Size,shape,charge and“stealthy”surface:Carrier properties affect the drug circulation time in vivo
,
State Key Laboratory of Toxicology and Medical Countermeasures, Beijing Institute of Pharmacology and Toxicology, Beijing 100850, China
ABSTRACT The present review sets out to discuss recent developments of the effects and mechanisms of carrier properties on their circulation time.For most drugs,sufficient in vivo circulation time is the basis of high bioavailability.Drug carrier plays an irreplaceable role in helping drug avoid being quickly recognized and cleared by mononuclear phagocyte system,to give drug enough time to arrive at targeted organ and tissue to play its therapeutic effect.The physical and chemical properties of drug carriers,such as size,shape,surface charge and surface modification,would affect their in vivo circulation time,metabolic behavior and biodistribution.The final circulation time of carriers is determined by the balance between macrophage recognitions,blood vessel penetration and urine excretion.Therefore,when designing the drug delivery system,we should pay much attention to the properties of drug carriers to get enough in vivo circulation time to arrive at target site eventually.This article mainly reviews the effect of carrier size,size,surface charge and surface properties on its circulation time in vivo,and discusses the mechanism of these properties affecting circulation time.This review has reference significance for the research of long-circulation drug delivery system.
Keywords:Drug carrier Circulation time Physical and chemical properties Macrophages Phagocytosis
1.Introduction
Free drug administration faces many difficulties,such as the poor solubility and/or poor permeability of drugs [1–3],which make them difficult to be dissolved in body fluid and hard to cross the physiological barriers.In addition,only small amount of drug can arrive at target organs or tissues,while most of the drug is apt to distribute into vital organs and cause adverse reactions.The development of drug carrier provides a good method to meet the above challenges [4,5] .More than 100 years ago,Paul Ehrlich envisioned an ideal drug carrier with following characteristics:long circulation duration/time,targeting ability,drug loading and drug release capacity,and bio-compatibility and non-toxicity [6] .Based on this assumption,with the continuous development of science and technology,various types of drug carriers have been prepared,such as nanomaterials [7–16],controlled-release microspheres [17–22],natural cell membranes [23–31] etc.These drug carriers can deliver not only small molecule drugs but also biological macromolecules,such as peptides and proteins.
The high bioavailability of the administrated drugs relies on their sufficient circulation time and efficient targeting delivery.Drug carriers tend to undergo non-specific uptake in healthy tissues.The drug carriers will be cleared by the mononuclear phagocyte system (MPS) previously known as the reticuloendothelial system and composed of bone marrow progenitor cells,blood mononuclear cells,tissue macrophages and dendritic cells in the systemic circulation [32] .Avoiding the removal of the carrier by MPS and other scavenging organs is the basis of long circulation [33–36] .The recognition of drug carriers by macrophages is mainly mediated by two factors.Firstly,macrophages themselves have receptors to recognize drug carriers.There are many kinds of receptors on macrophage membrane,and these receptors can recognize their corresponding ligands.Therefore,if the ligand is contained in the carrier,it will enhance the recognition and phagocytic metabolism of the carrier by macrophages,shorten thein
vivo
half-life of the carriers and reduce the efficacy of drugs [37] .For example,scavenger receptor is a group of heterogeneous molecules existing on the surface of macrophages [38,39] .It can recognize acetylated lipid proteins,polyanionic macromolecules,bacterial polysaccharides,gold nanoparticles and silicon dioxide,etc.In addition,there are also a large number of galactose particle receptors and mannose receptors on the membrane of macrophages[40–42] .Therefore,carriers modified with lactose,galactose and mannose can also be specifically recognized by macrophages.Another factor is that when the carriers are deliveredin
vivo
,they will gradually absorb serum proteins to form the protein corona and be affected by the opsonin,which enhances their recognition by macrophages.The protein corona is an important factor that mediates the recognition and phagocytic metabolism of the carriers by macrophages.The proteins adsorbed onto carrier surfaces are recognized by the corresponding receptors of macrophages,mainly immunoglobulin (Ig) and complement proteins [43,44] .After the protein corona is formed on the surface of the carrier,it is generally recognized by macrophages in three ways though it has complicated formation process and action mechanism.Firstly,the conformation changes after the opsonin protein is adsorbed on the surface,and it is activated from the resting state to the activated form,binds specifically to the macrophage receptor with the exposed site on the surface.Secondly,most of the opsonin protein structures contain a hydrophobic core region,which interacts with carriers through hydrophobic interaction to stimulate the uptake of macrophages.Thirdly,after the carrier enters the body,it will produce antibodies and activate the complement system.When deliveredin vivo
,phospholipid,cholesterol and polyethylene glycol (PEG)often produce corresponding antibodies.After the antibody recognizes and binds to carriers,it can enhance the recognition and phagocytosis of Fc receptors on the surface of macrophages to carriers.Meanwhile,the adsorption of Ig will also enhance the effect of apolipoprotein receptor and complement receptor system on carriers.Liposomes,carbon nanotubes,block copolymers and polystyrene nanoparticles can stimulate the complement system and induce the recognition by macrophage complement receptors [45,46] .After the carriers enter blood circulation,the complement proteins are adsorbed onto the carrier surfaces and activated by a series of proteolytic enzymes.The activated complement C3 proteins are adsorbed onto the surface of the carriers by covalent bonds with amino,sulfhydryl or hydroxyl groups,enabling them to be captured and metabolized by macrophages with C3 protein receptors [47] .Although macrophages are considered to be the first element involved in the metabolism of carriersin
vivo
,other liver cells,such as hepatic sinusoidal endothelial cells and hepatic stellate cells[36,48–50],also play an important role in the metabolism of carriers.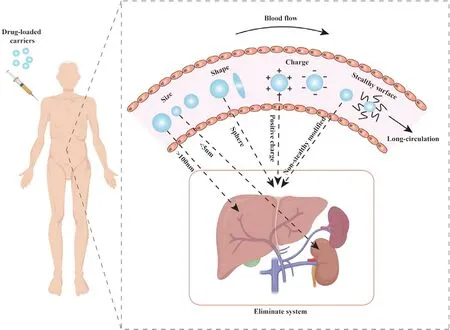
Fig.1–Physical and chemical properties that affect the long circulation of the carrier:size,shape,charge,“stealthy”surface.The carrier with size larger than 100 nm is mainly cleared by liver,and the carrier with size smaller than 5 nm is mainly cleared through kidney.Spherical,positively charged and non-stealthy modified carriers are easily cleared by the eliminate system.
Up to now,various delicate and complicated drug carriers have been designed to deliver drug to target sites.On the other hand,successful strategies for getting long circulation time,such as PEGylation,are still very limited.With the deepening of research,a lot of work started to focus on the long circulating effect brought by the physical or chemical properties of drug carriers.The widely studied properties of drug carriers [51–54] mainly included size,shape,surface charge and surface modification.Appropriate physical or chemical properties can prevent drug carriers from being recognized by the MPS or from formation of protein corona,resulting in longer blood circulation time [55–57] .
This review mainly discussed the influence of the physical and chemical properties of the drug carriers,including size,shape,surface charge and surface modification (Fig.1),on the blood circulation time,and explained how these properties affect thein
vivo
distribution and ultimate fate of the drug carriers.This review aimed to lay a theoretical foundation for the design of drug carriers with prolongedin
vivo
circulation time and efficient targeting delivery.2.Size
Particle size effect of drug carrier has become a hot topic in drug delivery field for over two decades.Firstly,the particle size of drug carriers is closely related to their circulation velocity and aggregation in blood,which will affect thein
vivo
permeability and distribution of drug carriers.Secondly,the size of particle affects the immunogenicity and plasma half-life of the particle,thus affecting the circulation time of the drug carrier and the drug therapeutic effect.The immunogenicity of larger particles is enhanced,thus,they are easy to be recognized and cleared by the immune system,such as macrophages,resulting in shortened blood circulation time [58] .In addition,the size of particles influences their endocytosis pathway,thereby affecting the intracellular distribution of the drug and the drug efficacy [59] .The size of the carrier directly determines its surface area in contact with the internal environment.Therefore,it is an important means for drug delivery to design particles with appropriate size by virtue of the guiding principle of carrier particle size effect.2.1.Behavior of particles with different sizes in vivo
The size of drug carriers has an important effect on theirin
vivo
circulation time.Long-circulation carriers can be used to treat chronic diseases,while the short-circulation carriers can reduce its toxicityin
vivo
.Jasinski et al.prepared RNA nanoparticles with diameter of 5,10 and 20 nm.Their study found that thein
vivo
circulation time increased with the increase of particles size,exhibiting a positive correlation [60] .Chen et al.synthesized particles with size of 80,172 and 243 nm,and investigated their half-life in blood circulation.Their study found that the size of particles was positively correlated with the uptake amount of macrophages.The larger the particle sizes are,the easier they were internalized by macrophages,leading to a significant reduction in circulation time [61] .However,this does not mean that the infinite increase of particle size results in an infinite extendedin
vivo
circulation time.The positive correlation between the size and the circulation time only established in a certain particle size range.Some scholars have proved that when the size of liposome is less than 1000 nm,the phagocytic capacity of macrophages to liposome is positively correlated with the size of liposome [62] .It has been reported that particles with a size of about 2 μm are more likely to be cleared by macrophages than larger or smaller particles[63,64] .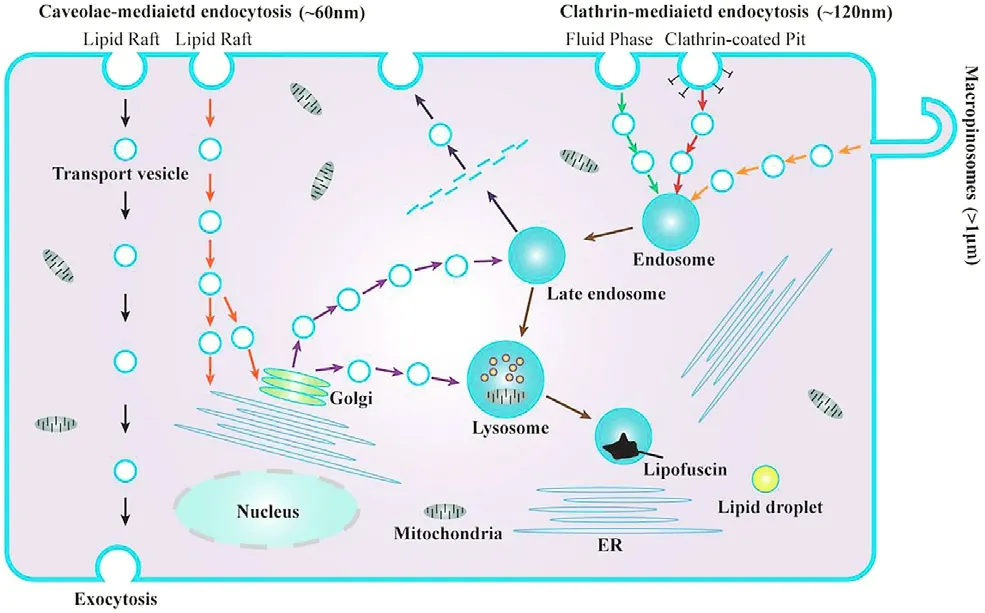
Fig.2–Three endocytosis pathways and the corresponding endocytosis pathways of particles with different particle sizes:clathrin-mediated endocytosis (~120 nm),caveolae-mediated endocytosis (~60 nm),macropinocytosis(> 1 μm).
There are several explanations for how particle size affects thein
vivo
circulation time.For the first,the excretion of drug carriersin
vivo
is mainly through liver and kidney elimination,while kidney elimination is faster than that of liver.Particles smaller than 5 nm are mainly expelled through kidney [60,65],while larger particles up to 100 nm are mainly expelled through liver due to that the larger particles have more opportunities to be taken in by macrophages [60,66] .Small particles are not easily cleared by the phagocytes of MPS,but small particles can be quickly eliminated by the kidney.The final circulation time of drug loaded particles in the body is determined by the balance between macrophages and urine excretion [60] .As a result,the particles with the size between 5 and 100 nm generally have longer circulation time.Another explanation is that when particles enter into blood,they absorb proteins onto their surfaces to form protein coronas [67] .The size of particles determines the amount and characteristics of protein coronas,which affects the circulation time andin
vivo
excretion route of the particles[60,68] .For example,a larger surface area to volume ratio permits more protein to bind onto smaller particles in contrast to large-sized particles [32] .Moreover,the third explanation is that the clearance of particles of different sizes is related to the spacing of membrane ruffles of macrophages.The spacing of membrane ruffles affects the adhesion of particles to the surface of macrophages [62,63] .In addition,the particle size distribution of particles also has a relatively important effect on pharmacokinetics and body distribution.Wide particle size distribution,increased liver uptake and faster blood clearance[69] .2.2.Endocytosis pathways of particles with different sizes
The size of the particles affects the phagocytic capacity,endocytosis speed and endocytosis mechanism of the cell.As shown in Fig.2,the currently reported endocytosis pathways mainly include clathrin-mediated endocytosis(CIME) [52],caveolae-mediated endocytosis (CaME) [70] and macropinocytosis [71–73] .Yue et al.used several biochemical inhibitors to inhibit several known phagocytic pathways.When promethazine was used to inhibit CIME,42% of the phagocytosis of the 430 nm nanoparticle group was inhibited,indicating the important role of CIME in the phagocytosis of nanosized particles;similarly,it was found that CaME was also involved in the endocytosis of nanoparticles with size~60 nm,and macropinocytosis was involved in the process of endocytosis of particles with a size smaller than 1.9 μm.In addition,when latrunculin B was used to disrupt actin filaments,it was found that the phagocytosis process of both nanosized and microsized particles were inhibited,indicating the role of actin in the phagocytosis process [59] .The phagocytosis of particles by phagocytes greatly depends on the remodel of the actin cytoskeleton.If the required structure of actin for the phagocytosis process is not established,the particles will not be internalized,and it will simply extend on the surface of the particle instead of being internalized [74] .
2.3.Size affects particles passing through physiological barriers
In addition to the effect of pinocytosis and phagocytosis of MPS on the circulation time of drug granule carriers,various barriers in the body also block the absorption and circulation of drug particles carriers.The common physiological barriers of drugs include blood-brain barrier [75–78],blood-retinal barrier [79],blood-pancreas barrier [80],blood-gas barrier[81],blood-placental barrier [82] and gastrointestinal mucus barriers [83,84] .At present,the blood-brain barrier is a hot spot in the research.Since the loss of life and disability caused by non-fatal cases of central nervous system diseases impose a heavy burden on society,the delivery of nanoparticles into the brain is particularly worthy of attention.The cells in the blood-brain barrier have the characteristics of no perforation and low endocytic activity [85] .Therefore,the drug or carrier with a suitable particle size is one of the key factors that determine whether it can pass through the blood-brain barrier.Maksymilian et al.compared the situation of 100,200 and 500 nm polystyrene spherical nanoparticles crossing the blood-brain barrier [86] .The results of the study found that different particle sizes have a non-monotonic dependence across the blood-brain barrier,that the transport of particles with a particle size of 200 nm is better than 100 and 500 nm.The exact reason for this non-monotonic dependence is unclear.Particles of different sizes may cross through cells along different paths.In addition,Mucus is the body’s protective coating against foreign invaders,but it is a major barrier for non-invasive administration of drugs.Fig.3 shows that the purple particles are bigger than the mesh space between mucus fibers,so it cannot penetrate the mucus layer.On the other hand,the green particles are smaller than mesh space,thus could theoretically diffuse through the pore.However,the actual situation is that,in the mucus layer,the particles will absorb the mucin or non-mucin proteins or other mucus constituents onto their surfaces.As a result,the spread of the green particles will be slowed down [87] .
2.4.Summary
It can be seen that the particle size has a significant influence on the circulation time andin
vivo
clearance of the particles.Particle size is an important factor affecting the pharmacokinetic and biological distribution of drug delivery systemin
vivo
.On one hand,the particle size and its distribution affect the adsorption of plasma proteins and the phagocytosis and clearance of mononuclear macrophages.On the other hand,the size of the gap between endothelial cells at specific tissue sites also affects the uptake of different particle size delivery systems at specific sites.Smaller particles between 10 and 20 nm are quickly removed by the kidney [88],while larger particles can activate the complement system[89–91] and are easily consumed by the MPS [63,92] .A range between 20 and 150 nm should be considered as suitable size [93,94],which can reduce the liver clearance and kidney filtration,and prolong the circulation timein
vivo
.In addition,the particle size determines its endocytosis pathways.The endocytosis pathways corresponding to 60 nm,120 nm and 1 μm are CaME,CIME and CC,respectively.Moreover,the size of the particle also determines whether it can get past the physiological barrier.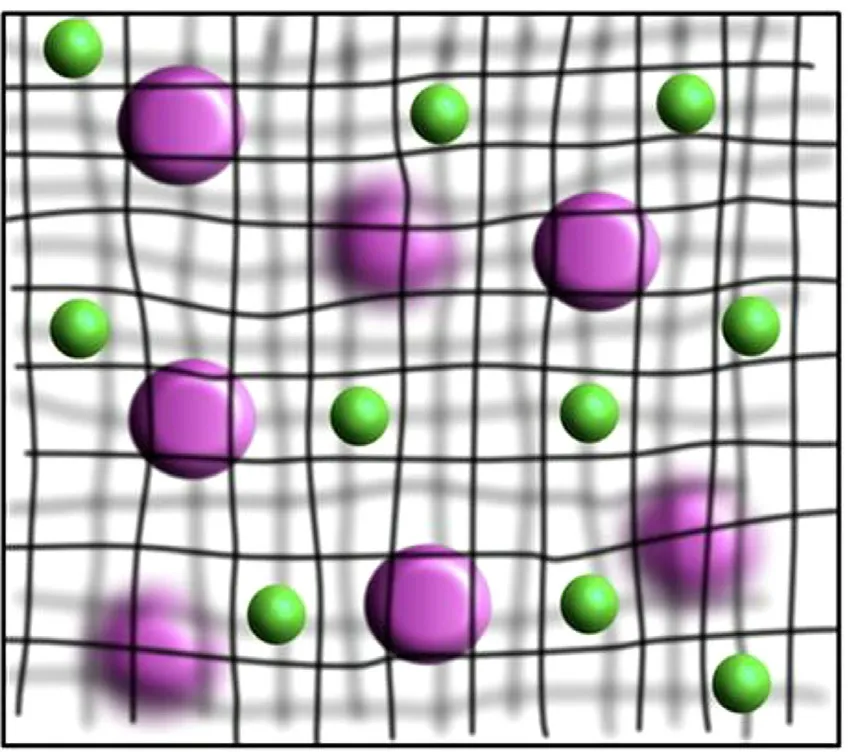
Fig.3–Schematic representation of the size filtering mechanism.The purple particles have the bigger size than green particles,so they trapped with the network.Reprinted with permission from [84] .Copyright 2017 Elsevier.
3.Shape
In previous studies,most of the drug carriers are spherical,which causes researchers to ignore the influence of carriers’shape onin
vivo
circulation.Interestingly,shape greatly affected thein
vivo
circulation time of the carriers and the internalization of the carriers by the phagocytic system [52] .Some viruses and bacterial pathogens are initially recognized by their shape.The non-spherical appearance of viruses and bacteria tend to evade recognition by the immune system,so a reference to natural biological systems suggests that non-spherical particles may have an advantage over spherical particles in prolonged circulation timein
vivo
.3.1.Particles with different shapes have different behaviors in vivo
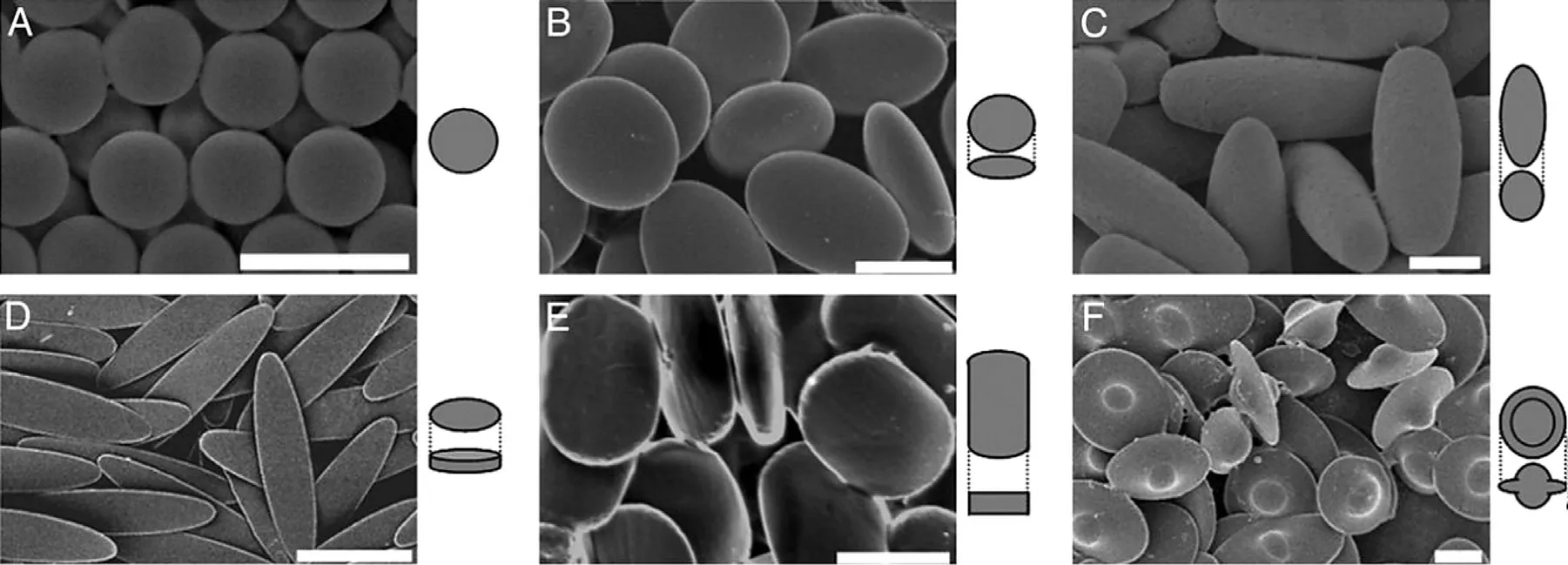
Fig.4–Scanning electron microscopy of six shapes particles.(A) spheres.(B) oblate ellipsoids.(C) prolate ellipsoids.(D)elliptical disks.(E) rectangular disks.(F) UFOs.Reprinted with permission from [74] .Copyright 2006 National Academy of Sciences.
With the development of science and technology,researchers have reported drug carriers with various shapes.The effect of carrier shapes onin
vivo
circulation has also attracted much research attention.In addition to spheres,other shapes have been reported (Table 1),including oblate ellipsoids,prolate ellipsoids,elliptical disks,rectangular disks and UFOs(Fig.4) [74] .The phagocytosis of particles with different shapes by alveolar macrophages was compared by timelapse video microscopy.The time-lapse video images showed that the spherical IgG-adsorbed particles were internalized by the cells,while the worm-like IgG-adsorbed particles exhibited different behaviors.The alveolar macrophages initially attached to one side of the worm-like particle,but did not internalize it [95] .The spherical particle was internalized in 2 min,while the worm-like particle was still not internalized in 32 min.And after 22 h of incubation with cells,the degree of internalization of worm-like particles was significantly lower than that of spherical particles [95] .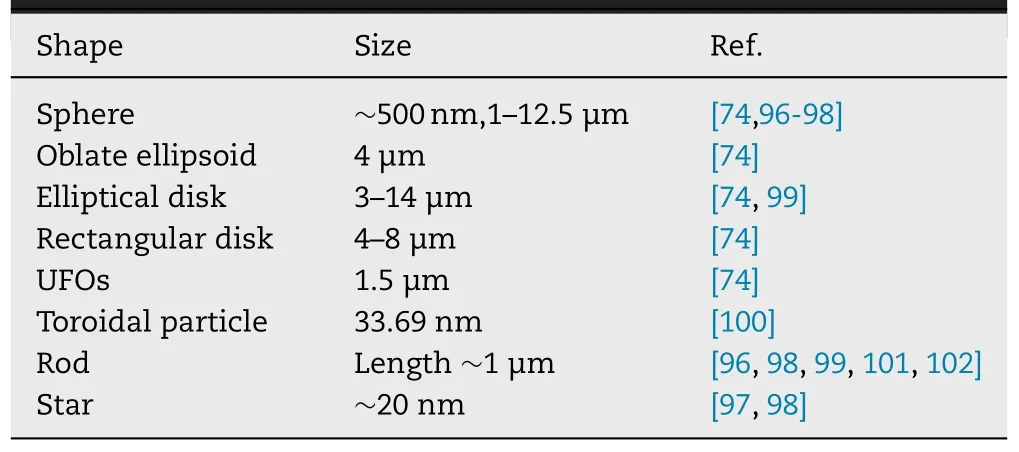
Table 1–Particles of different shapes prepared in the literature.
Table 2–Summary of physicochemical properties of carriers influencing the behavior in vivo .
Arnida et al.compared the distribution and circulation of gold nanospheres and nanorods in mice,and the result showed that between 30 min and 6 h after injection,the plasma concentration of gold nanorods was obviously higher than that of nanospheres.After 6 h,the concentration of gold nanospheres in the blood is lower than 1%,while 11% of the nanorods remained in blood.Moreover,the accumulation of gold nanospheres in the liver was significantly higher than that of nanorods.As we know,the primary phagocytes-Kupffer cells present in the liver,so the high accumulation of nanoparticles in the liver increases its clearance.In addition,the accumulation of nanorods in the tumor is significantly higher than that of nanospheres,and the interaction of nanospheres with bovine serum albumin is stronger than that of nanorods,which increased the clearance of nanospheres by macrophages [53] .
3.2.Mechanism of shape effect
To sum up,it can be concluded that shape has a great influence on the carrier’s clearance by the macrophagesin
vivo
and the long-circulation.How did shape affect the phagocytic behavior of the carriersin
vivo
? Several potential mechanisms are listed in the following.3.2.1.Fluid
dynamics
hypothesis
Geng et al.injected fluorescent-labeled filomicelles into mice and showed that the filomicelles circulatedin
vivo
for up to one week.At the same time,the researchers compared the interactions between spherical particles and filomicelles with phagocytesin
vivo
.The result showed that spherical particles were internalized by phagocytes,while filomicelles could avoid the rapid phagocytosis of phagocytes due to their shape being easily carried away by fluid flow,so that they could achieve the long circulationin
vivo
[103] .And they used fluid flow to simulate blood flow in the spleen and observed the endocytosis of filomicelles by macrophages in the fluid flow state.They found that macrophages had a strong endocytosis of flowing spherical micelles or vesicles,but could not phagocytosis of flowing filomicelles.Under the condition of same blood flow rate,filomicelles can generate a faster flow rate than other particles.The shear force caused by the strong flow rate reduced the adhesion between filomicelles and macrophages,and thus reduced the internalization of the macrophage.3.2.2.Local-shape
hypothesis
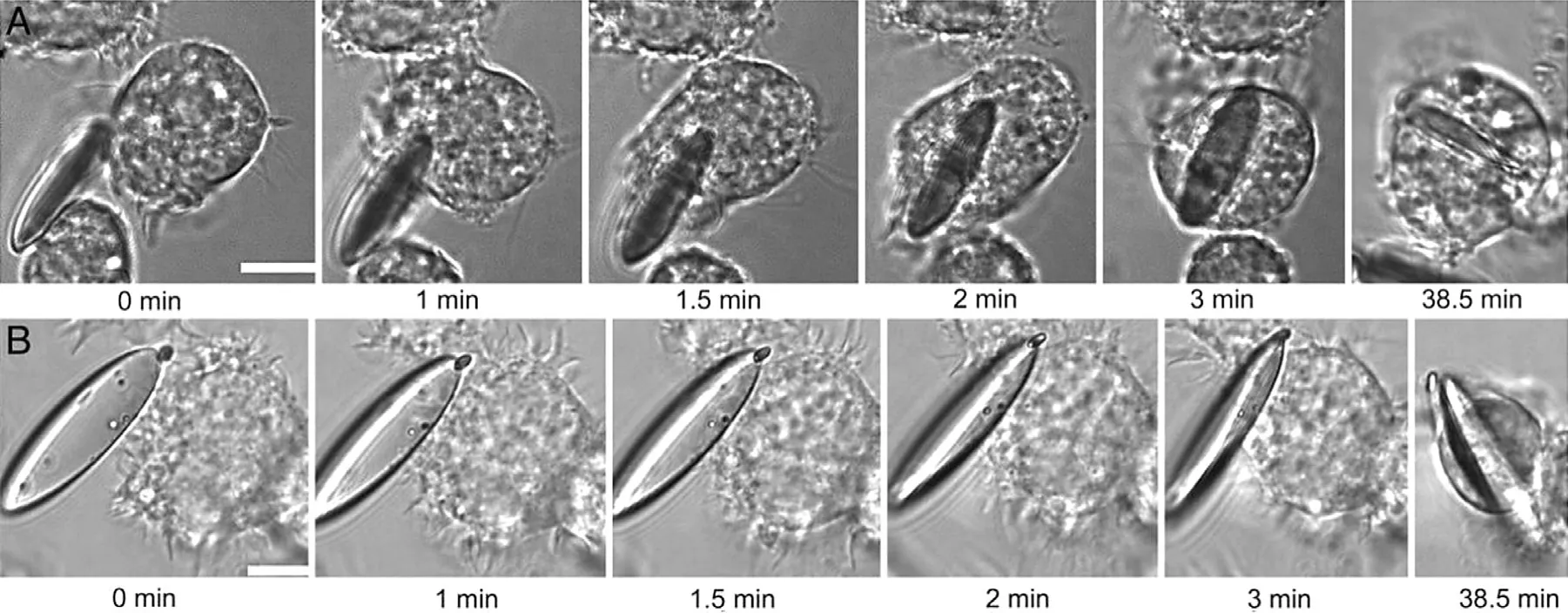
Fig.5–Time-lapse microscopy of macrophages interacting with ED particles.(A) macrophage attaches along the major axis.(B) macrophage attaches from the flat side.Reprinted with permission from [74] .Copyright 2006 National Academy of Sciences.
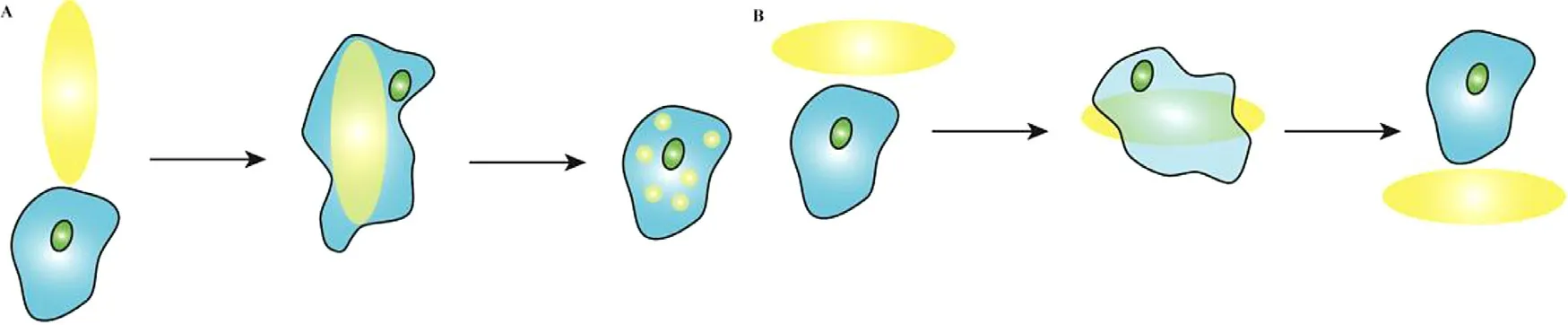
Fig.6–Schematic representation of macrophages attach from different directions.(A) invaded from the side with higher curvature.(B) invaded from the side with lower curvature.
The other explanation is that it is not the shape of the intact particle that influents whether the carrier is internalized,but its local shape -the shape of the particle when it first contacts the macrophage or other phagocyte.Fig.5 shows how different contact directions affect phagocytosis of rodshaped particles by macrophages.As can be seen from the time-lapse video microscopy,macrophages attached along the major axis of elliptical disks (EDs) can internalize them within 6 min,whereas they did not internalize the EDs even after 2 h along the minor axis [74] .Other researchers have come up with similar results [51,103] .All these studies have come to this conclusion that it is not easy for particles or drug carriers with high aspect ratio to be internalized by phagocytes.As shown in Fig.6,if the phagocytes invade the carrier from the side with higher curvature,the carrier will be more likely to be internalized by macrophages.On the contrary,if the carrier is invaded from the side with lower curvature,the carrier will not be easily internalized.The curvature is related to the contact direction,thus macrophages can show different internalization behaviors for different contact directions.And Champion et al.introduced a feature angle parameters“Ω
”and established the corresponding mathematical model to quantitatively illustrate the geometry of the particles of the biological effect [74] .WhenΩ
is 0 °,the long axis of the elongated particles is perpendicular to the cell contact surface,which is conducive to cell phagocytosis;whenΩ
is 90 °,the long axis of the elongated particles is parallel to the cell contact surface,which is not conducive to cell phagocytosis.3.3.Summary
The studies above have shown that the shape of carriers have significant effects on theirin
vivo
circulation time and clearance,and carrier shape plays an important role in drug distributionin
vivo
.Spherical particles had a higher accumulation rate in organs responsible for clearance than non-spherical particles.The phagocytosis of the drug carriers can be regulated by changing the intact shape and the local shape of the drug carriers.Studies have shown that other shapes,such as rods,elliptical carriers,worms,have less phagocytosis than spherical shapes.Although the mechanism of how the shape of drug carriers affects theirin
vivo
behavior has not been thoroughly studied,more and more studies have paid attention to the shape factor.It is believed that with the continuous improvement of technology and theory,it will not be long before the key mechanism of shape is clearly explained.4.Surface charge
The surface charge affects the distribution of drug carriersin
vivo
.The surface charge properties of drug carriers affect the intracellular endocytosis rate and the intracellular distribution of drug carriers.Since the cell membrane is negatively charged,positively charged carriers are relatively easy to be absorbed by targeted cells,but also easy to be trapped by macrophages of MPS,which makes it difficult to realize the longin
vivo
circulation time of drug carriers [104–106] .Therefore,negatively charged or uncharged drug carrier particles can realize their long circulation in the body,but at the same time bring some negative effects.For example,it is difficult to achieve targeted transmembrane delivery of drug under the repulsive effect of electrostatic force between the carriers and cell membrane.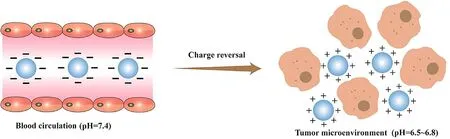
Fig.7–Schematic representation of the behavior of charge-reversal particles in vivo.In the normal physiological tissue,the charge reversal particle exhibiting negative charge can avoid the interaction with opsonin and the clearance by the MPS.While in the slightly acidic environment of tumor,the charge of particle reversed to be positive,which can interact with the negatively charged cell membrane.
4.1.Charge-reversal particles
Positively charged particles can avoid being cleared by phagocytes,but they tend to interact with components in blood,causing hemolysis and toxic side effects on normal cells[107] .The application of charge reversal materials solved the negative effects mentioned above.In the normal physiological tissue,the charge reversal particle exhibiting negative charge can avoid the interaction with opsonin and the clearance by the MPS.While in the slightly acidic environment of tumor,the charge of particle reversed to be positive,which can interact with the negatively charged cell membrane (Fig.7).The reversion of carriers’ surface charge allows the carrier to interact with the target cell and allow the drug to enter the cell,thus improving the therapeutic effect.Hu et al.fabricated charge-reversal nanoparticles that can change the surface charge characteristics,which not only effectively increased the uptake rate of positive drug carriers by tumor cells,but also realized long circulationin
vivo
under the original conditions (pH 7.4),because the particle surface charge was negative [108,109] .At present,the commonly used charge-reversal materials are carboxymethyl chitosan [110],polyurethane [111],amino acid polymer [112,113],etc.The isoelectric point of such substances is between the pH of normal physiological environment and the tumor microenvironment (pH 6.5–6.8).Under the normal physiological conditions,the charge of drug carriers made of these materials are negative,and the slight acidity makes it protonation in the tumor microenvironment,its surface charge changes from negative to positive and the positively charged carriers can interact with the negatively charged tumor cell membrane,promoting the internalization of drugs.It is worth noting that the design of the chargereversal carriers puts forward high requirements for the synthesis of polymer materials.The polymer materials used to prepare drug carriers should be sensitive to the differences of physical and chemical properties between the normal physiological environment and the tumor environment in order to make charge reversal behavior faster.
4.2.Effect of the value of absolute charge
Moreover,the cationic particles with low absolute positive charge on their surfaces can reduce the absorption of opsonin.Particles absorbed opsonin are easily recognized by receptors on the surface of macrophages thus mediating the phagocytosis of phagocytes in the MPS.Xu et al.prepared cationic particles with low positive charge (+3.28 mV in PBS and+5.46 mV in deionized water) and anionic particles with high negative charge (−25.4 mV in PBS and −36.3 mV in deionized water).And they studied the percentage of surface cationic and anionic hemoglobin-loaded polymeric nanoparticles (HbPNPs) absorbed by macrophages compared with untreated HbPNPs,and the results showed that the absorption rate of cationic HbPNPs was significantly lower than that of untreated HbPNPs,while the absorption rate of anionic HbPNPs was significantly higher than that of untreated HbPNPs.And the blood clearance rate of cationic HbPNPs was significantly lower than that of untreated HbPNPs,while the anionic HbPNPs had the opposite effect.Moreover,thein
vivo
distribution results showed that the accumulation of untreated HbPNPs in liver after 15 min of injection was about 5 times of that of cationic HbPNPs [114] .They concluded that cationic particles with low absolute positive charge can avoid phagocytosis by phagocytes.The same results showed that the phagocytosis of cationic and anionic particles increases with their absolute potential,and there was no significant difference between the phagocytosis of cationic and anionic particles with the same potential [115] .With the increase of the absolute value of surface potential,the uptake of the carrier by the liver and spleen is increased,and the clearancein
vivo
is accelerated.Therefore,it can be considered to cover the surface of the carrier with hydrophilic long-chain PEG or poloxamers,which can shield the strong electricity on the surface of the carrier,and it is conducive to avoiding or delaying the binding and recognition of the opsonin protein in the blood and prolonging the circulation time of the carrierin
vivo
.4.3.Summary
Positively charged particles tend to be early cleared from the blood by the MPS,while negatively charged particles are less likely to be internalized by target cells.Charge-reversal particles cleverly combined the advantages of positive and negative charges to provide a technical platform for the study of tumor-targeted drug delivery systems.In addition,the opsonin protein in the blood has a weaker opsonization to the electrically neutral drug delivery system,and the stronger to the charged drug delivery system.Thus particles with a lower absolute charge can weaken the adsorption force with opsonin,and achieve longin
vivo
circulation time.The influence of surface charge should be fully considered when designing drug carriers.In the process of particle charge reversal,the change of charge sometimes causes some changes in other physical properties of the particle,such as significant reduction in size [116] .Therefore,the comprehensive effect of physical properties should be considered when designing drug carriers.5.“Stealthy”surface
In order to prolong thein
vivo
circulating time of drug carriers,the introduction of the "stealthy" molecule is a conventional method.The presence of "stealthy" molecule allows the carrier to evade the recognition of MPS,and makes the carrier not be cleared by the human body quickly,and makes it have the function of "stealth".Therefore,the chemical grafting and ligand modification can be used to regulate the surface properties of drug carriers to avoid MPS uptake,and significantly enhance the ability of carriers to actively target tumor sites.The hydrophilicity and lipophilicity of the surface of the nanoparticles will affect the strength of the adsorption binding force between the particles and opsonin,thus affect the phagocytic rate by the phagocytic cells.Generally speaking,the greater the surface lipophilicity of particles,the stronger the binding force to the opsonin and the phagocytic effect of phagocytes.Therefore,to prolong the circulation time of particlesin
vivo
,it is necessary to increase the hydrophilicity of their surface,which is a necessary condition for selecting materials for surface modification of particles.However,the hydrophilic surfaces are not sufficient to guarantee long circulation.There is a class of particles with hydrophilic surfaces,which are fabricated from hydrophilic polymers such as polysaccharides.Although the surface of polysaccharide particles is hydrophilic,they tend to be cleared by the MPS because they can promote adaptive immunity through a variety of mechanisms,activating the complement system [117] .For example,hyaluronic acid plays an important role in antigen presentation,which can promote T cell activation by binding to CD44 [118] .Polysaccharides can activate the complement system by alternative pathway.C3b formed in the alternative pathway can bind with amino and hydroxyl groups [119] .Polysaccharides contain a large number of hydroxyl and amino groups,which play an important role in the activation of complement system[120,121] .Therefore,polysaccharide particles with hydrophilic surface such as chondroitin sulfate,hyaluronic acid,and chitosan and its derivatives are easily to cleared by MPS.All in all,surface hydrophilicity is an important factor to achieve long circulation,but not a determinant.
Most of the "stealthy" molecules are made of materials with hydrophilicity,good biocompatibility,low protein absorption or negative charge.At present,the molecules commonly used in surface modification are PEG,surfactants,block polymers and ganglionic glycosides.
5.1.PEG and derivatives modification on drug carrier surfaces
PEG is a common "stealthy" molecule with greater hydrophilicity.The PEGylation on the particle surface can reduce the electrostatic charge of the particle,because it forms an aqueous layer on the surface,thus preventing the renal clearance of particles [122] .In addition,PEGylation can improve its stability and steric hindrance.PEG chains have the characteristics of flexibility and stretch.The attraction between opsonin and particles causes the PEG chains to be compressed,and the condensation of the chain changes the original conformation,forming a conformation with steric hindrance [123],thus cancelling out the attraction between opsonin and particles,and thus avoiding the uptake by MPS[122,124] .Lv et al.compared the circulation time of PEGylated and un-PEGylated nanoparticles in blood,and the results showed that the half-life of PEGylated nanoparticles was more than 30 h,while that of un-PEGylated nanoparticles was only 8 h,indicating that PEGylated nanoparticles could reduce the phagocytosis of MPS,prolong the blood circulation time and realize the function of long circulation [125] .There are other similar studies.Mustafa et al.modified polylactic acidglycolic acid (PLGA) nanoparticles with PEG and water-soluble chitosan (WSC) coatings to investigate their circulatory behaviorin
vivo
.The results showed that compared with the unmodified PLGA nanoparticles,the presence of PEG and WSC coating significantly prolonged the circulation timein
vivo
,and the concentration of modified nanoparticles in the liver decreased significantly.The combination of PEG and WSC on particle surfaces formed a brush like structure,which reduced the particle binding to blood protein and avoids phagocytosis[126] .The molecular weight,density and conformation of the PEG chain can significantly affect the protein opsonization of the PEGylated carrier and the phagocytosis and clearance of MPS,thereby affecting the stealth effect of the carrier in the body [127] .A large number of studies have shown that when the molecular weight of PEG is greater than 2000 Da[128],the carrier can effectively avoid the phagocytosis of MPS,and the circulation time in the body is prolonged.Increasing the molecular weight of the PEG chain can reduce the protein opsonization in the blood and reduce the phagocytosis of MPS,and the circulation half-life of the carrier in the body can be further extended.When the density of the PEG chain on the surface of the carrier is low,there is no interaction between the PEG chains,and the PEG chain has a large range of activity,so it is easy to form a mushroom structure on the surface.Since a large amount of surface space is not covered by PEG,it is easy to interact with opsonin and cleared by MPS.On the contrary,when the density of PEG on the surface of the carrier is high,the movement of the PEG chain is greatly restricted,and a brush-like structure is formed on the surface of the nanoparticles,and the formed PEG layer is thick,so it is not easy to combine with opsonin and cleared by MPS[123,129,130] .Therefore,the PEG on the surface of the carrier needs to reach a certain coverage rate to reduce the protein’s conditioning effect,so that the carrier has a better stealth effect in the body.
However,a growing number of studies have shown that the existence of PEG has caused the body to produce an immune effect,promoting the exhalation of particles.Studies have shown that the existence of PEGylation has caused the body to produce anti-PEG antibodies [131–134] .PEG,as an exogenous substance,will gradually produce pegrecognizing IgMin
vivo
after injection.PEGylation particles will rapidly interact with IgM,and then accelerate the recognition and metabolism of PEG by macrophages [135] .So,researchers used other polymers instead of polyethylene glycol such as hyperbranched polymers [136],zwitterionic coatings [137],etc.In the past decade,biomaterials such as polyoxazoline have received more attention.Given its simple synthesis and rich chemical composition,it provides more options for adjusting the properties of biomaterials.Polyoxazoline is an amphiphilic polymer with good water and organic solubility.Therefore,it can be considered to prepare polymer particles,because of its good hydrophilicity stealth properties,to achieve a long circulation effect.Woodle et al.prepared early amphipathic polymer-lipid conjugates with polyoxazoline to escape the recognition of the reticuloendothelial system and achieve long circulation[138] .Koshkina et al.studied Poly(2-ethyl-2-oxazoline) (PEtOx)instead of PEG,and the results showed that PEtOxylation significantly reduced phagocytosis of phagocytes.They compared the phagocytosis of nanoparticle POS-NET3,POSCOOH,POS@PEG and POS@PETOx,and found that among these four types of nanoparticles,POS@PETOx were the least taken up by macrophage-like cells THP-1 M [139] .The polymer carrier polyoxazoline has great advantages in drug research and development.At present,the drug SER-214 based on polyoxazoline is in clinical trials [140] .Compared with PEG,the research on the polyoxazoline is still a minority,but we believe that if the SER-214 clinical trial can achieve better results,then polyoxazoline will usher in more extensive research and continue to narrow the gap with PEG.5.2.Surfactant modification on the surface
In addition to PEG modification on particle surfaces,some researchers used other nonionic surfactant to modify the particle surface.Joseph et al.applied a layer of polysorbate 80 on nanoparticle surfaces,and the results showed that the surfactant decreased the aggregation of the nanoparticles in liver when compared with un-coated nanoparticles,and it reduced the phagocytosis of the nanoparticles by phagocytes[141] .The reason was similar to the modification of PEG on nanoparticle surfaces.The presence of surfactants increased the hydrophilicity of nanoparticles,reduced the adsorption of opsonin,thereby avoid being recognized by MPS and phagocytosis by phagocytes.Hydrophobic particles were reported to be more easily recognized by phagocytes [142–144] .For the similar reason,some polysaccharose,such as dextran,was also used to modify the surface of particles.The brush structure of dextran can reduce the adsorption of opsonin onto particles,so prevent the particles being recognized by MPS.
5.3.Protein modification on nanoparticle surfaces
On one hand,the PEGylation of carrier surface significantly reduced the uptake of MPS.It prolongs the circulation time of the drug delivery system,but on the other hand,it significantly weakens the interaction between the target cells and the drug delivery system,resulting in insufficient uptake of the target cells to the drug delivery system.Therefore,the modification of specific molecules on the carrier surface can be considered.Macrophages can distinguish the selfsubstances and unself-substances of organism by CD47 protein,and the CD47 protein carried by self-substances can interact with the signal regulatory proteinα
(SIRPα
) expressed by macrophages,thus macrophages identify them as selfsubstances.Unself-substances do not carry CD47 proteins and they are easily cleared by phagocytes.Therefore,CD47 proteins can be modified onto particle surfaces,pretending the particles to be the self-substance for avoiding being cleared by macrophages.Kim et al.modified CD47 protein on the surface of drug nanocarriers and found that the presence of CD47 significantly reduced the clearance of the carriers by macrophages [145] .So,a strategy based on pretending to be the self-substance can effectively avoid being earlier cleared by macrophages,thus prolong thein
vivo
circulation time of particles.Moreover,using the cell membrane of somatic cells as drug carriers,the cell membrane loaded with a pharmaceutical preparation is sent directly back into blood,which can also avoid recognition by the immune system.Lee et al.used zr-89 labeled red blood cell membranes,and the result showed the CD47 proteins rich on the membrane allowed the drug loaded red blood cells to persistin
vivo
for a long time [146] .Hu et al.coated the erythrocyte membrane on PLGA nanoparticles,and confirmed by fluorescence labeling that erythrocyte membrane coating could significantly prolong the circulation time of nanoparticlesin
vivo
,and was significantly better than conventional PEG-modified nanoparticles [147] .It is an effective method to prolong the half-life of pharmaceutical preparations by using living cells as the carrier of nanomaterials.Living cells can escape the phagocytic action of phagocytes.The drug is adsorbed on the surface of living cells,and then the living cells delivery the drug to the target site.Anselmo et al.adsorbed PLGA particles on the surface of red blood cells,which significantly increased the content of the pharmaceutical preparation in the lungs and extending the half-life of the drug [148] .However,the strategy using the membrane of somatic cells as drug carriers still has defects,such as blood collection before preparation,strict conditions that preparation process requires and poor controllability of released drugs.The cell membrane is difficult to be accurately controlled and is easy to be damaged,which is liable to lose the previous characteristics of small immunogenicity.In addition,the method can onlybe used in self-infusion because of the immunological effect.These defects make the somatic cell membrane carriers still stay in the laboratory research stage,and limit their clinical transformation.
5.4.Summary
Based on the considerations of safety,controllability and low cost,surface modification of carriers based on novel polymer excipients will still be the preferred strategy for constructing long-circulation drug delivery systems.PEGylation is a common strategy to make the carrier avoid the clearance by macrophages.The modification of other macromolecules can avoid the immune effect caused by PEGylation.Living cells or cell membranes as the carriers is the current research frontier to realize the long-circulation.Under such strategies,drug delivery depends on the intrinsic properties of cells or membranes.In the future,we can conduct directional transformation of cells through the gene or cell engineering technology,to build a more intelligent bionic carrier system,it is expected to further expand the application potential of this strategy.
6.Synergistic effect of physicochemical properties
In the above,we discussed the effect of a single physicochemical property on the behavior of the carrierin
vivo
.It can be seen that the properties of the carrier have an important impact on its fatein
vivo
.However,the biodistribution of the carrierin
vivo
,the identification of MPS,and the pharmacodynamics are not determined by single property.One property changed is always accompanied with another.The properties of carrier show a synergistic effect on itsin
vivo
circulation.For example,the modification of stealthy molecules on the surface may change the surface charge of the carrier and also affect the size of the carriers.To some extent,the change in shape also changes the size of the carrier.At present,the change of physical and chemical properties of the drug carrier can significantly alter its biological distribution in the body,avoid rapid elimination by MPS,and significantly improve the drug therapeutic effect.Table 2 summarizes several studies that the behavior of the carrierin
vivo
changed by synergistic effect of physicochemical properties of the carrier.7.Conclusion and perspectives
In order to improve the bioavailability of drugs and prolong the circulation time of drug carriers,it is a conventional strategy to optimize the physical and chemical properties of drug carriers.Optimal physical and chemical properties determine the interaction of the carriers with MPS and other physiological barriers and prevent the drug carriers from being quickly cleared from the circulation.
The carrier larger than 150 nm were mainly taken up by phagocytes in MPS and mainly accumulated in liver and spleen.The size between 20 and 150 nm may be a suitable range to get longerin
vivo
circulation time.Therefore,the study of size-reversal particles can combine both the advantage of small and large particles,which the small particles can avoid being cleared by MPS and the large particles have a long retention time in the diseased tissue.As to carrier shapes,spherical carriers are easier to be cleared by phagocytes than other shapes.In addition,the initial contact point and the shear force caused by fluid flow are also important causes of phagocytosis.Surface cationic particles tend to bind to the negatively charged macrophage cell membranes and then be internalized.And the study of the carriers with charge-reversal function prolong the circulation time of carrier on the basis of better therapeutic effect.Other studies have found particles with low absolute charge on their surfaces have a lower blood clearance rate.PEGylation on particle surfaces is a common strategy for prolonging the circulation time,but the accelerated blood clearance coming from the immune effects of PEGylation casts a shadow over its application.More and more studies begin to focus on the substitutes of PEG to modify the nanocarrier surfaces.Moreover,the strategy based on pretending to be self-substance can effectively avoid being earlier cleared by the immune system.In view of the above characteristics,each parameter should be precisely controlled to achieve the ideal circulation time when design drug carriers,instead of simply adding the physical and chemical properties.It is noteworthy that the change of one property sometimes causes changes of other properties,so we should give full consideration to their synergistic effects.In addition to the properties mentioned above,there are still other physicochemical properties worth to be studied,such as materials,which provide a broad space for the design of a new generation of drug carriers.The long circulation is realized on the basis of the synergistic effect of various properties rather than acting independently.The drug carrier designed with optimal physical and chemical properties will have great potential of clinical transformation.
Conflicts of interest
None.
Acknowledgement
This study was supported by Military Medical Innovation Project (16CXZ032),National Science and Technology Major Projects for“Major New Drugs Innovation and Development”(No.2018ZX09J18107-03,2018ZX09721003-005-009).
 Asian Journal of Pharmacentical Sciences2021年4期
Asian Journal of Pharmacentical Sciences2021年4期
- Asian Journal of Pharmacentical Sciences的其它文章
- The utility of endogenous glycochenodeoxycholate-3-sulfate and 4 β-hydroxycholesterol to evaluate the hepatic disposition of atorvastatin in rats
- Chondroitin sulfate-mediated albumin corona nanoparticles for the treatment of breast cancer
- Integrated computer-aided formulation design:A case study of andrographolide/ cyclodextrin ternary formulation
- Targeting the resolution pathway of inflammation using Ac2–26 peptide-loaded PEGylated lipid nanoparticles for the remission of rheumatoid arthritis
- The effect of ethanol on the habit and in vitro aerodynamic results of dry powder inhalation formulations containing ciprofloxacin hydrochloride
- Macrophage membrane-mediated targeted drug delivery for treatment of spinal cord injury regardless of the macrophage polarization states
