多活性位点的磁性氮掺杂石墨烯活化过一硫酸盐研究
杨 柳,刘 丹,刘世昂,吴西林,陈建荣
多活性位点的磁性氮掺杂石墨烯活化过一硫酸盐研究
杨 柳,刘 丹,刘世昂,吴西林*,陈建荣
(浙江师范大学地理与环境科学学院,浙江 金华 321004)
通过简单的一锅法制备Fe2O3、Fe3N、单原子Fe(SA-Fe)和N掺杂的磁性石墨烯材料(Fe-MNG)应用于催化活化过一硫酸盐(PMS). 结果表明,Fe-MNG/PMS体系可在宽的pH范围(3-10)氧化降解磺胺异恶唑(SIZ),降解率均达到99%以上. 经过五次循环使用其对SIZ的降解率仍保持在95%以上. Fe-MNG中的SA-Fe、N等活性位点可高效催化活化PMS产生各种活性氧物种(ROS). 淬灭实验和电子顺磁共振波谱分析表明Fe-MNG/PMS体系中产生多种ROS,包括硫酸根自由基(SO4•–)、羟基自由基(HO•)和单线态氧(1O2),证明存在自由基和非自由基两种氧化过程. 此外,Fe-MNG具有大的比表面积(446.18m2/g),能将水中有机微污染物吸附富集到材料表面,同时在Fe-MNG表面催化PMS产生大量ROS,实现对有机微污染物的原位、高效氧化去除. Fe-MNG还具有磁性,易于分离和回收,具有潜在的应用前景.
高级氧化;过一硫酸盐;石墨烯;吸附;单原子催化
磺胺类抗生素是一大类抗菌药物和生长促进剂[1].磺胺呈弱酸性,溶于水,不能被人和动物完全代谢,也不能在常规污水处理厂中有效去除[2].因此,磺胺很容易排放到地表水中,并转移到水生环境中[3-4].磺胺的使用量很大,2016年美国对磺胺类抗生素的使用量为369t/a,2013年中国的使用量为7890t/a)[5].大量的研究表明,磺胺类药物已经广泛存在于各种环境介质,包括地表水[6],地下水[7]和沉积物[8]中. 磺胺类药物在环境中累积将导致抗生素的耐药性增加,诱导产生“超级细菌”,将对生态系统和人类健康构成巨大威胁.因此,高效去除水中的磺胺类抗生素具有重要的意义[9].
近年来,基于过硫酸盐的高级氧化工艺(AOPs),由于其强的氧化能力和绿色、无污染等优点,被广泛的研究应用于有机污染物的降解[10-13].与HO•相比,SO4•–具有更强的氧化性、更长的半衰期(30~ 40μs)[14].尽管过渡金属离子(Mn2+、Fe2+、Co2+、Ni2+和Cu2+等)能有效地活化过硫酸盐产生SO4•–[15-16],但使用过渡金属离子易造成二次环境污染且消耗大量的金属催化剂[17].因此,研究开发具有高催化活性、高稳定性、环境友好、易于固液分离和重复使用的催化材料[18],具有重要的应用价值.
纳米催化剂具有大比表面积、高催化活性和易于调控等优点,被广泛的应用于非均相催化领域.纳米材料催化的非均相高级氧化技术具有催化效率较高和环境友好等优点[19].过渡金属氧化物纳米粒子能高效地活化过一硫酸盐(PMS)降解水中的有机污染物[20-21].在各种过渡金属氧化物中,铁氧化物如Fe2O3、Fe3O4具有较好的生物相容性、较低的毒性和磁性等优点[22],在环境催化领域具有潜在的应用价值.此外,负载型金属催化材料也被广泛研究应用于环境催化.例如,钴酞菁负载的还原氧化石墨烯、CuFe2O4纳米颗粒负载的氮掺杂氧化石墨烯等均能有效催化活化PMS[20,23-24].石墨烯负载的金属催化剂具有金属催化和石墨烯吸附双重优势,有望实现对水中有机污染物的高效吸附富集和催化降解.然而,上述石墨烯基催化材料仍存在制备方法复杂、难以回收利用和稳定性较差等缺点.
为了解决上述问题,本文采用简单的一锅法制备了具有多活性位点的Fe2O3、Fe3N和单元子Fe负载的氮掺杂磁性石墨烯(Fe-MNG),并成功应用于高效催化活化PMS.本研究包括以下几个部分:1)探究初始pH、催化剂用量和PMS用量等对Fe-MNG/PMS体系降解磺胺异噁唑(SIZ)的影响;2)研究Fe-MNG的稳定性和循环使用性能;3)通过各种表征手段探明Fe-MNG催化PMS的活性位点,识别活性氧(ROS)物种,推测Fe-MNG/ PMS体系中ROS产生机理.
1 实验部分
1.1 试剂与仪器
三聚氰胺(M)由鼎盛鑫化学工业有限公司(中国天津)提供.美罗培南(MEM)、糠醇(FFA)、氯化血红素(Hemin)和聚乙烯亚胺(PEI)购自阿拉丁试剂公司(中国).磺胺异恶唑(SIZ),磺胺甲恶唑(SMX)购自东京化学工业有限公司(日本东京).三聚氰酸(CA)购自阿法埃莎公司(中国).过一硫酸盐(PMS)、盐酸(HCl)、氢氧化钠(NaOH)、甲醇(CH3OH)、异丙醇((CH3)2CHOH)购自国药化学试剂有限公司(中国).实验用0.45μm水系聚醚砜过滤膜购于天津津腾实验设备有限公司.
用透射电子显微镜(TEM,JEM-2100,日本)、X射线光电子能谱(XPS,Thermo Escalab 250,美国)、X射线衍射仪(XRD,Shimadzu XRD-6000,日本)、激光拉曼光谱仪(Raman, Horiba Jobin Yvon LabRam,法国)对材料进行表征.使用比表面分析仪(Quantachrome Instruments version 11.03)测试材料的比表面积和孔径.采用总有机碳(TOC)分析仪(Elementar,Liqui TOC II,德国)测定反应溶液中有机物总量.电子顺磁共振(EPR,JES-FA200,Japan)测定体系中的活性氧物种.溶液中的有机物含量由高效液相色谱仪测定(HPLC,Agilent 1200Infinity).
1.2 Fe-MNG的制备
前驱体由0.32g(2.5mmol)的三聚氰胺(M)、0.52g(4mmol)的三聚氰酸(CA)和0.50g的聚乙烯亚胺(PEI)在20mL去离子水中自组装得到.将得到的乳状胶体用0.45μm滤膜过滤,在60℃下真空干燥10h,即得到PEI-MCA前驱体.将血红素(0.05g)溶于5.0mL乙醇中并与1.25g PEI-MCA前驱体充分研磨.将得到的混合物置于管式煅烧炉中,在550℃、氮气气氛中煅烧1h,后升温至800℃煅烧2h.待管式炉冷却至室温,即得到Fe-MNG材料.
1.3 催化降解实验
将3.0mg催化剂粉末超声分散到30mL的SIZ溶液(20mg/L)中,搅拌60min达到吸附平衡.接着在悬浮液中加入3.0mg的PMS引发氧化降解反应.在一定的时间间隔,吸取0.5mL反应溶液加入到0.5mL甲醇中淬灭反应,并立即用0.45μm的过滤膜分离催化剂.溶液中的SIZ浓度通过高效液相色谱 (HPLC)测定.HPLC检测器为紫外检测器,检测波长为270nm.流动相的组成为乙腈:水(含0.15%三氟乙酸(TFA))=55:45,流速为0.7mL/min,进样量为20µL.循环实验保持上述所有条件不变,每次降解实验后,将Fe-MNG催化剂分离,用乙醇和去离子水分别清洗3次后真空干燥,再进行下一次循环.
2 结果与讨论
2.1 材料表征
透射电子显微镜(TEM)图片中[图1(a)],Fe纳米颗粒分布在在褶皱的石墨烯薄片上.高分辨TEM (HRTEM)图像[图1(b)]显示Fe纳米粒子的晶格条纹间距均为0.23nm,对应于Fe2O3的(104)晶面[25].由图1c的TEM图片和图1d~1f的能量色散X射线能谱(EDX)分析,可以看出,C、N元素均匀分布在石墨烯片的整个范围内,而Fe颗粒分散在石墨碳基质中.Fe-MNG和石墨烯(graphene)的X-射线衍射(XRD) [图1(g)]显示在20°~30°之间存在一个宽峰,对应于石墨烯中石墨碳的特征峰[26].而Fe-MNG的XRD图谱中大多数的特征峰属于Fe2O3(JCPDS 16-0653)22和Fe3N(JCPDS 49-1664).图1h中,Fe-MNG的拉曼光谱中存在G带和D带[27]. G带(1580cm–1)由sp2碳的振动形成,D带(1350cm–1)由无序碳产生[28].D带与G带的强度比为1.37[29].表明该氮掺杂的石墨烯中存在大量的缺陷,从而提供额外的活性位点.

图1 样品的TEM图、能谱mapping图像、XRD谱图和Raman光谱图

图2 刻蚀后Fe-MNG的TEM、XRD和HAADF-STEM图片
Fe-MNG中除了存在Fe2O3和Fe3N外,还存在大量的单原子Fe(SA-Fe)分散在石墨烯载体中.为证明单原子Fe(SA-Fe)的存在,通过酸刻蚀后,Fe-MNG中的铁氧化合物以及铁氮化合物均被去除,仅检测到一个石墨稀的宽峰.由图2(a)中的TEM和HRTEM图片,以及图2(b)中的XRD谱图可以证明铁颗粒被去除.球差矫正高角环形暗场像扫描透射电子显微镜(HAADF-STEM)图片[图2(c)、2(d)]显示存在大量的金属原子亮点(白色虚线圆圈标注),说明存在SA-Fe活性位点.进一步证明Fe2O3、Fe3N、单原子Fe(SA-Fe)和N掺杂的磁性石墨烯材料(Fe-MNG)被成功制备.

图3 Fe-MNG的N2吸附—脱附及XPS谱图
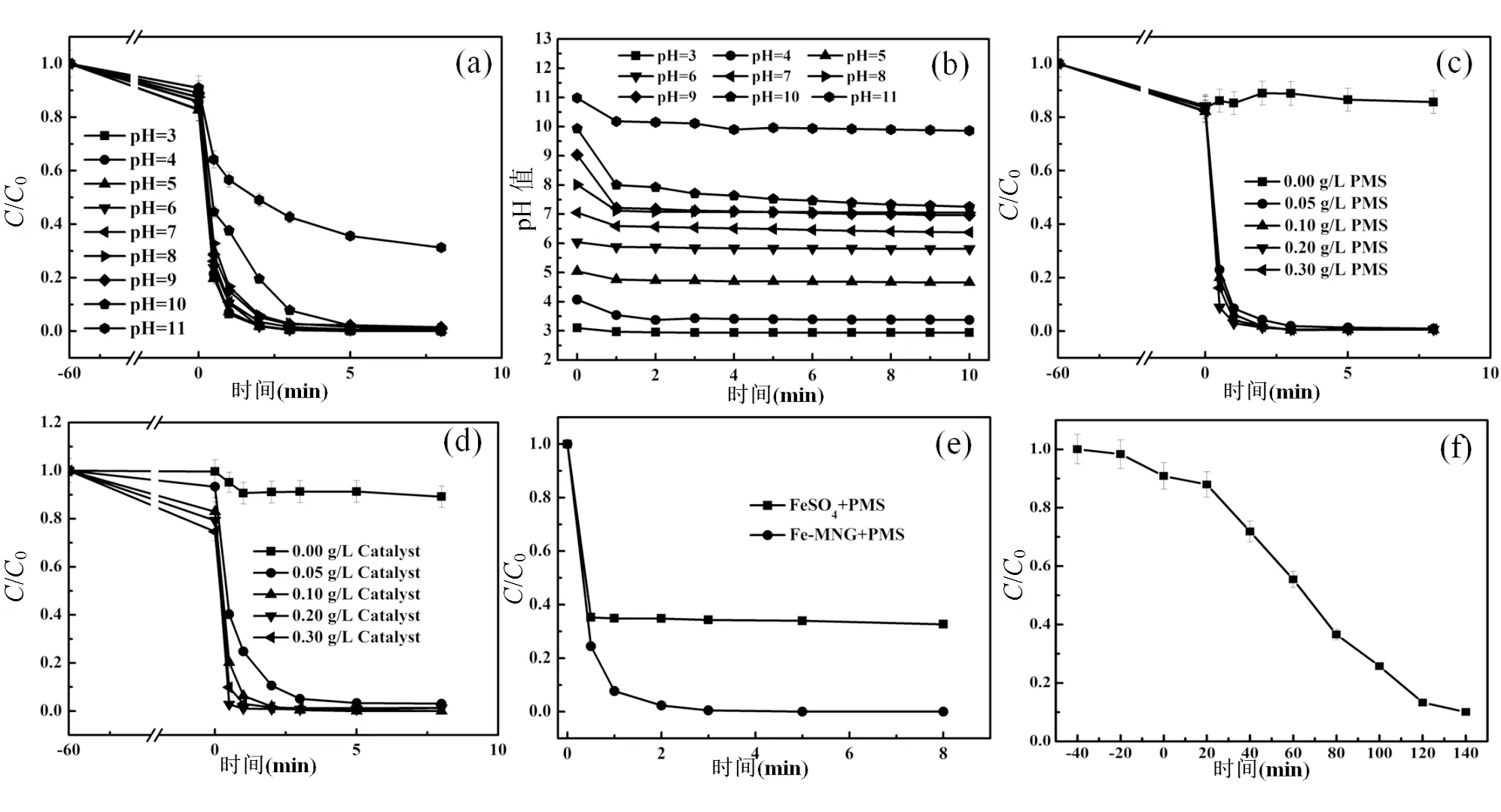
图4 pH值、PMS和Fe-MNG用量对SIZ降解的影响
[SIZ]=20mg/L,[PMS]=0.1g/L,[Fe-MNG]=[FeSO4]=0.1g/L,pH=3.0,=303K
如图3a所示,图中吸附等温线在相对高压力区域(/0>0.5)呈现出闭合的回滞环,证明存在大量的介孔.图3a中孔径分布也证明了介孔的存在[30].根据Brunauer-Emmett-Teller(BET)分析, Fe-MNG的比表面积为446.18m2/g,说明其比表面积巨大.该材料的多孔性质和大的比表面积赋予其大量的离子、分子传输通道和暴露更多的催化活性位点,从而极大提升非均相催化的效率[31-32].图3(b)为Fe-MNG和石墨烯的X-射线光电子能谱(XPS)图,可以看出Fe-MNG中存在C,N,O和Fe元素.其中N的峰最强,说明其中大量的N掺杂.这些N掺杂在石墨烯载体中提供了丰富的缺陷位点,同时为固定金属Fe原子提供优良的配位环境.Fe-MNG的高分辨N 1s XPS谱图在398.2, 399.6,400.6和404.0eV处的峰,分别对应于吡啶N(63.2%)、吡咯N(17.3%)、石墨N(19.5%)和氧化N[图3(c)][33-34].大量存在的吡啶N和吡咯N为Fe单原子提供锚定位点.此外,吡啶N和吡咯N上的孤对电子与石墨烯π电子耦合,极大促进了催化过程中石墨烯载体与金属活性位点之间的电子转移.高分辨Fe 2p XPS光谱中[图3(d)]存在4个峰,其中710.9和724.5eV对应于Fe2+,712.9和729.0eV处对应于Fe3+[35-36].Fe3+主要来源于Fe2O3,而Fe2+主要来源于单原子Fe,进一步证明Fe-MNG中存在多种Fe活性位点[37].
2.2 吸附、催化性能研究
对Fe-MNG催化活化过一硫酸盐(PMS)降解磺胺异恶唑(SIZ)的性能进行了研究.如图4(a)所示,在60min吸附过程,大约10-20%的SIZ被吸附.在宽的pH值范围 (pH 3.0~10.0),Fe-MNG/PMS体系中SIZ(20mg/L)的降解率均达到98%以上.在pH11.0时,SIZ的降解率下降到65%,说明在强碱性条件下Fe-MNG/PMS体系的氧化性能降低.在碱性条件下PMS易失去质子生成SO52−(式(1)),并进一步消耗PMS反应生成1O2(式(2)).
HSO5−- H+→ SO52−(1)
HSO5−+ SO52−→HSO4−+ SO42−+1O2(2)
SO4•–+ OH−→SO42−+ HO•(3)
尽管碱可以活化PMS,但是强碱性条件不利于SO4•–的生成(式(3)),导致在pH11时氧化效果变差.此外,在碱性条件下HO•自由基的氧化还原电位下降,导致其氧化性能下降.图4(b)展示了Fe-MNG/ PMS体系反应过程中的pH值变化,可以发现体系的pH值略有下降,说明加入了少量的PMS(0.1g/L)对体系的pH值略有影响.采用ICP-MS检测了不同pH值条件下铁离子的浸出,测得的铁离子浓度均小于0.1mg/L,说明该材料具有优异的稳定性,证明炭包裹可以减少金属的浸出.图4(c)和图4(d)表明在较低的Fe-MNG(0.05g/L)和PMS(0.05g/L)用量下,SIZ的降解率均能达到99%,说明该体系具有高的催化氧化效率,可以极大减少催化剂和氧化剂的用量.本文还比较了基于PMS的非均相催化氧化体系与均相催化氧化体系降解SIZ的效果,从图4(e)可以看出Fe-MNG/PMS比Fe2+/PMS能更好地降解SIZ,这可能由于Fe-MNG催化剂中有更多的催化活性位点且提高了PMS的利用率.此外,在均相体系中Fe2+逐渐被氧化为Fe3+且不易再生,使得其催化性能下降.污染物的矿化程度也是衡量高级氧化体系的重要指标.如图4(f)所示,在140min内,Fe-MNG/PMS降解SIZ体系的总有机碳(TOC)去除率达到90%,表明大量的中间产物降解为小分子H2O和CO2[38-39],证明Fe-MNG/PMS高级氧化体系具有优异的氧化能力,对污染物的矿化程度较高.
循环使用性能是衡量催化剂实用性的一项重要指标.如图5a所示,在Fe-MNG/PMS体系中,五次循环降解SIZ的去除率分别达到99.9%, 99.8%, 99.0%,98.0%和96.0%,表明该催化剂具有良好的循环使用性能.研究了Fe-MNG对SIZ的吸附性能(图5b).SIZ在Fe-MNG上的吸附等温线分别用Langmuir和Freundlich等温线模型进行拟合[40-41]. Langmuir模型数学公式如下:
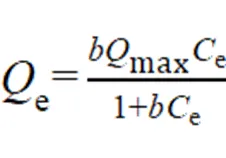
式中:e(mg/g)为平衡条件下单位质量Fe-MNG对SIZ的吸附量,e(mg/L)是吸附平衡时溶液中SIZ的浓度,max(mg/g)为计算得到的SIZ在Fe-MNG上的最大吸附量,而(L/g)为Langmuir模型的吸附常数[42].Freundlich模型的数学公式如下:
e=e1/n(5)
Freundlich常数和分别代表了吸附能力和吸附强度.表1为对应的拟合参数.结果表明Langmuir模型能更好的拟合实验数据,说明SIZ在Fe-MNG上的吸附属于单层吸附[43].SIZ在Fe-MNG上的最大吸附量达到263.27mg/g,表面该材料具有较大的吸附容量.此外,Fe-MNG易于在外加磁场下分离、回收和重复使用(图5b).
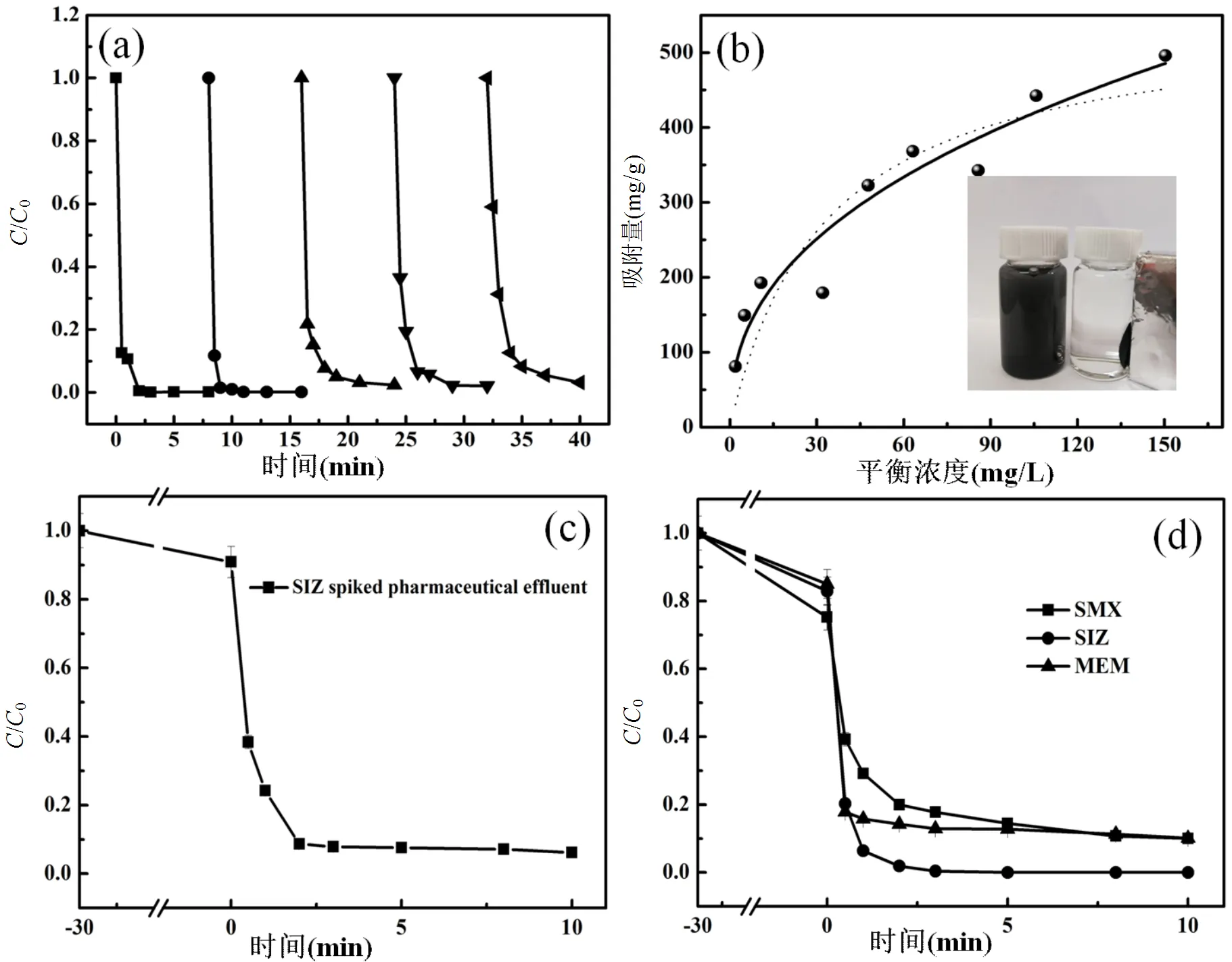
图5 Fe-MNG催化降解及吸附试验
[SMX]=[SIZ]=[MEM]=20mg/L,[PMS]=0.1g/L,[Fe-MNG]=0.1g/L,pH=7.0,=303K,=24h
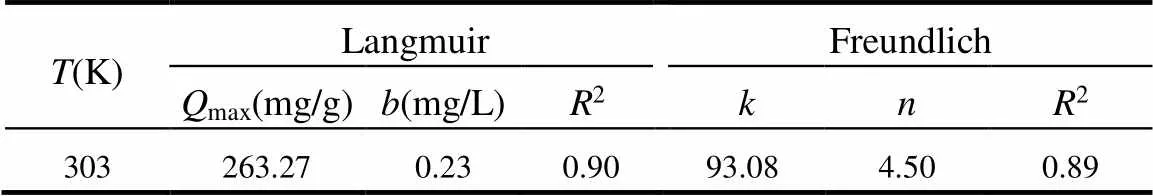
表1 Langmuir和Freundlich模型拟合吸附等温线参数
在制药厂污水处理厂出水中添加SIZ(20mg/L)模拟真实废水,进一步探究Fe-MNG催化活化PMS对实际废水的处理效果.结果表明[图5(c)],在10min内超过94%的SIZ被氧化去除.此外,Fe-MNG/PMS体系还能高效去除其它抗生素类有机微污染物[图5(d)],如磺胺甲恶唑(去除率88.7%)和美洛培南(去除率90.0%).上述结果表明,具有多催化活性位点的Fe-MNG材料能高效活化PMS;同时大比表面积的石墨烯载体赋予其优异的吸附性能,能有效富集水中低浓度的有机微污染物;通过吸附富集和催化氧化协同作用实现水中抗生素类有机微污染物的快速高效去除.此外, Fe-MNG还具有磁性和优异的循环使用性能,易于回收和重复使用,具有潜在的应用前景.
2.3 催化机理探究
猝灭实验可探究Fe-MNG/PMS体系中产生的活性物种.采用异丙醇(IPA)淬灭羟基自由基(HO•),甲醇(MeOH)淬灭HO•和硫酸根自由基(SO4•–),糠醇(FFA)淬灭单线态氧(1O2).如图6(a)所示,加入FFA、IPA和MeOH后,SIZ的降解率分别为29%、88%和58%,表明1O2、SO4•–和HO•均起到氧化降解SIZ的作用.因此,Fe-MNG对SIZ优异的降解效果归因于自由基(SO4•–,HO•)与非自由基(1O2)的共同作用.

图6 猝灭实验、EPR谱图和Fe-MNG/PMS体系降解SIZ示意
电子顺磁共振 (EPR)实验使用5,5-二甲基-1-吡咯啉N-氧化物(DMPO)作为SO4•–和HO•捕获剂,2,2,6,6-四甲基-4-哌啶醇(TEMP)作为1O2捕获剂.如图6(b)所示,在Fe-MNG/PMS体系中观察到相同强度的三重峰,对应于TEMP-1O2信号,表明在Fe-MNG/PMS体系中产生1O2物种[17,44].类似地, Fe-MNG/PMS体系中探测到DMPO-HO•(1:2:2:1四重峰)和DMPO-SO4•–(1:1:1:1:1:1六重峰)的信号[图6(c)],表明Fe-MNG可以有效活化 PMS产生活性自由基.上述结果进一步证明SO4•–,HO•与1O2共存于Fe-MNG/PMS体系中.
首先,活性Fe物种可以催化活化PMS产生自由基.其中,单元子Fe物种(≡Fe2+)可催化PMS产生SO4•–(反应式(6)和(7)).此外,产生的SO4•–可进一步生成HO•(反应式(8))[45].
HSO5–+ ≡Fe2+→ SO4•–+ ≡Fe3++ OH–(6)
≡Fe3++ HSO5–→ SO5•–+ Fe2++ H+(7)
SO4•–+ H2O→ SO42–+ HO•(8)
其次,丰富的N活性位点也可通过电子转移活化PMS(反应式(9)和(10)),产生SO4•–和HO•自由基[46].
HSO5–+ e–→ SO42–+ HO•(9)
HSO5–+ e–→ OH–+ SO4•–(10)
在Fe-MNG作用下,PMS产生大量的1O2(反应式(11)).上述结果表明,Fe-MNG可以显著活化PMS产生大量ROS(反应式(12)).最终,表面吸附的SIZ可以上述原位产生的ROS降解为小分子中间产物,并逐渐矿化为H2O和CO2[图6(d)].
2SO5•−+ H2O → 2HSO4−+1.51O2(11)
SO4•−+•OH +1O2+ SIZ → degraded products(12)
3 结论
采用简单的一锅法,合成出具有Fe2O3、Fe3N、SA-Fe和N掺杂的Fe-MNG材料.Fe-MNG复合材料兼具优异的吸附性能和催化效果,通过高效吸附富集和快速氧化降解,实现抗生素类有机微污染物如SMX、SIZ和MEM的高效去除.Fe-MNG活化PMS氧化过程包括自由基和非自由基两种途径,产生的SO4•−、•OH 和1O2多种ROS共同起到高效氧化和矿化有机污染物的作用.此外,Fe-MNG还具有磁性和优良的循环使用性能,易于回收和重复使用.本研究提供了一种廉价制备具有多活性中心的新型石墨烯复合材料的方法,将极大推动非均相类芬顿氧化技术的实际应用.
[1]Wang P, Zhou T, Wang R, et al. Carbon-sensitized and nitrogen-doped TiO2for photocatalytic degradation of sulfanilamide under visible-light irradiation [J]. Water Research, 2011,45(16):5015–5026.
[2]Du L, Cheng S, Hou Y, et al. Influence of sulfadimethoxine (SDM) and sulfamethazine (SM) on anammox bioreactors: Performance evaluation and bacterial community characterization [J]. Bioresource Technology, 2018,267:84–92.
[3]Guo M T, Yuan Q B, Yang J. Microbial selectivity of UV treatment on antibiotic-resistant heterotrophic bacteria in secondary effluents of a municipal wastewater treatment plant [J]. Water Research, 2013, 47(16):6388–6394.
[4]Kim S D, Cho J, Kim I S, et al. Occurrence and removal of pharmaceuticals and endocrine disruptors in South Korean surface, drinking, and waste waters [J]. Water Research, 2007,41(5):1013–1021.
[5]Liu M, Zhang D, Han J, et al. Adsorption enhanced photocatalytic degradation sulfadiazine antibiotic using porous carbon nitride nanosheets with carbon vacancies [J]. Chemical Engineering Journal, 2020,382:382–392.
[6]El-Ghenymy A, Rodriguez R M, Brillas E, et al. Electro-Fenton degradation of the antibiotic sulfanilamide with Pt/carbon-felt and BDD/carbon-felt cells. Kinetics, reaction intermediates, and toxicity assessment [J]. Environmental Science and Pollution Research, 2014,21(14):8368–8378.
[7]Du J, Xiao G, Xi Y, et al. Periodate activation with manganese oxides for sulfanilamide degradation [J]. Water Research, 2020,169:1–35.
[8]Lei K H, Lai H T. Effects of sunlight, microbial activity, and temperature on the declines of antibiotic lincomycin in freshwater and saline aquaculture pond waters and sediments [J]. Environmental Science and Pollution Research, 2018,26(33):33988–33994.
[9]Zhang R, Yang Y, Huang C H, et al. Kinetics and modeling of sulfonamide antibiotic degradation in wastewater and human urine by UV/H2O2and UV/PDS [J]. Water Research, 2016,103:283–292.
[10]Liu Y, Hu J, Wang J. Fe2+enhancing sulfamethazine degradation in aqueous solution by gamma irradiation [J]. Radiation Physics and Chemistry, 2014,96:81–87.
[11]Zhou Y, Jiang J, Gao Y, et al. Activation of peroxymonosulfate by benzoquinone: A novel nonradical oxidation process [J]. Environmental Science & Technology, 2015,49(21):12941–12950.
[12]Xu Y, Ai J, Zhang H. The mechanism of degradation of bisphenol A using the magnetically separable CuFe2O4/peroxymonosulfate heterogeneous oxidation process. [J]. Journal of Hazardous Materials, 2016,309:87–96.
[13]Chen W H, Xiong J H, Teng X, et al. A novel heterogeneous Co(II)- Fenton-like catalyst for efficient photodegradation by visible light over extended pH [J]. Science China Chemistry, 2020,63(12):1823–1836.
[14]Wang J, Wang S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants [J]. Chemical Engineering Journal, 2018,334:1502–1517.
[15]Oh W D, Chang V W C, Hu Z T, et al. Enhancing the catalytic activity of g-C3N4through Me doping (Me = Cu, Co and Fe) for selective sulfathiazole degradation via redox-based advanced oxidation process [J]. Chemical Engineering Journal, 2017,323:260–269.
[16]Li H, Wan J, Ma Y, et al. Degradation of refractory dibutyl phthalate by peroxymonosulfate activated with novel catalysts cobalt metal- organic frameworks: Mechanism, performance, and stability [J]. Journal of Hazardous Materials, 2016,318:154–163.
[17]Sun H, Peng X, Zhang, S, et al. Activation of peroxymonosulfate by nitrogen-functionalized sludge carbon for efficient degradation of organic pollutants in water [J]. Bioresource Technology, 2017,241:244–251.
[18]Yılmaz Baran N. Fabrication and characterization of a novel easy recoverable and reusable Oligoazomethine-Pd(II) catalyst for Suzuki cross-coupling reactions [J]. Journal of Molecular Structure, 2019, 1176:266–274.
[19]Yan D, Zhao H, Pei J, et al. Metal ion-mediated structure and properties of α-Fe2O3nanoparticles [J]. Materials Research Bulletin, 2018,101:100–106.
[20]Marinescu C, Ben A M, Hamdi A, et al. Cobalt phthalocyanine- supported reduced graphene oxide: A highly efficient catalyst for heterogeneous activation of peroxymonosulfate for rhodamine B and pentachlorophenol degradation [J]. Chemical Engineering Journal, 2018,336:465–475.
[21]Sajjadi M, Nasrollahzadeh M, Mohammad Sajadi S. Green synthesis of Ag/Fe3O4nanocomposite using Euphorbia peplus Linn leaf extract and evaluation of its catalytic activity [J]. Journal of Colloid and Interface Science, 2017,497:1–13.
[22]Tian Y, Hu X, Wang Y, et al. Fe2O3nanoparticles decorated on graphene-carbon nanotubes conductive networks for boosting the energy density of all-solid-state asymmetric supercapacitor [J]. ACS Sustainable Chemistry & Engineering, 2019,7(10):9211–9219.
[23]Li Z, Ma S, Xu S, et al. Heterogeneous catalytic degradation of organic pollutants by peroxymonosulfate activated with nitrogen doped graphene oxide loaded CuFe2O4[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2019,577:202–212.
[24]Yu X J, Qu J, Yuan Z, et al. Anisotropic CoFe2O4@graphene hybrid aerogels with high flux and excellent stability as building blocks for rapid catalytic degradation of organic contaminants in a flow- type setup [J]. ACS Applied Materials & Interfaces, 2019,11(37):34222– 34231.
[25]Abbas N, Shao G N, Haider M S, et al. Sol–gel synthesis of TiO2- Fe2O3systems: Effects of Fe2O3content and their photocatalytic properties [J]. Journal of Industrial and Engineering Chemistry, 2016, 39:112–120.
[26]Xiao P, Wang P, Li H, et al. New insights into bisphenols removal by nitrogen- rich nanocarbons: Synergistic effect between adsorption and oxidative degradation [J]. Journal of Hazardous Materials, 2018,345:123–130.
[27]Stobinski L, Lesiak B, Malolepszy A, et al. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods [J]. Journal of Electron Spectroscopy and Related Phenomena, 2014,195:145–154.
[28]Couzi M, Bruneel J L, Talaga D, et al. A multi wavelength Raman scattering study of defective graphitic carbon materials: The first order Raman spectra revisited [J]. Carbon 2016,107:388–394.
[29]Li B, Cao H, Shao J, et al. Superparamagnetic Fe3O4nanocrystals@ graphene composites for energy storage devices [J]. Journal of Materials Chemistry, 2011,21(13):5069–5075.
[30]Zhang X, Gao B, Creamer A E, et al. Adsorption of VOCs onto engineered carbon materials: A review [J]. Journal of Hazardous Materials, 2017,338:102–123.
[31]Yang Y J, Xu L J, Li W Y, et al. Adsorption and degradation of sulfadiazine over nanoscale zero-valent iron encapsulated in three- dimensional graphene network through oxygendriven heterogeneous Fenton-like reactions [J]. Applied Catalysis B: Environmental, 2019, 259:358–369.
[32]Ma Y Y, Lv X F, Yang Q, et al. Reduction of carbon tetrachloride by nanoscale palladized zero-valent iron@ graphene composites: Kinetics, activation energy, effects of reaction conditions and degradation mechanism [J]. Applied Catalysis A, General, 2017,542 (252–261):105–112.
[33]Long B, Lin J, Wang X. Thermally-induced desulfurization and conversion of guanidine thiocyanate into graphitic carbon nitride catalysts for hydrogen photosynthesis [J]. Journal of Materials Chemistry A, 2014,2(9):2847–3258.
[34]Song W L, Ge P, Ke Q, et al. Insight into the mechanisms for hexavalent chromium reduction and sulfisoxazole degradation catalyzed by graphitic carbon nitride: The Yin and Yang in the photo-assisted processes [J]. Chemosphere, 2019,221:166–174.
[35]Song X, Shi Q, Wang H, et al. Preparation of Pd-Fe/graphene catalysts by photocatalytic reduction with enhanced electrochemical oxidation-reduction properties for chlorophenols [J]. Applied Catalysis B: Environmental, 2017,203:442–451.
[36]Bicalho H A, Lopez J L, Binatti I, et al. Facile synthesis of highly dispersed Fe(II)-doped g-C3N4and its application in Fenton-like catalysis [J]. Molecular Catalysis, 2017,435:156–165.
[37]Huang X, Niu Y, Hu W. Fe/Fe3C nanoparticles loaded on Fe/N-doped graphene as an efficient heterogeneous Fenton catalyst for degradation of organic pollutants [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2017,518:145–150.
[38]Liao G, Chen S, Quan X, et al. Graphene oxide modified g- C3N4hybrid with enhanced photocatalytic capability under visible light irradiation [J]. Journal of Materials Chemistry, 2012,22(6):2721–2726.
[39]Morales-Torres S, Pastrana-Martínez L M, Figueiredo J L, et al. Graphene oxide-P25 photocatalysts for degradation of diphenhydramine pharmaceutical and methyl orange dye [J]. Applied Surface Science, 2013,275:361–368.
[40]王 浩,陈 枫,柯 倩,等.氮化硼负载磷钨酸铁对U(VI)的吸附及其机理研究 [J]. 中国科学, 2018,49(1):123–132.
Wang H, Chen F, Ke Q, et al. Adsorption of U(VI) by boron nitride-supported iron phosphotungstate: an experimental and mechanism study [J]. Scientia Sinica Chimica, 2018,49(1):123–132.
[41]李碧云,吴忆涵,唐 昊,等.氰基改性UiO-66的合成及其对Eu(III)的去除性能及机理研究 [J]. 中国科学, 2020,50(8):936–944.
Li B, Tang Y H, Tang H, et al. Synthesis of cyano-modified UiO-66 and its properties and mechanisms for Eu(III) removal [J]. Scientia Sinica Chimica, 2020,50(8):936–944.
[42]毕薇薇,陈 娅,马晓雁,等.磁性有序介孔碳的制备及其对水中双酚A的吸附 [J]. 中国环境科学, 2020,40(11):4762–4769.
Bi W W, Chen Y, Ma X Y, et al. Synthesis of magnetic ordered mesoporous carbon and its adsorption of bisphenol A in water. [J]. China Environmental Science, 2020,40(11):4762–4769.
[43]Li H, Lu T, Pan L, et al. Electrosorption behavior of graphene in NaCl solutions [J]. Journal of Materials Chemistry, 2009,19(37):6673–6679.
[44]Yang Z, Qian J, Yu A, et al. Singlet oxygen mediated iron-based Fenton-like catalysis under nanoconfinement [J]. Proceedings of the National Academy of Sciences, 2019,116(14):6659–6664.
[45]Liu Y, Guo H, Zhang Y, et al. Heterogeneous activation of peroxymonosulfate by sillenite Bi25FeO40: Singlet oxygen generation and degradation for aquatic levofloxacin [J]. Chemical Engineering Journal, 2018,343:128–137.
[46]孙 鹏,柳佳鹏,王维大,等.活性炭强化热活化过硫酸盐降解对硝基苯酚 [J]. 中国环境科学, 2020,40(11):4779–4785.
Sun P, Liu J P, Wang W D, et al. Active carbon enhanced thermal activation of persulfate for degradation of p-nitrophenol. [J]. China Environmental Science, 2020,40(11):4779–4785.
Magnetic N-doped graphene with multiple catalytic sites for efficient activiation of peroxymonosulfate.
YANG liu, LIU Dan, LIU Shi-ang, WU Xi-lin*, CHEN Jian-rong
(College of Geography and Environmental Sciences, Zhejiang Normal University, Jinhua 321004, China)., 2021,41(9):4127~4134
The Fe2O3, Fe3N, single-atom Fe (SA-Fe) and N-doped magnetic graphene (Fe-MNG) were prepared by a facile one-pot method and applied for the activation of perpxymonosulfate (PMS). The results show that Fe-MNG/PMS system was efficient for the oxidative degradation of sulfisoxazole (SIZ) over a wide pH range (3~10) with the removal percentages over 99%. After five cycles, degradation percentages of SIZ maintain over 95% by using the recycled Fe-MNG catalyst. The multiple catalytic sites such as SA-Fe and N of Fe-MNG can effectively activate PMS for the generation of various reactive oxygen species (ROS). Quenching experiment and electron paramagnetic resonance spectroscopy showed that SO4•–, HO• and1O2were produced in the Fe-MNG/PMS system, demonstrating the co-existence of free radicals and non-radicals processes. In addition, the Fe-MNG possesses large surface area (446.18m2/g), which can pre-concentrate organic micropollutants onto its surface by adsorption, simultaneously producing a large amount of ROS via PMS activation, leading to the in-situ and high-efficiency oxidation and removal of organic micropollutants. The Fe-MNG also possesses magnetic properties which can be easily recycled, indicating its great application potential.
advanced oxidation process;peroxymonosulfate;graphene;adsorption;single-atom catalysis
X703.5
A
1000-6923(2021)09-4127-08
杨 柳(1995-),女,新疆五家渠人,浙江师范大学硕士研究生,主要研究方向为高级氧化技术.
2021-02-02
浙江省自然科学基金(LGF19B070006);浙江省重点研发计划(2021C03163)
* 责任作者, 副教授, dbwxl@zjnu.cn

