Degradable Plastics Are Vulnerable to Cracks
Xuxu Yng ,Json Steck ,Jiwei Yng ,Yecheng Wng ,Zhigng Suo ,*
a John A.Paulson School of Engineering and Applied Sciences,Kavli Institute for Bionano Science and Technology,Harvard University,Cambridge,MA 02138,USA
b State Key Laboratory of Fluid Power and Mechatronic System,Key Laboratory of Soft Machines and Smart Devices of Zhejiang Province,Department of Engineering Mechanics&Center for X-Mechanics,Zhejiang University,Hangzhou 310027,China
Keywords:
ABSTRACT
1.Introduction
Degradable plastics were developed soon after the rise of the polymer industry,with applications including packaging[1],agriculture[2],and medicine[3,4].They are used to curtail plastic pollution by replacing nondegradable plastics[5].Degradable polymers gradually break down into low-molecular-weight polymer chains or small molecules in response to stimuli such as ozone[6],water[7],other molecules[8],pH[9],enzymes[10],mechanical load[11],and temperature[12].The kinetics of degradation must fulfill requirements—often conflicting ones—for function and degradation.
Degradation kinetics have long been characterized by the loss of weight and strength over time[13].For example,when collagen suture fibers are placed in an enzyme solution at 37°C,the weight and strength decrease by 50%and 10%within one day[14].When poly(tetrafluoroethene)samples are placed in a heated air flow with the temperature increasing by about 10°C·min-1,the weight decreases gradually below 550°C but dramatically at about 600°C[15].These methods of characterization are useful,but their exclusive use is misleading when plastics degrade heterogeneously.In fact, heterogeneous degradation is commonly observed.For example,subject to a small mechanical load,crack-like flaws may outrun erosion in speed by orders of magnitude[16].Such cracks,often called corrosion cracks,have been studied extensively in metals,ceramics,and inorganic glasses[17,18].An extensive literature also exists on corrosion cracks in polymers,also referred to as environmental stress cracking[19,20].For elastomers,a salient example is ozone cracking in natural rubber[6,21].For plastics,theoretical and numerical modeling of corrosion cracks has been studied[22,23].However,experimental characterization of cracking in degradable polymers has commenced only recently,with a 2020 study of corrosion cracking in a polyester elastomer[16].Plastics present distinct fracture property from elastomers due to different dissipation mechanisms.It is urgent to ascertain whether similar crack growth takes place in degradable plastics.
Of all degradable plastics,polyesters are the most widely used.Thus,we chose polylactic acid(PLA)as a model degradable plastic.We cut a crack using scissors in a PLA film,tore the film using an apparatus,and recorded the crack growth using a camera.We found that the crack velocity is insensitive to load in our testing range but is sensitive to humidity and pH.Because the surfaces of plastics inevitably contain crack-like flaws,and because many applications require plastics to carry loads,crack growth under the combined action of mechanics and chemistry is likely to be prevalent.We summarize the evidence of crack growth in various degradable polymers.These cracks can greatly affect the kinetics of degradation.When a degradable plastic is used inside the body of a patient,cracks may fragment the plastic into particles,which may cause medical complications—sometimes fatal ones.
2.Material and methods
PLA is a thermoplastic polyester,synthesized by the polycondensation of lactic acid or the ring-opening polymerization of lactide.PLA and its copolymers are biocompatible and bioabsorbable,and therefore stand at the forefront of medical and commercial use[24].A PLA chain consists of repeating units of condensed lactic acid,linked by ester bonds.Under no load and in the presence of water molecules,an ester bond in a PLA chain can react with a water molecule to form a carboxylic acid and an alcohol,resulting in two dangling PLA chains(Fig.1(a))[25–27].Such hydrolysis can cleave ester bonds at multiple sites,either in one chain or in several chains,subsequently creating more dangling chains as well as free-moving short chains.The dangling chains and freemoving short chains cannot sustain force and therefore weaken the mechanical properties of the PLA.When the free-moving chains leak out of the PLA,a loss of weight is measured.
We hypothesize that hydrolysis takes place more rapidly at a crack front than elsewhere.Even when the PLA carries a small load,the sharp crack front concentrates stress.The ester bonds at the crack front react with water molecules from the environment to form two dangling chains,which lose the capacity to withstand stress.Thus,the crack advances(Fig.1(b)).
We adopted a tear test to study hydrolytic cracking.We cut a crack with a length of 10 mm using scissors along the centerline of a PLA film(Model GC3X5,ClearBags,Canada)with the dimensions 50 mm×100 mm×40μm.The PLA film was then transferred to a chamber,in which one arm was fixed horizontally on the top of the chamber using double-sided tape,and the other arm was loaded vertically by hanging a constant weight from it(Fig.2(a)).The humidity and pH inside the chamber were controlled by either immersing the specimen in a solution(deionized pure water for pH 7,0.01 mol·L-1hydrochloric acid for pH 2,0.01 mol·L-1sodium hydroxide for pH 12,and 10–5mol·L-1sodium hydroxide for pH 9)or dry air(dried by calcium oxide particles,relative humidity(RH)=5%).The applied weight gave an energy release rate of G=W/t,where W is the weight and t is the thickness of the PLA film(in our test,the thickness is fixed at 40μm).The toughness of the PLA film was found to be 1500 J·m-2,as measured by tear at a rate of 10 mm·min-1using a tensile machine(Instron 3342 Single Column universal testing system(UTS),100 N load cell,USA).At this high tear rate,the scission of polymer chains is entirely mechanical,unaffected by hydrolysis.To study the combined action of mechanics and chemistry,we kept the energy release rate lower than the toughness of the PLA film,so that the crack did not advance rapidly and ester bonds were cleaved by hydrolysis.The chamber was made to be transparent so that the crack extension could be clearly recorded from the top using an optical microscope(5 MP Handheld Digital Microscope Pro,Celestron,USA)(Fig.2(b)).The camera periodically took a photo of the crack front.In one test,the PLA film was immersed in an alkaline solution of pH 12,and the crack extended by 4.5 mm in 10 min(Fig.2(c)).
3.Results and discussion
We first studied the effect of an applied load on crack extension.We fixed the PLA films in the chamber,applied various loads,and immersed the films in a sodium hydroxide solution(pH 12).Under varied load,the crack extension was recorded as a function of time(Fig.3(a)).For all loads,the cracks extended with time,reaching 4–8 mm within 20 min.Applying a larger load did not seem to increase the crack extension in the same amount of time.We further calculated the crack velocity from the slope of the curves to be 7.59(±3.48)×10–6m·s-1,insensitive to the applied load.We also tested crack extension under other pHs and found that the crack velocities were insensitive to the applied load under a single fixed pH,but were lower with a lower pH(Fig.3(b)).The crack velocity at a low pH varies over a range of nearly one order of magnitude.This large range can be attributed to measurement errors arising from the very slow crack velocities in low pH solutions.A crack takes hours to propagate one pixel-distance and be detected,and the resultant error is potentially large.Therefore,it is possible that the crack velocity at pH<12 is sensitive to load with a magnitude of change less than one order of magnitude over the load range tested here.

Fig.1.PLA hydrolysis.(a)An ester bond in the PLA polymer chain cleaves into carboxylic acid and alcohol end groups,resulting in two dangling polymer chains;(b)at the crack front of the PLA,hydrolysis of an ester bond advances the crack.
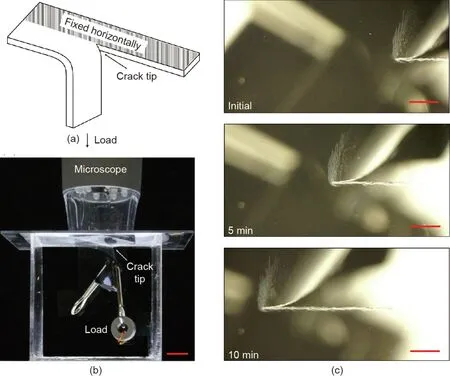
Fig.2.Tear test.(a)Schematic of the tear test;(b)photograph of the tear test;(c)the crack extends 4.5 mm in 10 min in an alkaline solution of pH 12.The scale bars represent 1 cm in(b)and 1 mm in(c).
We next studied the effect of humidity on crack extension.The number of water molecules available to react with the ester bonds at the crack tip may determine the kinetics of hydrolysis.To verify this,we tore PLA films by hanging a weight from the film(energy release rate of 1200 J·m-2)and placed the film and weight in either pure water or dry air with RH=5%.The crack extension was much slower in dry air than in pure water,advancing 0.2 mm in dry air after almost a week and about 0.25 mm in pure water after just two days(Fig.4).Note that the crack extension in air was not perfectly linear.The reason for this nonlinearity is unclear,but the deviation from linear trend line is comparable to the statistical scatter.We treat the crack extension as linear with time and calculate the average crack velocity as the slope of a linear fit.The average crack velocities were estimated to be 1.15×10–9m·s-1in pure water and 3.38×10–10m·s-1in dry air.This experiment confirmed that the hydrolysis of ester bonds governs the degradation of PLA.
The degradation kinetics of PLA may depend on the pH[28,29].A higher pH provides abundant hydroxide,which acts as a catalyst to accelerate the hydrolytic reaction and thus the crack velocity.To verify this,we tested the PLA films in different pH solutions under an energy release rate ranging from 1100–1450 J·m-2,and recorded the crack extension as a function of time(Fig.5).The crack extension increased with a higher pH.For example,at pH 2,the crack extended 0.175 mm in 6 h,while at pH 12,the crack extended 7 mm in just 15 min.The crack velocity was calculated as the average crack extension divided by the time(Fig.3(b)).The crack velocities varied over several orders of magnitude with pH.For example,the crack velocity in the pH 2 solution was 4.1×10–10m·s-1,whereas the crack velocity in the pH 12 solution was 6.7×10–6m·s-1,approximately four orders of magnitude faster.

Fig.3.The effect of applied load on crack extension.(a)PLA films are immersed in a solution with pH 12 under various loads(energy release rate:1100–1450 J·m-2);(b)the crack velocities as functions of energy release rate in different pH solutions.
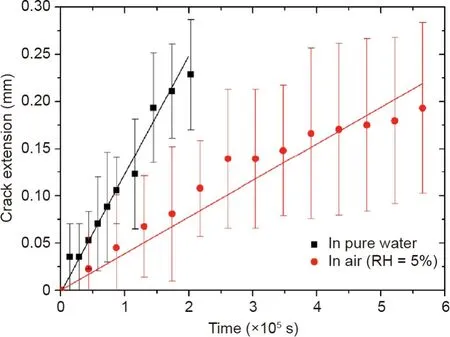
Fig.4.The effect of humidity on crack extension.Each data point represents the mean of 10–12 specimens and the error bars represent standard deviations.
We note that under a modest load,below toughness,a crack in PLA greatly outruns erosion.It has been reported that,in the absence of a mechanical load,PLA degrades through either surface or bulk erosion[30].At high pH,PLA degrades through surface erosion;while at low pH,PLA degrades through bulk erosion.It was reported that a cylindrical PLA sample,12.5 mm height and 1.4 mm diameter,lost 55%mass after 50 h in pH>13[30].We use this observation to estimate the erosion velocity at high pH.The initial sample has a surface area of 58.06 mm2and a volume of 19.24 mm3.We take the loss of mass to correspond to a loss of volume of 10.58 mm3.This loss of volume corresponds to the erosion of a layer of thickness 0.18 mm.The data indicates that the erosion is approximately linear in time;therefore,we estimate the erosion velocity to be about 1.01×10-9m·s-1.The erosion velocity at low pHcan be represented by the specific surface degradation rates(SSDR)reported for degradation of PLA in environmental conditions[31].In marine environments,the SSDR has been reported to be 2.38×10-13m·s-1[32].Since typical marine pH values range from 7.6 to 8.4,we compare this SSDR to our crack velocities at pH 7 and 9.At pH 12,the crack velocity is more than 1000 times faster than the estimated erosion velocity at pH>13.At pH 7 and 9,the crack velocities are,respectively,four and five orders of magnitude greater than the SSDR in marine environments.Because of the large discrepancy between the crack velocity and the erosion velocity,we therefore expect that local degradation by crack extension follows a different mechanism than surface or bulk erosion.
Here,we propose a possible reason for the discrepancy between bulk erosion and local degradation by crack extension.When the polymer chains on the PLA surface are cleaved by hydrolysis,the dangling chains—or polymer debris—are unable to sustain large mechanical loads,yet are still unable to dissolve[29,33].The polymer debris on a flat surface is behind the diffusion front and may inhibit water molecules from reaching the fresh PLA surface underneath(Fig.6(a)).However,under an applied load,a crack concentrates stress at the crack front,which breaks the debris layer and creates a path for water molecules to reach the fresh PLA surface underneath(Fig.6(b)).Once the load is large enough to break the debris,the local degradation rate by crack extension increases dramatically,much faster than the rate of bulk erosion.Increasing the load further can widen the path for water molecules to reach the crack tip and change the energy landscape of the hydrolysis reaction,but for the load range tested in this paper,the effect on the local degradation rate is negligible.During our tests,we observed that the PLA film,although fractured into two strips,remained in the form of bulk material.
We note that the observed load insensitivity of crack velocity is limited to loads between 1100 and 1450 J·m-2.A load sensitive regime may exist for loads outside of this range.For example,it has been observed that a brief load-sensitive regime of crack growth exists in a degradable poly(glycerol sebacate)elastomer before transitioning to a load insensitive regime[16].Similarly,both regimes were observed in a hybrid organic and inorganic molecular network,where a process zone separated the crack tip from the environment when the crack velocity was insensitive to small loads[34].It is possible that the mechanism of stress corrosion cracking in PLA is related to,among other factors,the crystallinity of the polymer,the pH,and the magnitude of the applied load.However,a detailed characterization of this mechanism is beyond the scope of this work.
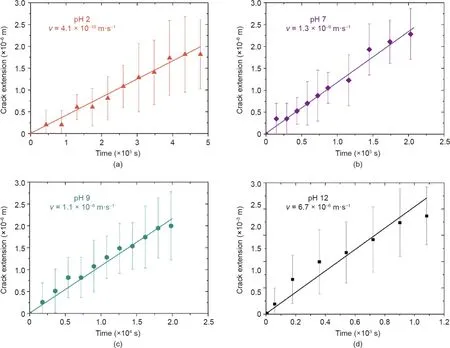
Fig.5.Crack extension as a function of time at different pH:(a)pH 2;(b)pH 7;(c)pH 9;and(d)pH 12.Each data point represents the mean of 10–12 specimens and the error bars represent standard deviations.

Fig.6.Bulk degradation versus local degradation.(a)A water molecule must pass through the debris layer to reach the fresh PLA surface;(b)at the crack tip,the debris breaks and a water molecule can easily reach the fresh PLA underneath.
Aliphatic polyesters are the most widely used environmentally degradable plastics[35].They include plastics such as PLA,polycaprolactone(PCL),polyhydroxybutyrate(PHB),and polyglycolic acid(PGA).All polyesters can degrade through the hydrolysis of ester bonds.Therefore,we expect that,like PLA,other aliphatic polyesters will also suffer crack growth under the combined action of a small mechanical load and water molecules.Indeed,cracks normal to the loading direction have been observed in polyethylene terephthalate(PET)fibers after in vivo implantation,indicative of stress corrosion cracking[36].Furthermore,hydrolytic degradation has been widely observed in a variety of other plastics besides polyesters;examples include the hydrolysis of glycosidic bonds in polysaccharides[37,38]and urethane bonds in polyurethane[39,40].In addition,materials that are not classified as degradable,such as poly(dimethyl siloxane),have been shown to exhibit hydrolytic crack growth under a mechanical load[41].Different degradation chemistries can lead to different degradation kinetics.Moreover,since the crack velocity corresponds to the rate of bond cleavage,sub-critical crack growth can be used to characterize the energy landscape of degradation[41].
4.Conclusions
In summary,we studied hydrolytic crack growth in PLA.We have confirmed the hypothesis that,when a load is applied at a magnitude below that which causes fast fracture,a crack in PLA outruns bulk erosion.The crack velocity is insensitive to the magnitude of the load but is sensitive to humidity and pH.We propose a mechanism for the large difference between the crack velocity and the erosion velocity due to bulk degradation.Our results suggest that other plastics that degrade through hydrolysis will also be susceptible to hydrolytic crack growth,and thus solicit further study.Hydrolytic crack growth can lead to premature failure and fragmentation of the material.Applications in packaging and medicine require accurate control of the mechanical properties of a degradable plastic throughout its lifetime,and fragmentation can lead to microplastics in the environment and complications in medical applications(e.g.,aseptic loosening).Furthermore,recyclable and self-healing materials are being developed that do not degrade through hydrolysis,yet may still suffer stress corrosion,including materials with dynamic covalent disulfide bonds[42]and degradable silyl ether monomers[43].Since the crack velocity corresponds to the rate of bond cleavage,degradation kinetics can be quantified by the kinetic theory of bond cleavage.This study develops a new path for studying degradation by cracking.Prompted by these findings,we have started the development of degradable polymers that resist hydrolytic crack growth.Taken together,this series of work may aid the development of degradable polymers for medicine and sustainability,where functional groups and triggers for degradation are not restricted to ester bonds and water molecules.
Acknowledgements
The work at Harvard University,USA was supported by National Science Foundation (NSF)Materials Research Science and Engineering Centers(MRSEC)(DMR-2011754).X.Yang was a visiting student at Harvard University supported by the China Scholarship Council.J.Steck acknowledges support from the NSF Graduate Research Fellowship(DGE1745303).
Compliance with ethics guidelines
Xuxu Yang,Jason Steck,Jiawei Yang,Yecheng Wang,and Zhigang Suo declare that they have no conflict of interest or financial conflicts to disclose.
- Engineering的其它文章
- Processing,Quality,Safety,and Acceptance of Meat Analogue Products
- Advanced Devices Ease Burden of Glucose Monitoring for Diabetics
- Functional Capsules Encapsulating Molecular-Recognizable Nanogels for Facile Removal of Organic Micro-Pollutants from Water
- Nanobubble Dynamics in Aqueous Surfactant Solutions Studied by Liquid-Phase Transmission Electron Microscopy
- Mechanically Strong Proteinaceous Fibers:Engineered Fabrication by Microfluidics
- China’s Latest Moon Mission Returns New Lunar Rocks

