An understanding of mitochondria and its role in targeting nanocarriers for diagnosis and treatment of cancer
National Institute of Pharmaceutical Education and Research (NIPER) Ahmedabad, Palaj, Opp.Air force station headqtrs, Gandhinagar 382355, India
ABSTRACT Nanotechnology has changed the entire paradigm of drug targeting and has shown tremendous potential in the area of cancer therapy due to its specificity.In cancer,several targets have been explored which could be utilized for the better treatment of disease.Mitochondria,the so-called powerhouse of cell,portrays significant role in the survival and death of cells,and has emerged as potential target for cancer therapy.Direct targeting and nanotechnology based approaches can be tailor-made to target mitochondria and thus improve the survival rate of patients suffering from cancer.With this backdrop,in present review,we have reemphasized the role of mitochondria in cancer progression and inhibition,highlighting the different targets that can be explored for targeting of disease.Moreover,we have also summarized different nanoparticulate systems that have been used for treatment of cancer via mitochondrial targeting.
Keywords:Mitochondrial targeting Cancer Nanocarriers Photodynamic therapy Photothermal therapy
1.Introduction
Cancer is a group of disease in which abnormal cells grow and divide in a rapid way without any control.In addition,these abnormal cells are also responsible for destruction of normal body tissues [1] .According to the prevailing theory of cancer,the normal cells convert into the cancerous cells,which eventually lead to abnormal cellular,molecular and biochemical networking of normal cells.These cancerous cells also spread or metastasized from a primary location to a secondary location in the body [2] .There are some common risk factors also which promotes unwanted growth of cells like tobacco-alcohol consumption,being overweight,exposure of chemicals and other substances,poor diet,etc.Aging and family history also act as a risk factor of cancer [3] .Till date,majority of cancer patients are being treated by the surgery,radiation therapy and chemotherapy.Generally,cancer tissue is removed by the surgery and then radiation therapy is given followed by chemotherapy.But these treatment strategies possess potential drawback due to their non-selectivity,which produces harmful effects on normal tissues too.Hence,to overcome these serious drawbacks there is need of novel targeting treatment approaches [1] .
Mitochondria are the powerhouse of cells responsible for the generation of adenosine triphosphates (ATPs),which is prerequisite for the proliferation as well as other proper cellular function.Mitochondria are comprised of different types of protein that have crucial role in the induction as well as prevention of cell death.Therefore,mitochondria play a critical role in determining the fate of a cell [4] .Also,for the growth of cancer cells,functional mitochondria are the most important component because it produces the Warburg effects or aerobic glycolysis which helps in mitochondrial dysfunction that produce a favorable environment for the cancerous cells.This effect also confirms unbalanced mitochondrial metabolism due to the mitochondrial DNA (mtDNA) mutation,creates an adaptable environment for cancer cells and increases tumorigenesis.This Mitochondrial metabolism dysfunction enhances the production of mitochondrial reactive oxygen species (ROS)and alters the cellular redox activities.Thus,by changing the functions of transcriptional factors such as HIFα
and FOS-JUN,they change the gene expression and stimulate cancer cell proliferation [5] .Since past few years,the use of different types of nanocarriers for tumor targeting has emerged as a newer approach for cancer treatment.Nanocarriers possess larger surface area with an unique electronic,mechanical,photonic and magnetic properties due to their nanosize range.To overcome the limitation of the conventional drug delivery system,specialised nanocarriers have been designed to achieve targeted cancer therapy by imparting unique properties [1] .Cancer cell are able to create multidrug resistance which can be successfully overcomed by using the multifunctional nanocarriers as a targeted drug delivery carrier [6] .Nanoparticles (NPs) drug delivery has been used to reduce the side effect and improve pharmacokinetics,and have longer circulation of half life.
2.Role of mitochondria in tumorigenesis
Mitochondria play an important role in tumorigenesis which was observed by Otto Heinrich Warburg in the year 1924.He found that there was a difference in the way that glucose was metabolised by cancer cells as compared to normal cells.In case of normal cells,in presence of oxygen,the glucose is metabolized by glycolysis in cytoplasm to form pyruvate followed by its oxidation in mitochondria via tricarboxylic acid cycle (TCA).This metabolism leads to the production of carbon dioxide,NADH [reduced form of nicotinamide adenine dinucleotide (NAD),which is used as a fuel for ATP production by oxidative phosphorylation (OXPHOS)] and minimum amount of lactate.In anaerobic conditions,glucose is metabolized via glycolysis into pyruvate,due to absence of oxygen pyruvate further cannot be oxidized,so it converted into lactate.This results in the production of a large amount of lactate inside the cell.In case of cancer cells,irrespective of oxygen availability most of the cells metabolize glucose by glycolysis and leads to overproduction of lactate,this is called as“aerobic glycolysis”.Apart from this,the intermediates produced in glycolytic pathway act as a raw material for the biosynthesis of proteins,lipids and nucleotides,which further helps in the proliferation of cancer cell [7] .It is observed that mitochondria play a major role in maintaining microenvironment in tumor cells more acidic than normal cells,which is very important for cell proliferation.The main reason for this acidic environment is the enhanced metabolism of glucose into lactic acid via aerobic glycolysis.This lactic acid releases Hions inside the cell and then these Hions diffuse outside the cell and lead to a decrease in pH of the microenvironment [8] .
Targeting mitochondria via nanocarriers is an appropriate approach for cancer treatment.These nanocarriers targets mitochondria by using different fate and mechanisms which includes macropinocytosis,caveolae-mediated endocytosis,phagocytosis,clathrin-mediated endocytosis,membrane fusion,receptor and protein-mediated,mitochondrial transmembrane-potential mediated accumulation,glutathione (GSH)-triggered triphenylphosphine (TPP)exposure,ROS-accelerated cellular internalization,hydrophobic-peptide-mediated [9],etc.Yamada et al.prepared a liposome-based carrier for the delivery of macromolecular cargo into an internal part of mitochondria via membrane fusion.They identified a liposome-based carrier which endorses its fusion with the mitochondrial membrane as well as releases the cargo into an internal part of the mitochondrial membrane [10] .In one such study,Chan et al.demonstrated dual ligand functionalized nanodiamonds (ND) for the treatment of cancer which circumvents the drug resistance.They reported that multifunctional NDs not only successfully distinguished normal cells and cancer cells but could also reach to mitochondria via a receptor and protein mediated targeting mechanism and successfully deliver the drug to cancer cell plasma membrane [11] .In another study by Zhou et al.,they have synthesized paclitaxel liposomes conjugated with D-α
-tocopheryl poly-ethylene glycol 1000 succinatetriphenylphosphine as mitochondrial targeting molecule to treat the multidrug resistant lung cancer via mitochondrial transmembrane-potential mediated accumulation [12] .Mkandawire et al.prepared conjugated gold nanoparticle with 3rd generation dendrimers for the induction of apoptosis in human cancer cells by targeting mitochondria via a specialised form of phagocytosis and caveolae-mediated endocytosis [13] .Xiang et al.developed resveratrol-loaded dual function titanium disulphide nanosheet which targeted and accumulated in mitochondria for photothermal triggered tumor chemotherapy via endocytosis [14] .3.Barriers in targeting mitochondria for cancer treatment

Fig.1–Different mitochondrial targets along with the drug for their targeting.
Targeting to mitochondria directly is very difficult due to its complex structure and many cellular barriers.Mitochondria possesess complex structure which includes mitochondrial outer membrane (MOM),mitochondrial inner membrane(MIM),intermembrane space and matrix.The crossing of MOM barrier is fully dependent upon the concentration,thus molecule can pass through passive diffusion [15] .The main barrier for the targeting mitochondria for treatment is,deficiency to reach therapeutics in the mitochondrial matrix owing to lack of their structural components.The major hurdles for the small molecule crossing through membranes are strong electronegative potential,which is−160 to–180 mV and presence of unusual phospholipid cardiolipin that exist across the membrane [16] .Apart from MOM and MIM,cytomembrane and cytoplasm are the main obstacles for targeting mitochondria.Cytoplasmic aqueous phase packed with vast macromolecular species (DNA,RNA,etc.),ions and small molecules and highly viscous cytosol retard the diffusion of targeting molecule to diffuse inside cells [17] .
4.Mitochondrial targets for cancer therapy
There are various functions that a mitochondrion performs in association with channels,enzymes and proteins,energy production in the form of ATP,destruction of ROS,proper glucose metabolism,etc.In Fig.1,different mitochondrial targets along with the drugs reported to bind with these targets are shown.All these functions are important for the cell survival and growth (Fig.2).In cancer therapy,targeting these channels,enzymes and proteins associated with mitochondria can lead to destruction of cancer cells.
4.1.Targeting HKIII
Cancer cells largely depend upon aerobic glycolysis for their energy production in the form of ATP.Hexokinase (HK) is an enzyme that is responsible for catalyzing the first step of glycolysis,i.e.
from glucose to glucose-6-phosphate (G6P).There are four isoforms of hexokinase,namely HKI,HKII,HKIII and HKIV.Out of these four isoforms,HKII is highly overexpressed in the cancer cells [18] .HKII binds to voltagedependent anion channel 1 (VDAC1) present on the MOM,which facilitates the interaction of VDAC1 with adenine nucleotide translocase on the MIM (Fig.3).This interaction favours the coupling of aerobic glycolysis with OXPHOS,which creates a working relationship between the two metabolic processes.This increases the rate of glycolysis by allowing HKII to exchange ADP for ATP from the mitochondria [19] .Hence,targeting HKII could lead to stopping of aerobic glycolysis and further resulting in cancer cell death.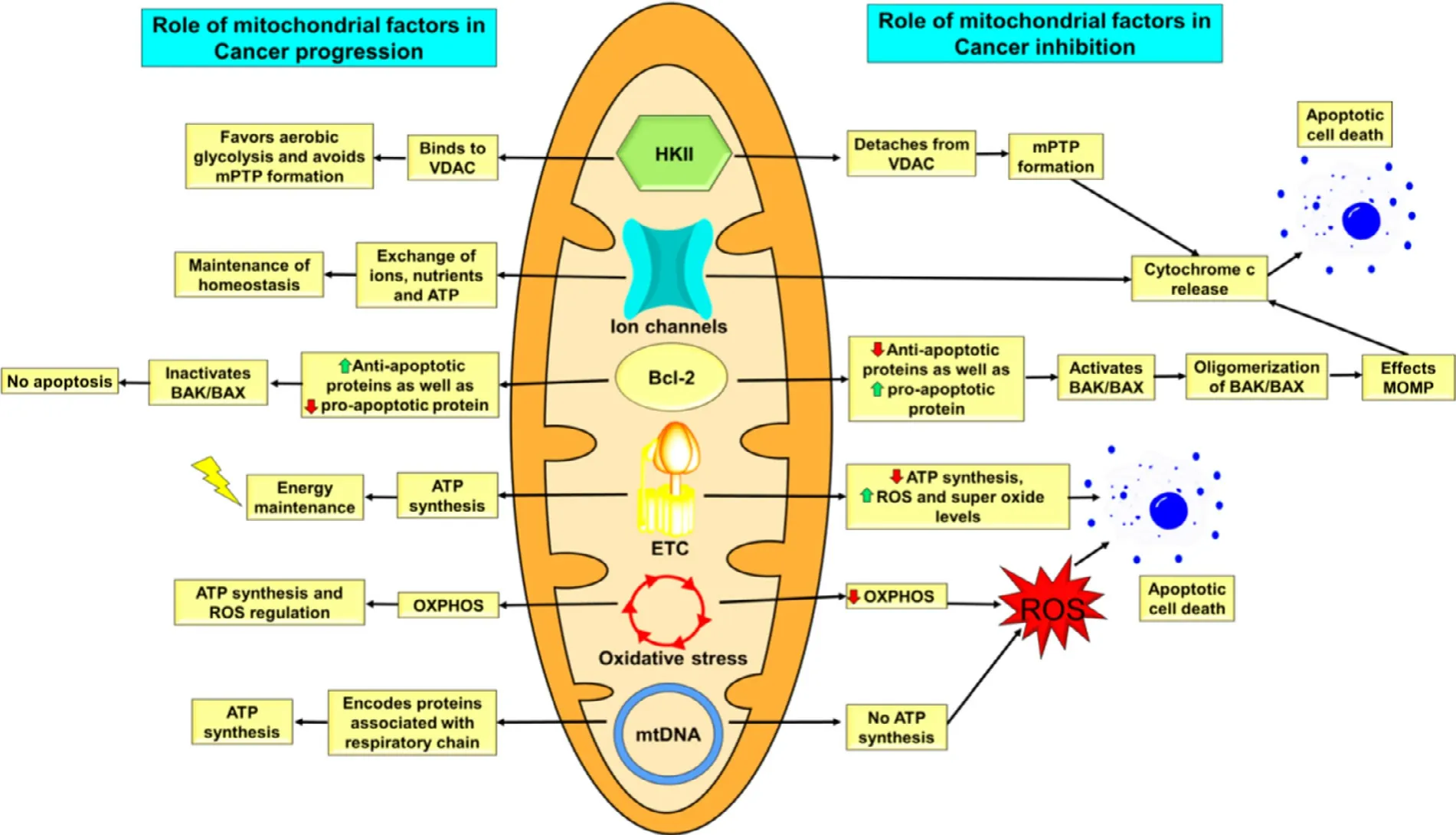
Fig.2–Overall summary of role of mitochondrial factors in cancer progression and cancer inhibition.
There are some examples of glucose analogs that used to target HKII,namely 2-deoxy-D-glucose (2-DG) and 3-Bromopyruvate (3-BPA) [20] .2-DG competes with glucose for its entry into the cell,and once gets entered into the cell,it undergoes phosphorylation by HKII to 2-deoxyglucosephosphate (2-DG-P) which cannot be used in further steps of glycolysis.Hence,2-DG-P gets accumulated inside the cells,resulting in product inhibition of HKII [21] .The binding of HKII to mitochondria is important to prevent the initiation of apoptosis.3-BPA acts as an alkylating agent and it covalently modifies sulphydryl groups of HKII.This modification leads to dissociation of HKII from mitochondria which trigger apoptosis and further cell death [20] .In a study carried out by Bao et al.showed that out of 13 steroidal derivative isolated from the mushrooms ofGanoderma
sinense
,(22E,24R) −6β
methoxyergosta-7,9(11),22-triene-3β
,5α
-diol was assumed to have a high HKII binding ability and was validated byin
vitro
microscale thermophoresis,enzyme inhibition and cell-based assays.It was the first natural compound that is reported to possess HKII inhibitory activity for cancer treatment [22] .Methyl jasmonate,an anticancer agent had shown a good binding affinity for HKII.It binds to HKII and detaches it from VDAC1,leading to mitochondrial permeability transition pore(mPTP) formation,through which cytochrome c comes out from mitochondria into cytosol.In the cytosol,cytochrome c triggers caspase activation and cell destruction [23] .4.2.Targeting ion channels
Ion channels are very helpful for any cell as they involve in the exchange of ions,fatty acids,cholesterol,etc.,and ultimately maintain the homeostasis of the cell.These ion channels are broadly classified into two classes,namely channels present on MOM and channels present on MIM.
4.2.1.Ion
channels
of
mitochondrial
outer
membrane
There are various ion channels that are present on MOM which are responsible for the entry of all the nutrients as well as the exit of many apoptosis mediators.Ion channels present on MOM are translocase of the outer membrane that is responsible for protein transport,mitochondrial apoptosis-induced channel that facilitates cytochrome c release and VDAC that transfers metabolite.These three channels help in the formation of mPTP.This mPTP facilitates the release of cytochrome c that further leads to apoptosis[24] .From the above-mentioned ion channels of MOM,VDAC is mostly targeted for cancer treatment.VDACs are also called as mitochondrial porins.These channels present on the MOM help in the transport of ions (Cl,K,Na,and Ca),ATP/ADP and NAD/NADH,fatty acids,cholesterol,ROS between inner mitochondrial membrane and cytosol through MOM [25] .There are three isoforms of VDAC that has been identified in mammals,namely VDAC1,VDAC2 and VDAC3 [26] .VDAC1 is abundantly present in the mammalian cell mitochondria and it plays a central role in mitochondrial apoptosis.It interacts with apoptosis regulatory proteins such as members of the B-cell lymphoma 2 (Bcl-2),Bcl-xL(anti-apoptotic proteins),apoptosis-suppressing glycolytic enzyme HK,etc.which inhibits the release of pro-apoptotic proteins [cytochrome c,apoptosis-inducing factor,second mitochondria–derived activator of caspases/direct IAPbinding protein (SMAC/Diablo)] to cytosol and helps in the survival of cell [19] .If the binding of apoptosis regulating proteins to VDAC1 is prevented,then it will lead to an increase in the MOM permeabilization.This will further help the pro-apoptotic proteins to cross MOM and enter into the cytoplasm where they will lead to apoptosis of cells [25] .
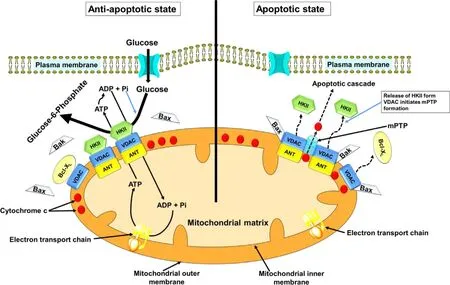
Fig.3–Regulation of apoptosis by HKII and VDAC.Right:During unfavorable conditions that exists in tumor microenvironment,caspase-mediated induction of apoptosis would be enabled by the activation of the MPTP because of release of HKII from VDAC.This pore complex is responsible for the transfer of caspase activator cytochrome c (located within the inter-membrane space).Bcl-2-related proteins (Bax and Bak) would likely overcome effects of the MPTP inhibiting protein Bcl-X L and helps in the release of cytochrome c.Left:On conjugation of HKII with VDAC,opening of the MPTP is inhibited.This causes inhibition of conjugation of VDACs to Bax and Bak,and the cytochrome c is then retained inside the mitochondria.Thus,HK II helps guarantee a highly malignant tumor’s proliferation,and its bypass from cell death,under conditions that would otherwise favor this process.
There are various compounds known as pro-apoptotic compounds that act on VDAC1 or HKII and inhibit their interaction or help in their dissociation,leading to cell death[27] .VDAC1 based peptides were synthesized namely Antp-LP4 and N-terminal-Antp,that selectively kill peripheral blood mononuclear cells obtained from chronic lymphocytic leukemia patients,but does not do any harm to normal cells obtained from healthy donors [28] .The same group of researchers had also synthesized one more cell-penetrating peptide to improve specificity,targeting and cellular stability,named as R-Tf-D-LP4.This peptide contains a transferrin receptor internalization sequence (Tf) that enhances the targeting of a peptide to cancer cells as there is an overexpression of transferrin receptors on cancer cells [29] .A study carried out by Yuan et al.observed that the endostatin causes the opening of mitochondrial permeability transition pore (mPTP) via VDAC1 and this leads to release of cytochrome c into the cytoplasm further causing a cascade of apoptosis and ending up with cell death [30] .
4.2.2.Ion
channels
of
mitochondrial
inner
membrane
MIM forms the surroundings of the matrix and is also allows the formation of an electrochemical gradient that is responsible for ATP generation.There are many ion channels that are present on the MIM which are responsible for the exchange of ions,the exit of ATP and cytochrome c from mitochondria to the cytoplasm [31] .These channels help in maintaining homeostasis of mitochondria and also regulates apoptotic cascade by regulating the release of cytochrome c in the cytoplasm.These ion channels are discussed below in more detail.
4.2.2.1.Mitochondrial
permeability
transition
pore
(mPTP).
mPTP is a pore that is commonly believed to be responsible for increasing the MIM permeabilization usually referred to as mitochondrial permeability transition (MPT) [32] .MOM has a highly complex structure consisting of VDAC and some proteins like HKII,while MIM consisting of adenine nucleotide translocase (ANT) and cyclophilin D (CyP-D)[33] .These channels get opened,when there is a high calcium ion concentration inside mitochondria and also at a time when there is ROS production inside mitochondria.Opening of mPTP causes depolarization,further leading to decrease of mitochondrial membrane potential that affects ion homeostasis,respiratory chain complexes activity and ATP production [27] .Binding of HKII with VDAC prevents the formation of mPTP in two different ways.First,the binding of HKII to VDAC causes conformational changes in VDAC,which further leads to conformational changes in ANT.These changes are not suitable for the formation of mPTP but it creates a passage through which ATP can be transferred.Second,binding of HKII to VDAC prevents the binding of Bax and other pro-apoptotic proteins to VDAC.Hence,VDAC cannot form its oligomeric structure which is necessary for mPTP activation [33] .Prevention of binding of HKII and other pro-apoptotic proteins to VDAC is necessary for the formation of mPTP which will lead to cell death.Furthermore,the reduced form of CyP-D closes mPTP pore,whereas oxidized from is responsible for its opening.In a study carried out by Floda et al.showed that auranofin,an oxidizing agent,increases the oxidation CyP-D in human leukemic T cells [34] .4.2.2.2.Mitochondrial
calcium
uniporter
(MCU)
complex.
Mitochondrial intra-cellular calcium ion (Ca) level plays a very noteworthy role in pathophysiological conditions.Normal Calevels are responsible for the proper regulation of the TCA cycle,stimulating oxidative phosphorylation,whereas high Cacauses apoptotic cell death by the opening of Mptp [35] .Once the Caions released from the endoplasmic reticulum into the cytosol,it can enter the mitochondria by crossing two membranes.It enters mitochondria through VDAC1 that are present on MOM,then it enters inside the mitochondrial matrix with the help of a complex containing pore-forming subunits MCU and MCUb(MCU isoforms ‘a’ and ‘b’) along with regulatory proteins like mitochondrial calcium uptake protein 1 (MICU1),MICU2,and EMRE (essential MCU regulator).MICU1 acts as an activator,whereas MICU2 acts as an inhibitor of MCU [27] .In normal conditions,both MICU1 and MICU2 acts as a heterodimer and are attached to MCU thereby it regulates Caentry inside the mitochondria.As cytosolic Calevel increases,this induces conformational changes in the dimer and MICU2 gets detached,leaving MCU-MICU1 complex.This complex gets stabilized by EMRE,and further,it allows the entry of Caions inside mitochondria [27] .Cais very essential for the proper functioning of mitochondria for ATP generation.A high amount of Caions because of the upregulation of MCU complex,leads to apoptosis;whereas,low Calevels resulting from downregulation of MCU complex,causes inefficient energy production.Hence,a balance should be maintained for the proper functioning of mitochondria.Therefore,the upregulation and downregulation of the MCU complex can be a possible way to eliminate cancer cells [36] .Bortezomib treatment for multiple myeloma causes an increase in the expression of MCU complexes,more Calevels in mitochondria followed by more ROS production and changes in the membrane potential [37] .
4.2.2.3.Voltage-dependent
potassium
channels.
Voltagedependent potassium channels (Kv) consist of a sixtransmembrane helix and a pore.There are total 12 families of Kv channels from Kv1 to Kv12,and each family has many isoforms.Amongst all the isoforms,Kv1.3 is most abundant in T lymphocytes,central nervous system,epithelial cells,kidneys,etc.[38] .Their presence on the plasma membrane governs cell proliferation;whereas when they are present on MIM,they are involved in apoptosis due to their ability to interact with pro-apoptotic Bcl-2 associated X (Bax) in lymphocytes [39] .Kv1.3 is overexpressed in cancer cells as compared to normal cells and by which they help the cancer cell in proliferation,migration and metastasis [27] .Inhibiting these channels will help in eliminating the cancer cells.Mostly permeant inhibitors (Psora-4,PAP-1 and clofazimine)were studied,which have the ability to cross the plasma membrane and have an effect on the MIM Kv1.3 channel.The inhibition of MIM Kv1.3 leads to excessive ROS production,followed by the opening of permeability transition pore,leading to depolarization of MIM,swelling of mitochondria,the release of cytochrome c and finally apoptosis [27] .In a study carried out by Zaccagnino et al.,it was observed that clofazimine was able to kill pancreatic ductal adenocarcinoma(PDAC) cellsin
vitro
and also it was helpful in reducing the tumor sizein
vivo
in a severe combines immunodeficient(SCID) mouse orthotopic xenograft PDAC model by 50% [40] .4.2.2.4.Ca
-dependent
channels
of
mim.
There are certain ion channels located on MIM,which are regulated by Caions and are referred to as Ca-dependent channels.One such channel is Ca-dependent potassium channels (K Ca),which are situated on the plasma membrane of both excitable and non-excitable cells [27] .Based upon their conductance,they are classified into three families:big-conductance potassium channel (BK Ca ;K Ca 1.1 or Slo1 or MaxiK),intermediateconductance potassium channel (IK Ca ;K Ca 3.1 or SK4 or IK1)and small-conductance potassium channels (SK Ca :K Ca 2.1,2.2,2.3 or SK1,2,3) [41] .BK Ca (K Ca 1.1) is highly expressed in human glioma cells.In a study carried out by Debska-Vielhaber et al.on LN229 human glioma cells,it was shown that,CGS7184 (ethyl 1-[[(4-chlorophenyl)amino]oxo] −2–hydroxy-6-trifluoromethyl-1H-indole-3-carboxylate) opens BK Ca (K1.1)channel.This causes increased cellular respiration,followed by mitochondrial membrane depolarization and further leads to cell death [42] .4.3.Targeting Bcl-2
Bcl-2 is a protein,belonging to the anti-apoptotic B-cell lymphoma 2 family,which contribute in tumor initiation,progression,apoptosis prevention and lack of response to chemotherapy [43] .Based upon their structure and function,Bcl-2 can be categorized as anti-apoptotic and pro-apoptotic members.Anti-apoptotic members contain four Bcl-2 homology domains (BH1,BH2,BH3,BH4) that includes Bcl-2 (the founding member),Bcl-X L,Bcl-w,Bcl-2-related protein A1 (Bfl-1/A1),myeloid cell leukemia 1 (MCL-1),and BCLB/Boo protein [43] .The pro-apoptotic members can be further divided into two subfamilies:the multi-domain pro-apoptotic ‘effectors’ (such as BAK,BAX and BOK) and those members which are known as ‘BH3-only proteins’(BAD,BID,BIK,BIM,BMF,HRK,PUMA,and NOXA),as they possess only the short BH3 domain [44],as shown in Fig.4 .Both pro-and anti-apoptotic proteins regulate apoptotic cell death.These proteins have a direct effect over the MOM permeabilization (MOMP).Activation of BAK/BAX leads to oligomerization of BAK/BAX,which forms a pore and thus,increases mitochondrial permeability leading to a cascade of events that eventually leads to apoptosis.Bcl-2 like antiapoptotic proteins inhibit the activation of BAK/BAX,which prevents apoptosis;whereas BH3-only proteins encourage oligomerization of BAK/BAX,leading to apoptosis [45] .In the case of cancer,anti-apoptotic proteins are increased;whereas pro-apoptotic proteins are found to be decreased.Recently,BH-3 mimetic compounds were developed and tested for their anticancer activity.ABT-263 (navitoclax) is an orally active BH-3 mimetic compound that selectively inhibits Bcl-2 and showed good results in clinical studies [19] .Venetoclax,by selectively targeting Bcl-2,was orally active against patients with relapsed small lymphocytic lymphoma (SLL) or chronic lymphocytic leukemia (CLL) [46] .
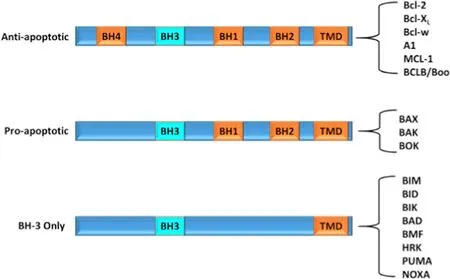
Fig.4–Different domains of B-cell lymphoma 2 family.Mammalian Bcl-2 proteins are categorised into three subclasses based on their function and the number of Bcl-2 homology (BH) domains:anti-apoptotic proteins,pro-apoptotic and BH-3 only proteins.Most of the members possess a C-terminal hydrophobic transmembrane domain(TMD) that can anchor proteins in the mitochondrial outer membrane.
4.4.Targeting electron transport chain complexes
Electron transport chain (ETC) acts as a central player for energy production by oxidative phosphorylation in mitochondria.ETC consists of five complexes namely CI,CII,CIII,CIV and CV that are embedded in the MIM.These complexes are responsible for energy production in the form of ATP.CI,CIII and CIV help in transportation of electron from NADH and succinate to molecular oxygen that forms water molecule.CI and CII transfer the electron to CIII via electron carrier’s ubiquinone (UbQ),followed by its transfer to cytochrome c.Cytochrome c transfers these electrons to CIV,which converts molecular oxygen into water molecules with the help of these electrons.During this process,energy is generated which is stored in the form of electrostatic and proton gradient across IMM.CV (the F1FO-ATPase) uses this energy for the conversion of ADP and inorganic phosphate into ATP,which provides the cell with its fundamental energy substrate [47] .Along with ATP,ETC also produces ROS,plus CI and CIII are primary sites for superoxide generation,which is responsible for maintaining cellular signaling.Higher levels of ROS and superoxide leads to apoptosis [47] .By inhibiting CI,CII and CIII,apoptosis can be induced leading to cancer cell death.A study carried out by Andrzejewski et al.showed that metformin which was used for the treatment of type II diabetes had also shown good anticancer activity by acting on ETC CI of mitochondria [48] .CII and CIII were inhibited by sorafenib (Nexavar○) along with inhibition of ATP synthase,causing activation of serine–threonine protein kinase PINK1-perkin pathway,further leading to Parkin-mediated apoptosis and cell death [49] .
4.5.Oxidative stress targeting
OXPHOS is one of the ATP producing mechanism in human beings.This mechanism is also responsible for controlling ROS in the mitochondria.Hence,it regulates apoptotic cell death.Targeting OXPHOS will lead to increased ROS generation plus decreased ATP production which ultimately initiates the cascade mechanism of apoptosis leading to cell death [50] .Targeting is done by using pro-oxidant agents that induce oxidative stress by generating ROS.Hu et al.demonstrated that rotenone causes activation of NOX-2 complex leading to excessive ROS generation through PI3K/Akt/mTORC1 signaling pathway,leading to cell death[51] .
4.6.mtDNA
mtDNA is double-stranded,circular DNA.There are more than 1000 mitochondrial proteins present,out of which,13 are encoded by mtDNA and remaining ones are encoded by nDNA (nuclear genome).These 13 proteins are associated with the respiratory chain,so increasing or decreasing the expression of mtDNA in tumor cells can interfere with energy production.Hence,mtDNA can be a good target for treating cancer.Vitamin K3 causes the inhibition of mtDNA polymeraseγ
,leading to cytotoxicity in cancer cells [52] .Table 1 .Briefly summarizes different mitochondrial targets used for therapeutics targeting.5.Nanocarriers for mitochondrial targeting in diagnosis and treatment of cancer
NPs have received much attention in recent years,especially for treatment of cancer due to their capability of enhancing safety,pharmacokinetic profile and bioavailability of chemotherapeutic agents [74] .The nanocarriers employed for mitochondrial targeting are enlisted in Table 2 .

Table 1–Briefly summarizes different mitochondrial targets used for therapeutics targeting.

Table 1 (continued)
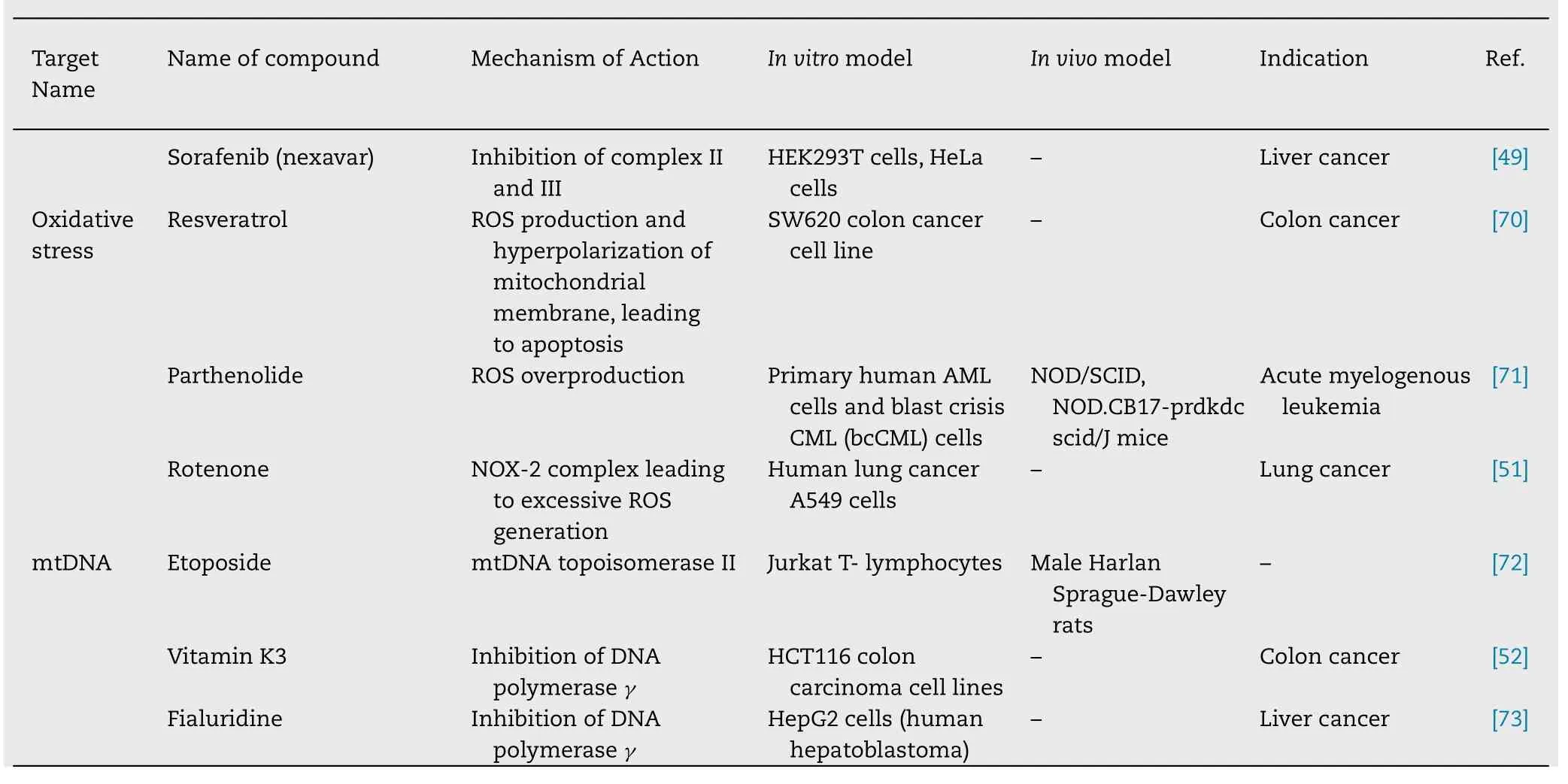
Table 1 (continued)
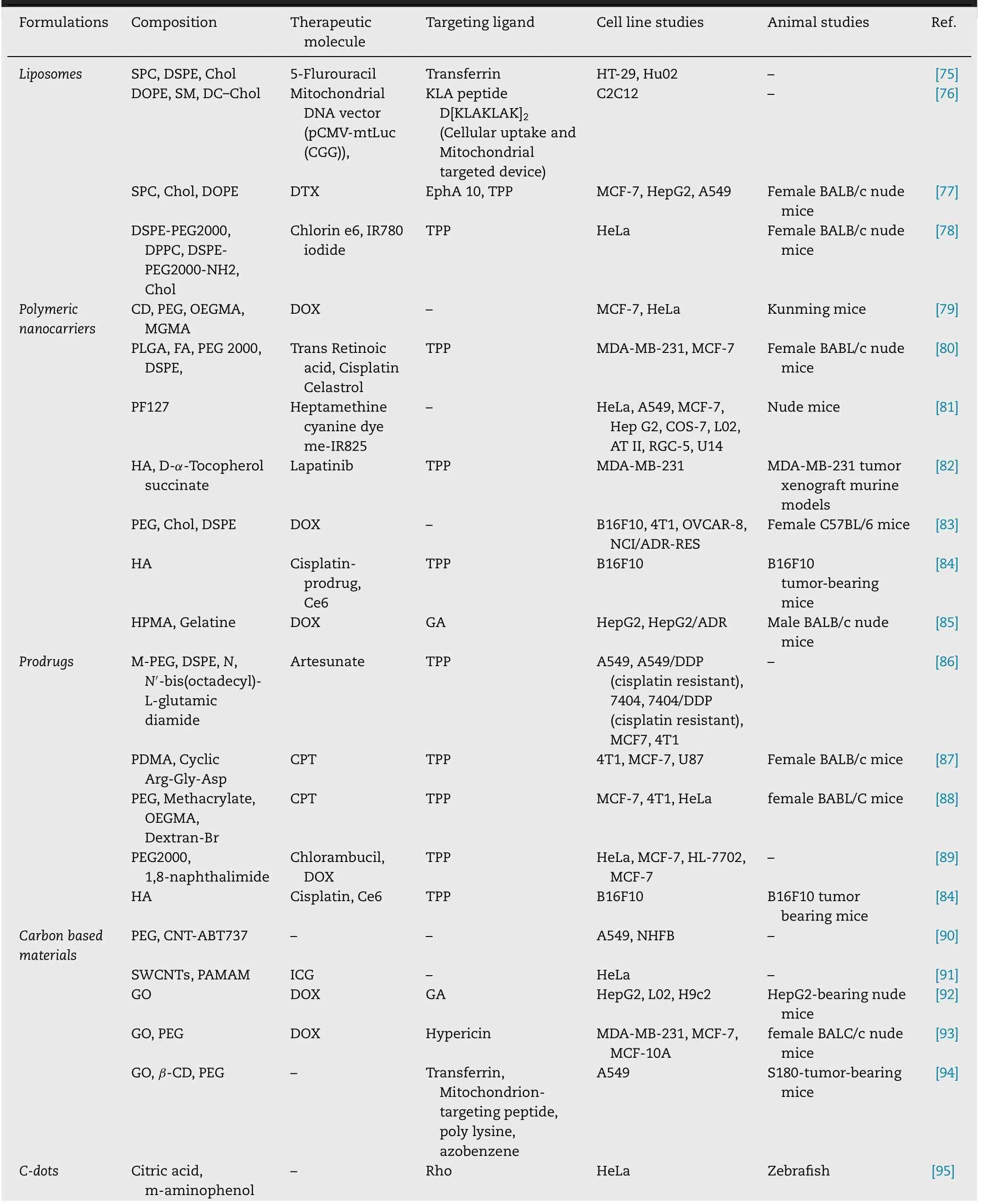
Table 2–The nanocarriers employed for mitochondrial targeting.
Table 2 (continued)
5.1.Carbon based materials
Carbon based nanomaterial such as carbon nanotubes(CNTs),graphene derivative and carbon dots (C-dots) have attracted significant attention over past decade because of their fascinating performance in biomedical field such as targeted drug delivery,chemo-photothermal therapy and real time bioimaging.Carbon based material have some unique physiochemical properties such as biocompatibility,supramolecularπ
-π
stacking,high adsorption capacity and excellent photoluminescence [102] .These materials are widely employed for targeting to mitochondria to kill cancer cells.The chemically functionalized CNTs show promising tumor-targeted accumulation,exhibit low toxicity,and good biocompatibility [103] .One of the early examples of mitochondrial targeting CNTs is peptidebased multi-walled CNTs (MWCNTs).The mitochondrial targeting sequence (MTS) functionalized MWCNTs were able to undergo selective accumulation in mitochondria of macrophages and Hela cell lines which was confirmed by different microscopic techniques such as confocal laser scanning microscopy,transmission electron microscopy,and fluorescent microscopy.The obtained results indicated that no significant cytotoxicity was produced by MTS functionalized MWCNTs,which further showed their promising utilization potential as a nanocarrier [104] .Recently,Wu et al.fabricated mitochondrial-targeted nearinfrared (NIR) light activable multitasking nanographene(GT/IR820/DP-CPG) forin
situ
trigger efficient triple punch approach for photodynamic therapy in cancer.They designed strategy based upon the modification of TPP on graphene which can serve as a carrier to deliver IR820 (photosensitive dye) to mitochondria.The photoactive compound generates ROS and photothermal heat to kill cancer cells by inducing mitochondrial collapse and cell apoptosis after irradiation of NIR laser.After the administration of immunostimulatory conjugate DP-CpGin
vivo,
they will significantly enhance the secretion of proinflammatory cytokine like interleukin-6,tumor necrotic factorα
,interferonγ
and remarkably increases immunogenicity of tumour.In
vivo
results demonstrated that GT/IR820/DP-CpG shows significant inhibition of tumor growth (tumor inhibition rate approximately 88%) which resulted from the combination of the photothermal effect of IR 820 and immunostimulatory activity of DP-CpG (Fig.5) [105] .5.2.Liposomes
The liposomes are enclosed globular vesicles made up of lipid bilayer with internal aqueous core region.Both hydrophobic and hydrophilic drugs can be loaded in lipid bilayers and aqueous core region respectively.Biswas et al.fabricated novel PEGylated phosphatidylethanolamine (PEGPE) conjugate with TPP attached with distal end of PEG block (TPP-PEG-PE).These conjugates were incorporated into the liposomal lipid bilayer and these modified liposomes were studied for their cytotoxicity,mitochondrial targeting capability and efficient delivery of paclitaxel (PTX) to cancer cell.The PTX loaded PEG-PE-TPP liposomes (TPP-PEG-L-PTX)showed higher PTX mediated cytotoxicity and anti-tumor action in bothin
vitro
andin
vivo
models as compared to PTX loaded unmodified liposomes (PL-PTX) (Fig.6)>
[106] .Boddapati et al.formulated targeted liposomes using stearyl TPP (STPP) bromide by film rehydration method followed by ultrasonication.By encapsulating rhodamine-PE dye,these liposomes were tracked in BT 20 mammalian breast cancer cell lines.From the obtained results,they found that liposomes were selectively co-localized in mitochondria [107] .Utilizing mitochondrial capability of STPP,Malhi et al.developed novel DOX loaded liposome for mitochondrial targeting with surface-functionalized with the dual ligand,folic acid (FA) for selective cancer targeting and TPP cation for mitochondrial targeting.The cytotoxicity,ROS generation study,and cellular uptake study of DOX loaded liposome were carried out by using KB cell.These dual-targeted liposomes produce more cytotoxicity and ROS generation as compared to non-targeted ones [108] .5.3.Polymeric nanocarriers
The polymeric nanocarriers made up from biocompatible and biodegradable polymer can serve as the most promising drug delivery system.By using an engineered composition of the hydrophilic and hydrophobic block,these polymers can be used to encapsulate both water-insoluble and watersoluble drugs to improve the bioavailability,retention time and aqueous solubility [109] .The polymeric NPs have many advantages such as higher stability and more controlled payload release.The most widely used biocompatible and biodegradable hydrophobic polymeric blocks are poly (lactic acid),poly(glycolic acid),and their co-polymer poly(lacticco-glycolic acid) and polycaprolactone.Most widely used,Food and Drug Administration (FDA) approved hydrophilic block polymer is PEG.The PEG has some unique features such as it prolongs retention time in body and allow further functionalization with drug or targeting moiety [110] .
Liu et al.designed selective mitochondrial targeting NPs system to overcome multi drug resistance (MDR) through enhanced ROS generation.Initially,doxorubicin (DOX) was conjugated to glycyrrhetinic acid (GA) and then attached to N-(−2-hydroxypropyl) methyl acrylamide (HPMA) polymer backbone via hydrazine bond (P-DOX-GA).Furthermore,(PDOX-GA) was grafted on the surface of gelatine NPs (GNPs).The GNP-P-DOX-GA would stay stable in blood circulation and then be degraded by extracellular tumor metal matrix protease 2 (MMP2) thereby releasing small size P-DOX-GA.The released P-DOX-GA was internalized by tumor cell through GA mediation and delivered to lysosome,DOX-GA get separated from HPMA polymer through hydrolysis of hydrazine bond at lower lysosomal pH.The separated DOX-GA could efficiently target mitochondria.As compared to the non-GA modified carrier,GNP-P-DOX-GA demonstrates nearly 4 times increased cellular uptake nearly 8.8 times mitochondrial targeting,and approximately 3 times increased ROS production level,thus causing significant decrease efflux rate in drug-resistant HgpG2/ADR cells.It also showed improvedin
vivo
antitumor activity on HgpG2/ADR heterotopic tumor nude mice.GNPP-DOX-GA based drug delivery system would be a promising approach for Anti MDR treatment and further enhanced ROS generation by mitochondrial targeting will be an effective approach to overcome tumor MDR [85] .5.4.Inorganic nanoparticles
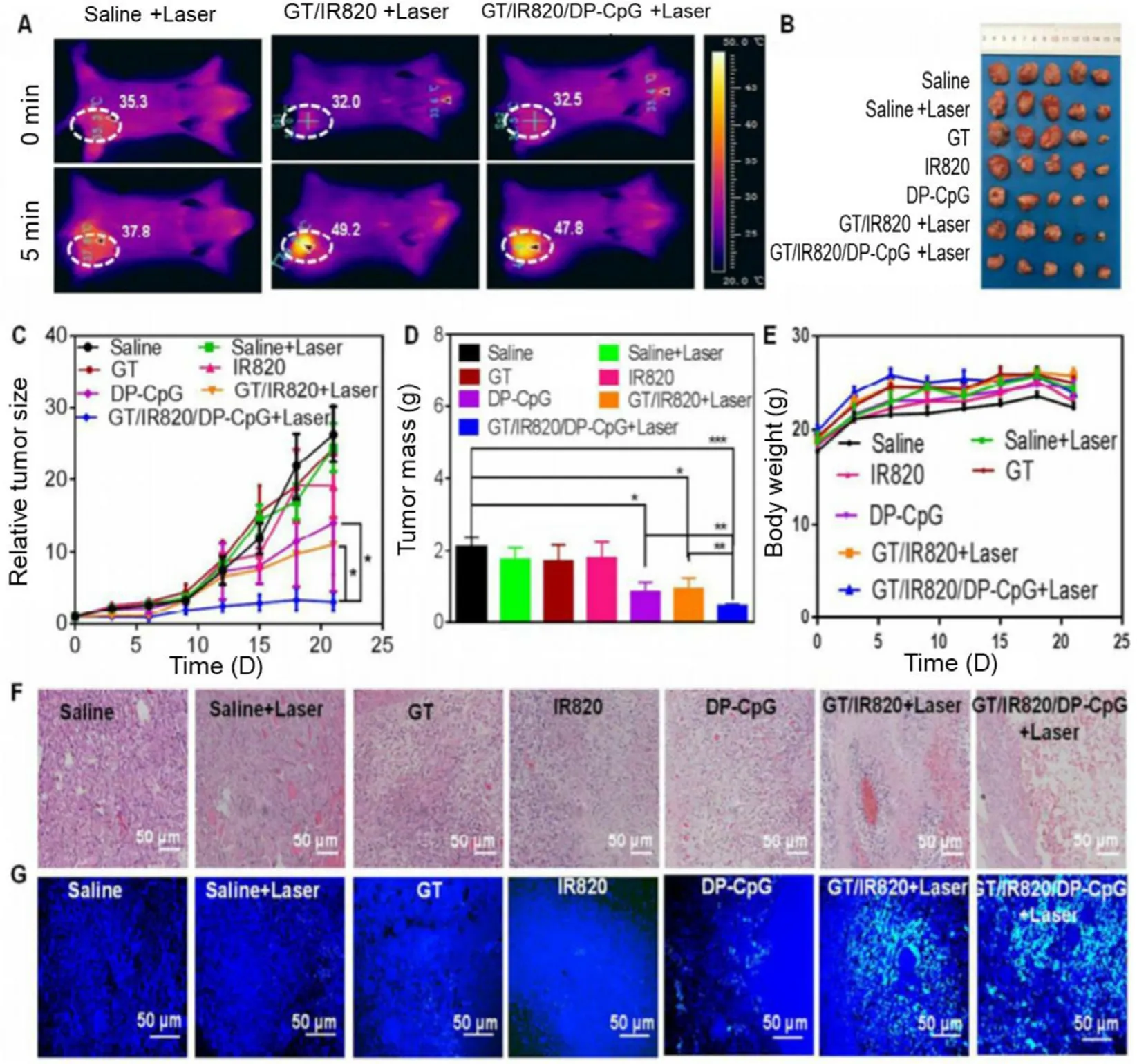
Fig.5–In vivo PTT effect of GT/IR820/DP-CpG in EMT6 tumor bearing mouse model.(A) Thermal images of mouse treated with saline,GT/IR820 and GT/IR820/DP-CpG before and after laser light irradiation.The represented circle shows size of the tumor.(B) Isolated tumor at the end of the treatment.(C) Tumor growth curve of different groups at the end of the treatment.(D) Images of the tumor weight after the treatment.(E) Comparative body weight of different groups of tumor bearing mouse after the treatment.(F) Haematoxylin and Eosin staining of different treatment group.(G) TUNEL staining images of tumor slices isolated form mice treated with saline (control group),saline plus laser (control+laser),GT,IR820,DP-CpG,GT/IR820 +laser and GT/IR820/DP-CpG.

Fig.6–Cytotoxicity of different formulations of PTX in HeLa and 4T1 cells.(A and B) HeLa and 4T1 cells were incubated with PL-PTX and TPP-PEG-PTX with different concentration of PTX at 24 h.(C and D) indicates cells were treated with 650 ng PTX in different formulations at 48 h.

Fig.7–(A) PTT imaging of tumor tissue at different time point after mouse treated with PBS,Lys Au and TPP-Au;PDT images of adjacent normal tissues and tumor tissue,when mice were treated with TPP-Au;whereas A represent adjacent normal tissue and T represents tumor tissue.(B) Demonstrates increased temperature of various groups after increasing laser irradiation time.(C) Schematic illustration of laser irradiation technique.This provides the boundary between adjacent normal tissue and tumor tissue.(D) Increased temperature of tumor tissue in comparison to normal tissue with increased laser irradiation time.(E) Represents the temperature of tumor tissue and normal tissue after the 10 min laser light irradiation.
The inorganic NPs possess an advantage due to their smaller and more uniform particle size as compared to organic NPs.Till date,number of inorganic materials have been used to prepare mitochondrial targeting NPs.Hydroxyapatite(HAP) [Ca(PO)(OH)] is one of the main constituents of mammalian hard tissue,which shows biocompatibility and high drug loading capacity.Most importantly,HAP NPs enter mitochondria of tumor cells and induces apoptosis via changing mitochondrial membrane potential and leakage of cytochrome c [111] .Sun et al.synthesized rod-shaped HAP NPs (approximately 10 nm in width and 50 nm in length) by aqueous precipitation method.The cellular uptake of HAP NPs takes place via caveolae-mediated endocytosis in both lung cancer cell lines (A459) and normal bronchial epithelial cells (16 HBE).But importantly,more NPs are uptaken by A459 cell and caused sustain increase in intracellular calcium in contrast to the transitory increase in normal cells (16 HBE).This further caused increased cellular uptake and increased intracellular calcium which resulted in nearly 40% tumor growth inhibition in a lung cancer nude mice model without any side effects [112] .Ma et al.designed TPP functionalized gold NPs (TPP-Au) for selective mitochondrial targeting.They found that TPP-Au NPs selectively accumulated in mitochondria of tumor cells,and subsequent interparticle plasmonic coupling effect was responsible for hyperthermia in mitochondria and subsequent antitumor effect.But in normal cells,negligible amount of accumulation of TPP-Au NPs takes place,which is not sufficient to reach threshold for plasmonic effect as well as hyperthermia.As a consequence of this,irradiation would not damage normal tissue.Thein
vivo
study demonstrated that an increase in temperature after irradiation was found approximately 4 times greater than normal tissue,which proves that TPP-Au NPs shows high selectivity for cancer therapy with fewer side effects[113] (Fig.7).5.5.Dendrimers
Dendrimers are a hyperbranched,star-shaped macromolecule that are in nanometre in size range.It exhibits some exclusive features such as high aqueous solubility and drug encapsulation capacity,biodegradability,low toxicity,high retention time,specificity and allows surface modification.Biswas et al.reported,for the first time,novel mitochondrial targeted 5th generation poly(amidoamine) (PAMAM)dendrimer (G-(5)-D).To achieve this goal,initially,the fraction of cationic charge of G-(5)-D was neutralized by partial acetylation of primary amine groups (G-(5)-D-Ac) and at last TPP was conjugated on the surface of G-(5)-D-Ac via acid amine coupling reaction (G-(5)-D-Ac-TPP) [114] .Wang et al.fabricated TPP modified dendrimer as a polymeric vector for efficient gene delivery to mitochondria.The TPP conjugated dendrimer could effectively target mitochondria and showed a higher amount of gene transfection capacity and low cytotoxicity in HeLa and COS-7 as compared to commercially available transfection reagent SupreFect and comparable efficiency to Lipofectamine 2000 [115] .MDR is a major leading problem in the failure of PTX chemotherapy.To avoid this problem,Ma et al.fabricated glucose transporter-mediated and MMP-2 triggered mitochondrial-targeted conjugate.The conjugate composed of PAMAM dendrimer core co-modified with TPP via amido bond and PTX disulphide bond and further conjugation was done with long circulating PEG layer via MMP sensitive peptide (GPLGIAGQ).It was reported that the conjugate was able to reach to mitochondria via TPP followed pathway and releases PTX by the breakdown of the disulphide bond.The conjugate selectively accumulates in mitochondria and shows higher cytotoxicity in MCF-7/ADR cell,which significantly reverse of MDR of MCF-7/ADR cells[116] .
6.Peptides and their role in mitochondrial targeting
Mitochondrial targeted peptides are small biomolecules consist of numerous amino acids ranging from 2 to 50,and that are linked by a peptide bond.Apart from this,functional modification with a targeting moiety,the therapeutic peptide demonstrates better targetability and therapeutic efficacy[117] .Peptide shows faster elimination rate because of their rapid renal clearance due to enzymatic degradation.They have some significant advantages such as small size,easy to synthesize and modify,good tumor targeting and penetrating capacity and biocompatibility.They can be used as a directly cytotoxic agent through several mechanisms or can specifically act as a carrier for the various cytotoxic agent by targeting specific organ [118] .Bae et al.reported MTS-hybrid cationic oligopeptide (MTS-H 3 R),which shows double roles of mitochondrial targeting carrier along with anti-tumor activity.MTS-H 3 R 9 demonstrates significant cellpenetrating and internalization activity,efficient endosomal escape tendency and improved mitochondrial targeting ability in the HeLa cell line as compared to unmodified MTS.Most importantly,MTS-HRcauses cell death by a higher amount of ROS generation and a decrease in mitochondrial membrane potential as well as an increase in anticancer activity in 3D spheroid and 2D cell culture model.MTS-H 3 R 9 provides dual potential both as a carrier for targeted drug delivery and anticancer agent for cancer treatment [119] .
7.Prodrug as a promising strategy for treatment of cancer via mitochondrial specific drug delivery system
Prodrugs are bio reversible derivative of a drug molecule that must undergo enzymatic and chemical transformation into active parent molecule with the objective to overcome the physiochemical,biopharmaceutical and pharmacokinetic barriers.These barriers include solubility,stability,permeability,pre systemic metabolism,and targeting limitation [120] .It is estimated that about 10% of drugs approved worldwide can be classified as prodrugs.Prodrugs are chemically modified versions of pharmacologically active agents that must undergoin
vivo
metabolic bioconversion to release active drugs.Non-toxic prodrugs that are activated by tumor microenvironment along with photodynamic therapy show synergistic anticancer activity to achieve this goal [121] .Wang et al.designed a mitochondrial-targeted camptothecin(CPT) polyprodrugs system for tumor-specific drug delivery.The system consists of an amphiphilic polyprodrug of dextran P (OEGMA-co-CPT-co-TPP) (DCT).The disulfide bond in polyprodrug is cleaved by intracellular reductants such as glutathione and leads to the release of active drugs.CPT molecule in polyprodrug system shows enhanced DNA damage and mitochondrial apoptosis which further accelerate apoptosis and cell death as compared to nontargeted prodrug.The mitochondrial-targeted polyprodrug demonstrates increased therapeutic efficacy by inhibiting tumor growth and promoting the cancer cell apoptosis (Fig.8)[88] .To avoid the problems associated with polymeric drug conjugate,Chen et al.fabricated mitochondrial-targeted NPs based on the modification of long aliphatic chain methoxy–polyethylene glycol-distearyl phosphatidylethanolamine(mPEG-DSPE) with artesunate-N,N-bis(octadecyl)-Lglutamic diamide (ART-LGC12) prodrug and this fabricated prodrug self-assembled to the NPs with further surface modification of NPs facilitated by TPP (NPs-TPP).NPs-TPP demonstrates significant enhancement of cytotoxicity compared with free ART in various cancer cell line including cisplatin-resistant cancer cells [122] .8.Immunotherapy based approaches in mitochondrial targeting
Cancer immunotherapy is quickly growing to the fourth most important cancer therapy after chemotherapy,surgery and radiation therapy.It is also referred to as biologic therapy,which boosts the body’s natural defence system to target and eradicate cancer cells thus resulting in durable anticancer action,reduce metastasis and recurrence of cancer.In contrast to other cancer treatment,immunotherapy primarily aims to prevent metastatic spread of cancer and to improve the quality of life of patients [123] .Approaches that are applied in immunotherapy are based on stimulation or complementation of immunity system via plethora of compound such as lymphokines,vaccines and antibodies[124] .Marrache et al.reported that stimulation of host immune response can be done by using light activated NPs containing zinc phthalocyanine (ZnPc) as photosensitizer.The NPs (T-ZnPc-NPs) were fabricated by encapsulation of ZnPc into biodegradable polymer i.e.poly (D,L lactide-co-glycolide)-b-poly(ethylene glycol)-TPP (PLGA-b-PEG-TPP).Thein
vitro
results demonstrated that upon 660 nm laser irradiation,TZnPc-NPs generate tumour antigen form breast cancer cells to activate dendritic cell to produce high level of interferongamma.The remarkableex
vivo
dendritic cell stimulation ability of this tumour cell supernatant was responsible for interleukins (IL) −12 and IL-18 autocrine effect.Theseex-vivo
studies suggested that secreted compound from T-ZnPc-NPs treated cancer cells also might be useful as cancer vaccines[125] .The checkpoint blockade-based cancer immunotherapy has recently emerged as promising approach for cancer treatment.But its clinical application has been obstructed by poor tumor penetration of nanocarriers and activation of immune response.To overcome these obstacles,Dai et al.designed tumor acidity-responsive,mitochondrial nanocomplex co-loaded with programmed cell death ligand 1(PD-L1)-blocked siRNA and photosensitizer for synergistic combination of PDT and immunotherapy [126] .
9.Stimuli-responsive mitochondrial targeting for diagnosis and treatment of cancer
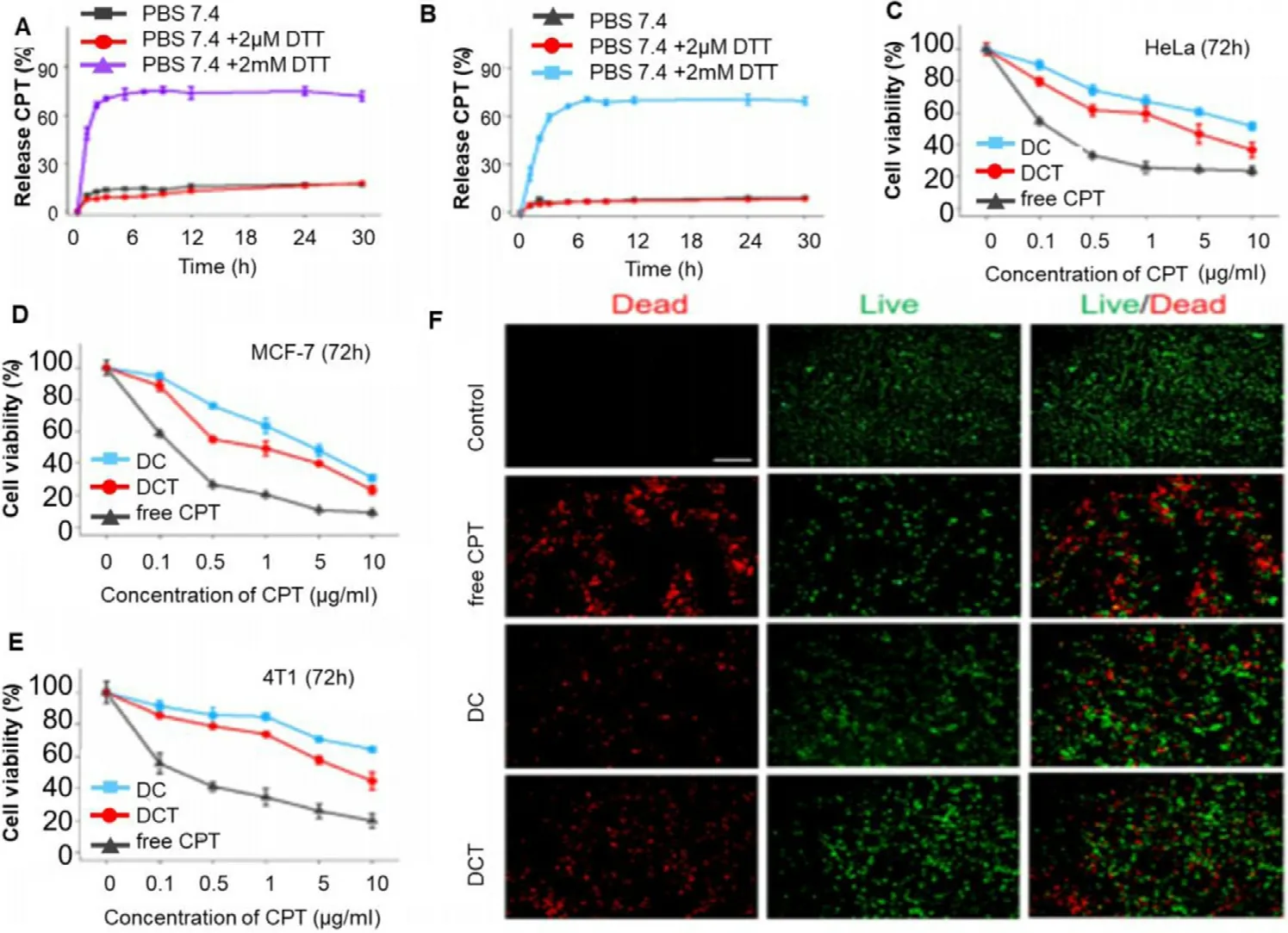
Fig.8–Demonstrates in vitro release behaviour,cell viability and apoptosis.CPT release curve of (A) DC and (B) DCT in PBS with different DTT concentrations (0,2 μM,2 mM).The cell viability assay of (C) HeLa cell line,(D) MCF-7 cells and (E) 4T1 cells incubated with free CPT,DC micelles and DCT micelles at different concentration for 72 h.(F) Dead and live cell staining of HeLa cells incubated with free CPT,DC micelles and DCT micelles with CPT concentration 30 μg/ml for 24 h at 37 °C.Untreated cells were used as control group.
Stimuli responsive drug delivery system releases the drug only when they are triggered by certain stimuli like ultrasound,light,external magnetic field,pH or enzyme.These systems help in delivering the drug at tumor site and also avoid its leakage from nanocarrier.These systems possess theranostic function (both therapeutic as well as diagnostic function)[127] .
9.1.Ultrasound mediated mitochondrial targeting
Ultrasound is used as a stimulus to release the drug from nanocarriers because it possesses minimal invasiveness along with deep tissue penetration as well as it is cheaper and nonradioactive.Also,it does not cause any harm to neighboring cells [128] .Sonodynamic therapy is one of the approaches,which is based on ultrasound waves.In sonodynamic therapy,when a high energy wave is bombarded on sonosensitizers(agents that get activated by ultrasound),they get activated and then they transfer energy to nearby oxygen molecules that ultimately lead to ROS generation [129] .Zhang et al.carried out a study by taking IR780 as sonosensitizer and preparing its nanodroplets.In
vitro
studies demonstrated that IR780-nano droplets had shown mitochondrial targeting,and cancer cell death was observed due to apoptosis induced by overproduction of ROS in mitochondria [130] .9.2.Redox triggering as a theranostic approach for mitochondrial targeting
For the survival as well as for proliferation of cancer cell,it is very much necessary for them to regulate their microenvironmental conditions like low pH,high level of GSH and ROS,and some more special enzymes which are not present in normal cells [131] .A high level of GSH in tumor tissue is responsible for providing a reduced environment.In the case of disulphide bond containing redox triggered drug delivery system (nano-drug delivery system),the disulphide bond gets reduced to sulphydryl groups by GSH present in tumor cells,leading to bond breakage and release of drug from nanocarrier [132] .Zhou et al.prepared PTX loaded lipid-polymer hybrid NPs that contain PLGA,TPP-containing amphiphilic polymer (C-PEG 2000 -TPP)and reduction-responsive amphiphilic polymer (DLPE-S-S mPEG 4000).Once the PTX-PLGA/CPT/DSSP gets entered into the cancer cell,PEG 4000 gets removed because of reduced conditions provided by GSH leading to enhanced targeting towards mitochondria and apoptotic cell death [133] .
9.3.Magnetically delivering diagnostic and therapeutic agent to mitochondria
As it is widely known,providing elevated temperature to tumor cells makes them sensitive towards chemo-and radiation therapy leading to a decrease in the viability of tumor cells.Also,there should be a localization of drug at the tumor site in order to avoid side effects on normal cells and more specific destruction of tumor cells [134] .To fulfil this purpose,magnetic NPs were used for targeting as well as for drug delivery by producing hyperthermia at the targeted site by external application of alternating magnetic field [135] .Shah et al.developed magnetic coreshell nanoparticles (MCNP) for the delivery of amphiphilic tail-anchoring peptide (ATAP),a pro-apoptotic peptide,used for targeting mitochondria in cancer treatment.Targeting of MCNP-ATAP to malignant brain and metastatic breast cancer cells was done by applying the external magnetic field as well as by attaching internalizing RGD (a tumor targeting peptide),and hyperthermia was induced by an alternating magnetic field,overall concluding to synergistic cell death.MCNPs based hyperthermia causes inactivation of anti-apoptotic Bcl-2 inhibition leading to overproduction of pro-apoptotic Bcl-2 proteins,leading to cell death by apoptosis;whereas,MCNP-ATAP increasing permeability across MOM leading to dysfunction of mitochondria [136] .
9.4.Light responsive mitochondrial targeting
Light triggered stimuli include photothermal,photodynamic and radiation therapy.In photothermal therapy,photosensitizers are first localized to the targeted area then bombarded by near-infrared energy.Photosensitizers converts this NIR energy into heat,leading to an increase in the temperature of above 42 °C that causes cancer cell death[137] .In photodynamic therapy,photosensitizers gets excited when light is bombarded on them in presence of oxygen,a photodynamic reaction takes place that can produce singlet oxygen (O) and ROS that are highly cytotoxic leading to cancer cell death [138] .Li et al.prepared X-ray responsive nanoradiosensitizers that unlocks a cascade of reactions leading to cancer cell death.They have modified titanium dioxide NPs (TiONPs) with gold NPs (Au NPs)and TPP was attached to the surface of TiO 2 for targeted delivery of nanoradiosensitizers to cancer cells mitochondria.On X-ray exposure,these nanoradiosensitizers produce ROS and leading to a cascade of apoptosis.The use of nanoradiosensitizers in radiotherapy decreases overall radiation exposure,treatment time and side effects associated with radiation therapy [139] .
9.5.pH mediated targeting to mitochondria
Different tissues and organs have different pH in the body.By taking this into consideration,nanocarrier drug delivery systems can be developed that will release the drug only when they will reach the targeted site having a particular pH.pHsensitive polymers can be used to release the drug at the targeted site.If pH of the targeted site is acidic then the pH of polymer should be alkaline so that,the polymer can dissolve in the acidic environment of targeted site and release the drugvice
versa
in case where the targeted site pH is alkaline the polymer will be acidic in nature [140] .The pH of mitochondria in tumor cells is weakly alkaline and it is around 8.0 [141] .The solubility of an alkaline drug is high in an acidic environment and solubility of an acidic drug remains high in an alkaline environment.Hence,an acidic drug can be incorporated into the nanocarriers for targeting to the mitochondria [142] .9.6.Enzyme triggered mitochondrial targeting
Enzymes are biocatalysts that carry out catalysis of several metabolic as well as biological processes.Apart from catalyzing these processes,they also act as a stimulus for nanocarriers to release the drug at the targeted site[143] .There is an overexpression of CD44 receptors in case of cancer cells as compared to normal cells and these receptors interact with several ligands (hyaluronic acid (HA),collagens,osteopontin,etc.) that are present in extracellular matrix leading to enhancement of cancer cell migration and metastasis.Also,there is an overexpression of hyaluronidase enzyme that causes degradation of HA in tumor cells acidic environment [144] .By considering this,Naz et al.prepared mesoporous silica nanoparticles (MSN) and attached TPP to it for mitochondrial targeting.Then DOX was loaded into it followed by capping it with HA.Here,HA was used as capping agent to prevent DOX from pre-leakage before it reaches to the targeted site as well as to increase CD44 targeting.The prepared MSN-DPH (consist of DOX loaded,TPP attached,HA capped mesoporous silica nanoparticles)acts as an enzyme responsive nano-drug delivery system.When this enters inside the cancer cells,it specifically targets mitochondria because of presence of TPP and hyaluronidase enzyme and causes degradation of HA that leads to release of DOX and eventually cancer cell death [145] .
10.Future perspective and regulatory constraints

11.Conclusion
Mitochondria play a key role in different cellular functions.In fact,it is responsible for providing the energy in the form of ATP needed to carry out all biological processes.Moreover,the growth of neoplastic tissue is often supported by anaerobic hyperactive glycolytic machinery.A thorough characterization study of the metabolic process involved in tumour has clearly indicated the dependency of tumorigenic cell populations on mitochondrial respiration compared to their dependency on glycolysis,and its depletion resulting in markedly limited tumorigenic potential.Hence targeting the mitochondria appears a promising strategy which can be used to easily kill the cancer cells by choking the much-needed energy supplement,essential for their growth.There are many reported mechanisms by which mitochondrial targeting can be achieved,however before that,it becomes essential to have a thorough understanding of the molecular mechanisms and types of factors present in mitochondria that are involved in tumorigenesis.The two most commonly used approaches for mitochondrial drug delivery:direct conjugation of the targeting ligand to drugs and attachment of the targeting ligand to a nanocarrier,have their own advantages and limitations.In the case of direct conjugation of the targeting ligand to a drug (eg.TPP based conjugates which exhibit strong mitochondrial targeting ability),many aspects should be considered,including the type of linkage and the type of targeting ligand.Despite the fact that direct drugtargeting ligand conjugation is simple and easy to control,and the drugs can readily reach the mitochondria;there is equally good probability that the conjugation procedure can diminish the therapeutic effects and solubility of the drugs.Apart from this,nanomedicine mediated targeting to mitochondria is significantly improving since the last decade due to their numerous advantages like higher surface area,smaller particle size (helps in enhanced permeability and retention mediated targeting as cancer blood vessel possesses fenestration,which helps in entry of NPs in tumor tissue from blood vessels),ease in functionalization and many more.Furthermore,attachment of targeting ligand to NPs really helps in accurate and effective mitochondrial targeting that will reduce the toxicity associated with conventional anticancer therapy.In the case of the nanocarrier system,there is lesser concern of loss of therapeutic effect as most of the approaches tackle the physical interaction and solubility issue very well,but optimization still pose a major bottleneck due to the use of many different compositions to prepare the nanocarrier,which may hinder mitochondrial delivery.In this review we have summarized all the mitochondrial factors that have role in cancer induction and inhibition including strategies to achieve targeting via direct or indirect methods specifically highlighting the different nanocarriers that have been employed for the treatment of cancer using mitochondria as a target.Though most of these studies are in initial stages,the day is not far when these advancements in cancer delivery can be moved from bench side to bed side provided exhaustive further in-depth studies are carried out.
Conflicts of interest
There is no conflict of interest and disclosures associated with the manuscript
Acknowledgement
The corresponding author would like acknowledge the Department of Science and Technology and SERB (INSPIRE Grant no:IFA-LSBM-13 and EMR/2016/007966/HS) for project funds.All other authors are grateful to the Ministry of Chemicals and Fertilizer for providing fellowship as allowing the conduct research on cancer at NIPER Ahmedabad.
 Asian Journal of Pharmacentical Sciences2021年4期
Asian Journal of Pharmacentical Sciences2021年4期
- Asian Journal of Pharmacentical Sciences的其它文章
- The utility of endogenous glycochenodeoxycholate-3-sulfate and 4 β-hydroxycholesterol to evaluate the hepatic disposition of atorvastatin in rats
- Chondroitin sulfate-mediated albumin corona nanoparticles for the treatment of breast cancer
- Integrated computer-aided formulation design:A case study of andrographolide/ cyclodextrin ternary formulation
- Targeting the resolution pathway of inflammation using Ac2–26 peptide-loaded PEGylated lipid nanoparticles for the remission of rheumatoid arthritis
- The effect of ethanol on the habit and in vitro aerodynamic results of dry powder inhalation formulations containing ciprofloxacin hydrochloride
- Macrophage membrane-mediated targeted drug delivery for treatment of spinal cord injury regardless of the macrophage polarization states
