring single-phase Aurivillius multiferroics.
2. Experimental details
Bi5Fe1−xNixTi3O15(BFNT-x,x= 0, 0.1, 0.2, 0.3, 0.4,0.5)ceramics were synthesized by the sol-gel auto-combustion method.[27,28]A detailed process is described as follows. Stoichiometric amounts of Bi(NO3)3·5H2O (5 wt% excess) and Fe(NO3)3·9H2O were dissolved in 4 M nitric acid solution,and citric acid C6H8O7·H2O was added. Dripping aqueous ammonia (28% mass fraction) into the above solution to adjust pH value to 6–7. Here the molar ratio of metal ions to citric acid was 1:1. The solution was transferred to an oil bath with stirring at 80°C to obtain dried xerogel. After that, the xerogel was burned at 400°C for 4 h, followed by ground and pre-sintered at 750°C for 6 h. The obtained powders were ground again,subsequently pressed into pellets,whose diameter and thickness were 10 mm and 1 mm,respectively.Finally,the pellets were calcined at 900°C for 4 h in air.
The microstructures of all the samples were analyzed by x-ray diffractometer (XRD, XRD-7000, SHK, Japan), scanning electron microscope (SEM, TESCAN VEGA3, Czech),and Raman scattering spectra (Renishaw, USA). The valence states of ions were investigated by an x-ray photoelectron spectroscopy(XPS,ESCALAB 250Xi,Thermo Fisher Scientific, USA). The magnetic properties were measured by a vibrating sample magnetometer (VSM, EV7, ADE, USA). To test the electrical properties, the calcined pellets were polished to a thickness of about 0.2 mm, and Ag electrodes were deposited on both sides. The ferroelectric properties of the samples were characterized by using a Precision LC ferroelectric analyzer (Radiant Technology product, USA).The dielectric constant and loss tangent dependent on temperature were measured with a broadband dielectric spectrometer(Novocontrol Technologies,Germany). The thermomagneto-gravimetry (TMG) measurements were performed using thermo-gravimetric analysis (TGA, Q5000IR, USA)technique to measure the samples’weight dependent on temperatures in an applied magnetic field. The measurements were carried out in nitrogen atmosphere with a 0.02 T magnetic field. The ferroelectric transitions were studied by the differential scanning calorimetry (DSC, STA 449 F3 Jupiter,Netzsch,Germany)in the process of a heating and cooling.
3. Results and discussion
Figure 1(a) shows the XRD patterns of the BFNT-xceramic samples. All the diffraction peaks of all samples can be detected according to the standard PDF file of Bi5Fe1Ti3O15(JCPDS No.38-1257,space groupA21am). All BFNT-xsamples have a four-layered perovskite structure without other identifiable impurity phases,[12–14]which suggests that Ni successfully entered into B-site to replace Fe. To confirm this,the Bi5Fe0.8Ni0.2Ti3O15was refined using Topas based on the orthorhombic space groupA21am,as exhibited in Fig.1(b),and good agreement between the experimental and calculated pattern is obtained withRwp=8.11%andRp=6.18%.

Fig. 1. (a) XRD patterns of Bi5Fe1−xNixTi3O15 (x=0–0.5) ceramics,and(b)XRD pattern of Bi5Fe0.8Ni0.2Ti3O15. Circles represent the experimental data,and the calculated data are the continuous line overlapping them. The middle curve indicates the difference between the experimental and calculated data. The lowest vertical bars show the expected reflection.
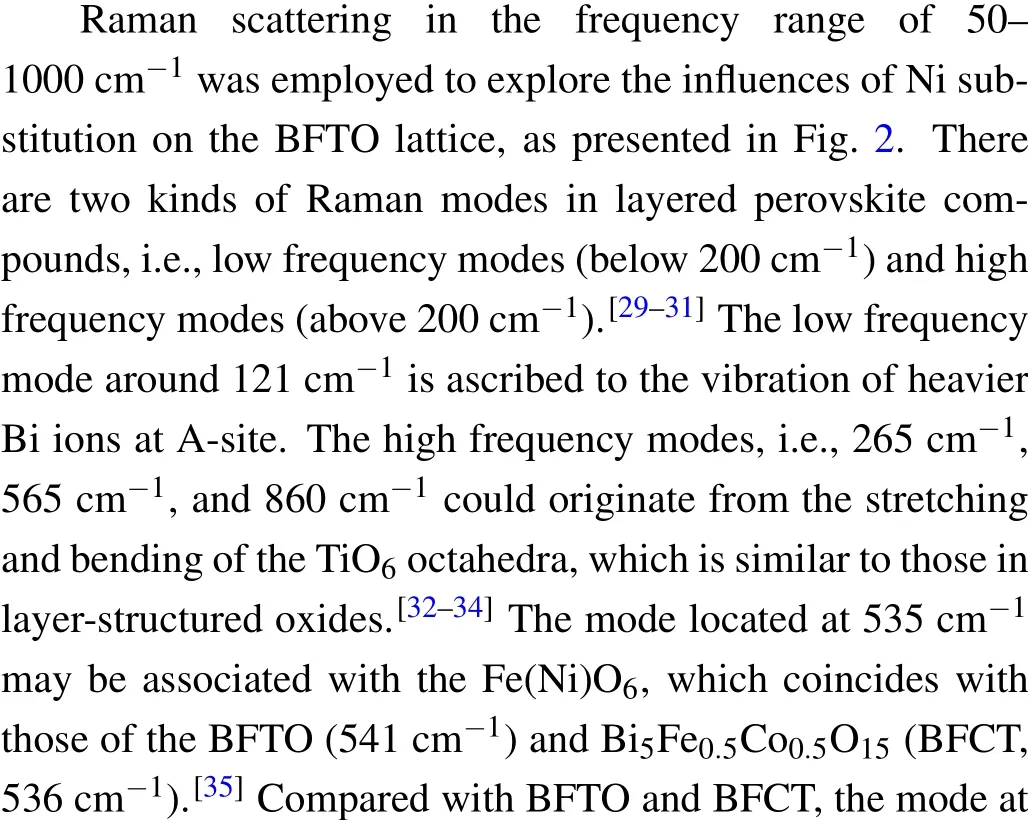


Fig.2. Raman scattering spectra of BFNT-x ceramics.
Figure 3 displays the surface SEM images of BFCT-xsamples. All samples are homogeneous,dense,and randomly oriented,implying good crystallinity. Meanwhile,all the samples have flaky grains with obvious anisotropy. This is the typical characteristics of layered perovskite Aurivillius oxides due to its preferential growth inabplane.[27,28]With the increase of Ni contentx,the grain size firstly increases and then decrease, revealing that Ni doping has an effect on the grain growth.
To gain the electronic structure information of elements in the samples, x-ray photoelectron spectroscopy (XPS) of BFNT withx=0,0.1,0.3,and 0.5 was carried out,as shown in Fig. 4. According to the report,[36]the Fe 2p3/2peaks located at 709.3 eV and 710.7 eV are ascribed to Fe2+and Fe3+ions, respectively. The experimental Fe 2p3/2peaks shown in Fig.4(a)are located at 710.5–910.6 eV,which means there are both Fe3+and Fe2+ions in the samples. Using the Lorentzian–Gaussian fitting and calculating, the atomic ratio of elements can be determined quantitatively by the ratio of area below the corresponding peak. The calculated ratio of Fe3+to Fe2+is listed in Table 1. It can be seen that a small amount of Ni doping (x<0.1) can inhibit the valence variation of Fe ions. As for the spectra of the Ni 2p3/2in BFNT-0.3 and BFNT-0.5(exhibited in Fig.4(b)), the peaks at 855.1 eV are close to the binding energy of Ni 2p3/2in Ni2O3, illustrating that the Ni ions are in the ionic state of +3.[37]Normally,the oxygen vacancies may be accompanied with the valence change of cations and affect the electrical and magnetic properties.[38]Therefore,the O 1s core-level spectra were analyzed and presented in Fig. 4(c). The deconvoluted peaks located at 529.7 eV and 531.7 eV are assigned to the lattice oxygen (denoted with O[1]) and oxygen vacancies (denoted with O[2]),[39,40]respectively.Here the peaks marked with O[3]are attributed to the absorbed oxygen species. The relative amount of oxygen vacancies can be qualitatively ascertained byIO[2]/IO[1],as shown in Table 1. It is concluded that Ni substitution can largely improve the stability of oxygen ions and reduce the oxygen vacancy concentration.

Fig.3. Surface SEM images of BFNT-x samples for(a)x=0,(b)x=0.1,(c)x=0.2,(d)x=0.3,(e)x=0.4,and(f)x=0.5.

Fig.4. 4 High resolution XPS spectra and fitted curve of(a)Fe 2p,(b)Ni 2p,and(c)O 1s of BFNT-x.

Table 1. The calculated atomic ratios of Fe2+/Fe3+ and IO[2]/IO[1] dependent on Ni content.
Figure 5(a)exhibits the magnetic hysteresisM–Hcurves of all samples measured at RT, and their enlarged view of central parts are shown in the inset. Forx= 0, 0.4, and 0.5 samples, the linearM–Hplots imply the feature of antiferromagnetism (AFM) or para-magnetism (PM). The otherM–Hplots exhibit typical hysteresis, which clearly indicate the presence of FM moment. However, the magnetization is not saturated even under the higher magnetic field~1 T, revealing that there also exist AFM interactions in these samples.Figure 5(b)summarizes the dependence of remnant magnetization(2Mr)and saturated magnetization(Ms)on Ni content. With the increase of Ni content, both of them firstly increase (x ≤0.2) and then decrease with further more Ni content. The changing trend of the magnetism with increasing Ni content is consistent with that of Bi7Fe3−xNixTi3O21ceramics.[23]Whenx=0.2, 2MrandMsreach the maximum of 0.24 emu/g and 0.68 emu/g,respectively. In this kind of oxides,the magnetism may be mainly affected by the following factors. Firstly,the Fe(Ni)–O6octahedral tilting and distorted crystal structure would bring about canted spin structure. And spin canting of AFM coupling of Fe–O and Ni–O sublattices based on Dzyaloshinsky–Moriya (DM) interactions leads to the present weak ferromagnetism.[15,16,18–24]Secondly, there exist oxygen vacancies in the samples confirmed in XPS results. The magnetic ions are apt to integrate with oxygen vacancies to form the bound magnetic polarons (BMPs),and the interactions between BMPs are ferromagnetic.[41]As discussed above, the oxygen vacancy concentration was depressed by Ni doping,which would decrease this kind of ferromagnetic interactions. Both the aspects above result in the variation of the magnetic properties dependent on Ni content.
To clarify the magnetic interactions in BFNT,the dependence of the sample’s weight under a magnetic field on temperature was investigated. Figure 6 gives thermo-magnetogravimetry(TMG)and the corresponding differential thermomagneto-gravimetry(DTMG)curves of BFNT-xsamples withx= 0.1, 0.2, and 0.3. Each sample has only one peak,which corresponds to the FM-to-PM phase transition. This means that there does not exist other magnetic impurity phase,demonstrating that the room-temperature weak FM comes from single-phase BFNT.The peaks of BFNT-0.1,BFNT-0.2,and BFNT-0.3 at approximately 824 K, 796.3 K, and 774 K,respectively, are far above RT, leading to the occurrence of weak ferromagnetism at RT.The magnetic transition temperature decreases as the Ni content increases. Generally,the two main factors affecting magnetic transition temperature include lattice distortion and magnetic interaction. Less Ni substitution (x ≤0.1) can dramatically enhance the magnetic transition temperature from 80 K to 824 K and realize the weak ferromagnetism at RT. More Ni content (x>0.1) decreases the magnetic transition temperature,which may be ascribed to the decreased order of the B-site ions and the relative stability of the magnetic structure.[42]As for the lattice distortion,it is affected by the changes of the bond angle and length between coupling magnetic ions,which is also related to the transition temperature from FE to paraelectric phase transition.[14]As reported,the higher ferroelectric transition temperature means the larger structural distortion. Therefore,we will discuss the temperature dependence of the dielectric properties and DSC curves of all samples below.

Fig.5. (a)Magnetic hysteresis M–H curves measured at RT of SBNT-x samples. (b)The variation of 2Mr and Ms with Ni doping content.

Fig.6. Weight loss under a magnetic field and DTMG curves of BFNT samples with x=0.1,0.2,and 0.3.
Figure 7 shows the temperature dependence of dielectric constantεand loss tanδof all BFNT samples from 370 K to 1080 K at different frequencies. All the samples exhibit a similar behavior, and a set of anomalies are observed around 600 K and marked with rectangle. The set of dielectric peak locations of dielectric constant and loss shift toward higher temperature with the increase of frequency, exhibiting a typical thermal-activated relaxation behavior. Whereas,it is difficult to determine the dielectric peak position due to the wide dielectric loss peak. In order to understand the mechanism of this abnormal dielectric behavior, the temperature dependent imaginary partM′′of dielectric modulus for BFNT was studied, depicted in Fig. S1. According to the point defect relaxation theory,the activation energyEaof relaxation units can be calculated by the Arrhenius lawfr=f∞exp(Ea/kBT), wherefrandf∞are the relaxation frequencies of characteristic peak and at infinite temperature,respectively.Tis the temperature of theM′′-peak, andkBis the Boltzmann constant. As displayed in Fig. S2, the activation energiesEaare evaluated to be 0.85 eV, 0.56 eV, 0.57 eV, 0.56 eV, 0.60 eV, and 0.55 eV for the BFNT samples withx=0, 0.1, 0.2, 0.3, 0.4, and 0.5,respectively. These values are close to the migration energy of oxygen vacancies (0.5–1.1 eV),[43,44]indicating that these dielectric anomalies around 600 K are associated with the hopping process of oxygen vacancies.Unfortunately,the FE Curie temperature is not found in the dielectric spectra dependent on temperature from 370 K to 1080 K due to the limit of the dielectric spectrometer. Then DSC traces of all BFNT samples will be studied as follows.
Figure 8 shows the DSC curves of all BFNT samples.There are two sets of endothermic peaks on heating and two sets of exothermic peaks on cooling for each sample,indicating reversibility of transitions. Both the sets of peaks exhibit thermal hysteresis.For the BFTO sample,the low-temperature endothermic peak located at 1030 K with its corresponding exothermic peak at 1020 K is in a good agreement with the ferroelectric transition accompanied with structural transition from orthorhombic to tetragonal phase.[45]The other hightemperature endothermic peak at 1126 K with its corresponding exothermic peak at 1083 K originates from the lattice expansion of the tetragonal phase. We summarize the temperature of all endothermic and exothermic peaks in Table 2,and define the transition temperature as the average value of the temperature of endothermic and corresponding exothermic peaks. From Table 2,it can be seen that the ferroelectric transition temperature decreases with the increasing Ni content,implying lessen structural distortion.

Fig.7. The temperature dependence of dielectric constant ε and loss tan δ of all BFNT samples ranging from 370 K to 1050 K at different frequencies.

Fig.8. DSC curves of all BFNT samples.

Table 2. The detected transition temperature of all BFNT samples.

Fig. 9. (a) Polarization versus electric field (P–E) hysteresis loops of BFNT.(b)The variation of remnant polarization(2Pr)and coercive field(Ec)dependent on Ni content.
Finally, the ferroelectric hysteresis loops of all samples were measured at RT,as illustrated in Fig.9.For the BFTO,its loop shows round and leaky. After Ni substitution, the ferroelectric remnant polarization as well as the breakdown electric field enhance largely. The values of remnant polarization(2Pr)and coercive field(Ec)firstly increase and then decrease with the increase of Ni content. Whenx=0.2,the 2Prreaches the largest value about 11.6µC/cm2,and the breakdown field also achieves the maximum of 220 kV/cm. As is well known, the ferroelectric property may be related to the following factors:(1)Structural distortion.[46,47]The substitution of Fe by larger radius ions,i.e.,Co and Ni,can reduce the displacement of the B-site ions,decreasing the structural distortion.The decreased structural distortion leads to the reduced FE Curie temperature,which is confirmed in Fig.8. The decrease structural distortion will reduce the larger remnant polarization. (2) Oxygen vacancy.[48]It was reported that oxygen vacancies usually aggregate at the ferroelectric domain walls and pin the domain switching, resulting in deterioration of the remnant polarization. Ni substitution decreases oxygen vacancy concentration discussed above and increases the polarization. (3)Grain size.[49]Larger grain can weaken the aggregation of the defects at the domain walls,lessening the pining of the domain walls and enhancing the remnant polarization. As shown in Fig.2,less Ni substitution increases grain size,which can enhance the remnant polarization. The above three factors bring about the variation of ferroelectricity dependent on Ni content.
4. Conclusions
The microstructures, ferroelectric, magnetic, and dielectric properties of BFNT-xceramics were investigated systematically. XRD and Raman spectra confirmed that Ni substitution does not change the four-layered perovskite structure. Ni substitution can improve ferroelectricity as well as magnetic properties. The largest remnant polarization(2Pr~11.6µC/cm2)and the highest remnant magnetization(2Mr~0.244 emu/g)were found in the BFNT-0.2 sample. The weak FM of the doped samples may mainly originate from the spin canting of Fe/Ni-based sublattices via the antisymmetric DM interaction.The enhancement of FE and magnetic properties is related to the structural distortion. Therefore,DSC tests were performed,detecting FE transition and lattice expansion of the tetragonal phase. The dielectric relaxation behaviors were observed in all samples, which may be caused by the migration of oxygen vacancies due to thermal activation. The present work is helpful for design of room-temperature multiferroics based on Aurivillius phase.
- Chinese Physics B的其它文章
- Physical properties of relativistic electron beam during long-range propagation in space plasma environment∗
- Heterogeneous traffic flow modeling with drivers’timid and aggressive characteristics∗
- Optimized monogamy and polygamy inequalities for multipartite qubit entanglement∗
- CO2 emission control in new CM car-following model with feedback control of the optimal estimation of velocity difference under V2X environment∗
- Non-peripherally octaalkyl-substituted nickel phthalocyanines used as non-dopant hole transport materials in perovskite solar cells∗
- Dual mechanisms of Bcl-2 regulation in IP3-receptor-mediated Ca2+release: A computational study∗

