氮氧同位素示踪南昌秋冬季降水中NO3-来源及其贡献
艾文强,肖红伟*,孙启斌,张永运,张泽雨,李静雯
氮氧同位素示踪南昌秋冬季降水中NO3-来源及其贡献
艾文强1,2,肖红伟1,2*,孙启斌3,张永运3,张泽雨2,李静雯2
(1.东华理工大学,江西省大气污染成因与控制重点实验室,江西 南昌 330013;2.东华理工大学水资源与环境工程学院,江西 南昌 330013;3.中山大学大气科学学院,广东 珠海 519082)
为确定南昌市秋冬季降水中硝酸盐来源及贡献,于2016年9月1日至2017年2月28日对南昌地区雨水进行采集,分析了其化学组成及NO3-同位素组成并利用贝叶斯混合模型对NO3-四种潜在来源贡献进行计算.结果显示NO3-浓度范围为7.3~99.5μmol/L,平均值为36.1μmol/L;δ15N-NO3-变化范围为-6.0‰~+8.3‰,平均值为-0.8‰,两者均呈现冬季高秋季低的变化趋势.NO3-浓度季节性变化可能是受到降雨量等因素的影响,而δ15N-NO3-变化可能是冬季降水中机动车尾气排放偏高和秋季降水中煤燃烧来源偏高双重因素作用的结果.同位素及贝叶斯混合模型源解析结果表明,南昌市降水中NO3-主要来源于生物质燃烧(32.5%)、机动车尾气排放(30.8%)和煤燃烧(23.1%),三者贡献超过86%;而机动车尾气排放和生物质燃烧释放均超过30%,这可能与近年来机动车快速增加和秋冬季野外生物质大量燃烧有关.煤燃烧虽然也是重要来源,但相对生物质燃烧和机动车尾气排放较小,这可能与近年我国减排措施有关.
南昌;NO3-;降雨;氮同位素;贝叶斯混合模型(MixSIA)
硝酸盐(NO3-)主要由NO(NO+NO2)转化形成,是大气气溶胶和酸雨的重要组分[1-4].近几十年来,随着工业化和城镇化的快速推进,人类活动产生的NO被大量释放至大气[4-5].研究表明,NO释放至大气后主要通过两种途径形成HNO3[6].白天,在光照条件下,NO发生Leighton循环(R1~R3),随后同光解产生的×OH反应生成气态HNO3;夜间,由于光照停止,R5成为夜间NO2消耗的主要反应,生成的NO3可与NO2结合形成N2O5(R6)并在气溶胶表面非均相水解形成液态HNO3(R7)[5,7-9].其中均相反应生成的HNO3,可改变大气的酸碱环境,也可在气溶胶表面经一系列反应生成颗粒态硝酸盐或与碱性物质反应形成颗粒物或吸附在碱性颗粒物上,改变其理化特性并使其粒径增长[6],造成灰霾现象.2013年以来,为控制酸雨和灰霾等大气污染现象,我国实施了一系列减排措施.研究发现,我国的减排措施虽有效控制了大气中SO2浓度及SO42-的形成,但大气中NO浓度下降并不明显,仍在高位波动,并表现为硝酸盐在细颗粒物和大气降水组成中占比不断增重要.因此,准确评估大气中硝酸盐来源及其贡献,实高[10-16],这使得硝酸盐对酸雨和气溶胶的贡献更为现NO精准控制,对缓解我国酸雨和灰霾等污染现象具有重要意义.
在大气中,硝酸盐主要来源于生物质燃烧、煤燃烧、机动车尾气排放和土壤中微生物作用产生的NO的二次转化,且不同NO来源N同位素特征值具有明显差异,如:煤燃烧源δ15N偏正( +13.7± 4.6‰)[17-19],机动车尾气排放源δ15N偏负(-7.3± 7.8‰)[19-22],生物质燃烧源δ15N偏正(+1.0± 4.1‰)[23-25],土壤排放源δ15N偏负( -33.8± 12.2‰)[20,26],因此,可根据硝酸盐δ15N组成范围判断其可能来源[1,3-5,9,27-28].近年来,国内外学者通过同位素示踪法对城市降水中硝酸盐来源解析已有越来越多的报道,如Fang等[1]的研究发现,广州降水中硝酸盐主要来源于煤燃烧; Li等[9]的研究发现,沈阳秋冬季降水中硝酸盐除了受供暖期煤燃烧影响,还受生物质燃烧影响;Liu等[29]发现机动车尾气也越来越成为城市降水中硝酸盐的重要来源.这些研究报道为揭示我国城市降水中硝酸盐来源提供了重要依据,但其主要集中在经济发达地区或北方城市.相比经济发达地区和北方城市,中部等经济正高速发展地区关于降水中硝酸盐来源解析的相关研究却鲜有报道.
江西地处长江经济带,是中部地区的重要省份,经济增速稳居全国前5.目前,对江西省大气降水的研究多集中于降水的化学特征分析,利用同位素示踪法和贝叶斯混合模型解析大气降水中硝酸盐来源及其贡献的研究几乎未见报道.因此,本研究以江西省会南昌市为例,采集了南昌市秋冬季降水,利用NO3-氮氧同位素和贝叶斯混合模型对降水中硝酸盐来源及其贡献进行解析,以期为中部地区治理大气硝酸盐污染提供科学的理论基础.
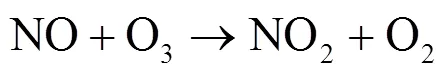

O+O2®O3(R3)
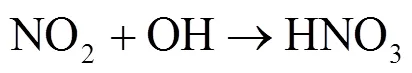

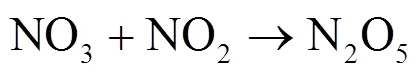
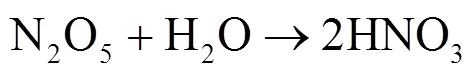
1 材料与方法
1.1 样品采集及处理
本研究于2016年9月1日至2017年2月28日期间对南昌市雨水进行采集,共采集33个降雨事件.采样点位于东华理工大学南昌校区校园内(28.72¢N,115.83¢E),周围无明显污染源.采样器采用90cm×40cm(长×宽)专用聚乙烯箱,在每次收集雨水前,用milli-Q(18.2MΩ)超纯水对采样箱内部进行反复冲洗;在不下雨期间,采样箱用专用箱盖封口避免大气干沉降及其他可能污染物的影响.雨水样品采集后,用孔径为0.45μm微孔滤膜进行过滤,过滤后取部分样品分装于两支15mL离心管用于测定雨水中阴、阳离子浓度,剩余样品分装于洗净聚乙烯瓶中用于测定同位素.所有样品在测定前均置于-20℃冰箱保存,仅在测定前取出.
1.2 分析方法
1.2.1 雨水样品中化学组成分析 降水中主要阴离子(F-、Cl-、NO3-、SO42-)浓度,采用ICS-90型离子色谱仪(美国Dionex)进行测定,检出限分别为1.58、0.845、1.29、0.781μmol·L-1,相对标准偏差为0.57%、2.55%、1.16%、1.36%;阳离子(Ca2+、K+、Mg2+、Na+)浓度,采用MPX型电感耦合等离子体-发射光谱仪(美国VISTA,ICP-OES)进行测定,检出限分别为0.0750、1.54、0.0206、0.870μmol/L,仪器相对标准偏差£1.5%.NH4+浓度采用紫外线分光光度计测定,仪器检出限为5.56μmol/L,相对标准偏差£5.0%.
1.2.2 NO3-氮氧同位素分析 降水中硝酸盐15N和18O值通过微生物反硝化法测定.反硝化法是广泛用于测定雨水、海水和气溶胶中硝酸盐15N和18O前处理方法[30-34].本方法主要步骤为:将过滤后,NO3-浓度为20nmol/L的雨水加入含有反硝化细菌(Pseudomonas aureofaciens,ATCC13985)培养基,随后反硝化细菌将NO3-还原为N2O,然后用GasBench II连续流质谱仪(IRMS, Thermo Delta V Advantage)测定N2O的氮氧同位素[35],所测得的N2O的15N和18O值即为硝酸盐的15N和18O.测定过程中采用两个国际标准USGS34、IAEA-N3和两个实验室工作标准对仪器进行校正,15N测试精度为±0.2‰,18O测试精度为±0.5‰分析方法具体流程参见Luo等[35],Xiao等[28].
1.3 后向轨迹模型及卫星火点地图
HYSPLIT模型是由美国国家海洋和大气管理局(NOAA)的空气资源实验室和澳大利亚气象局联合研发的一种用于计算和分析大气污染物输送、扩散轨迹的专业模型.本文利用TrajStat软件,通过美国空气资源实验室(NOAA ARL)提供的GDAS数据对每次降雨过程的气团后向轨迹进行模拟计算(http: //ready.arl.noaa.gov/HYSPLIT.php). 轨迹的起始高度为500m,起始时间为降水事件的0点,并向后推2d(-48h).卫星火点地图基于美国国家航空航天局(NASA)火灾信息资源管理系统(https://firms. modaps.eosdis.nasa.gov/map/)全球在线卫星监测火点数据制作.卫星火点数据取每个下雨事件前48小时及下雨当天采样点及气团轨迹途径区域数据.
1.4 贝叶斯混合模型(MixSIA)
1.4.1 模型简介 贝叶斯混合模型可以通过稳定同位素来评估源对混合物贡献的概率分布,同时明确地考虑到多个源、分馏和同位素特征间的不确定性[8,36].基于贝叶斯混合模型,可通过NO排放源15N特征计算NO来源贡献.本文通过内置同位素分馏计算模块的贝叶斯模型,计算煤燃烧、机动车尾气排放、生物质燃烧、土壤排放四种潜在来源贡献 .此模型基本计算原理可表达为:
(q∣数据)=(数据∣)×(q)/∑β(数据∣q) ×(q)
式中:β(数据∣q)为基于先验信息的混合数据的概率;(q)为基于先验信息的先验概率;∑(数据∣q)×(q)为数据的边缘概率的近似值,其具体原理参见文献[5,8].
1.4.2 数据选择 MixSIA模型计算硝酸盐不同来源贡献需要输入3类同位素数据,包括样品的15N、18O和源谱的15N信息.源谱信息为国内外学者实验测定的硝酸盐四种潜在来源15N的平均值和标准差,其中煤燃烧源、机动车尾气排放源、生物质燃烧源和土壤排放源的15N-NO3-分别为(+13.7‰± 4.6‰)[17-19]、(-7.3‰±7.8‰)[19-22]、(+1.0‰±4.1‰)[23-25]和(-33.8‰±12.2‰)[20,26].样品15N、18O信息为实验室测定的固定数值,其中样品18O主要用于计算硝酸盐形成途径,并基于硝酸盐不同形成途径贡献,计算硝酸盐氮同位素分馏,从而对硝酸盐来源进行准确的定量分析.
1.4.3 同位素分馏计算 大气中,硝酸盐形成过程(R1~R7)通常伴随着同位素分馏现象,为更精确计算硝酸盐不同来源贡献,对硝酸盐主要形成过程的同位素分馏进行计算,计算公式为E1~E7,其中E1~E3用于计算氮同位素分馏,E4~E6用于计算硝酸盐不同形成途径贡献, E7用于计算分馏系数,具体计算过程可参见文献[5,7-8].

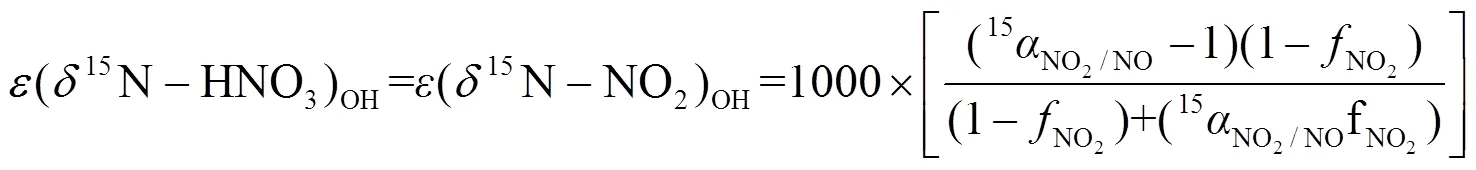
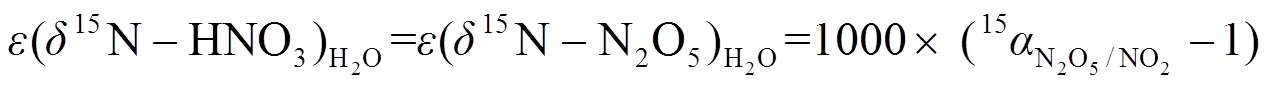
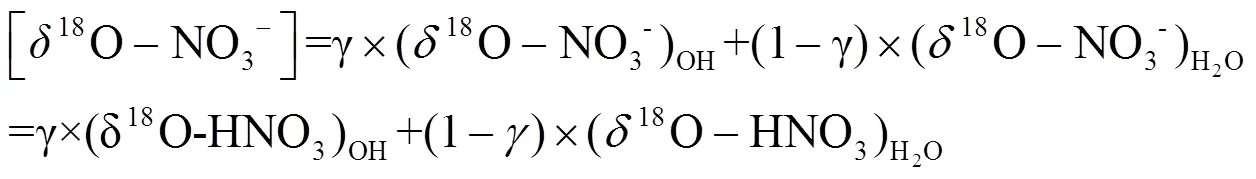

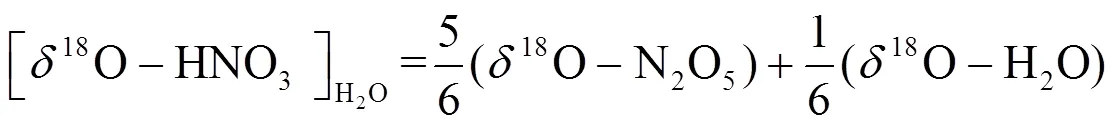

式中:为同位素分馏贡献占比;/z为物质和的分馏系数,=15或18;NO2取值范围为0.2~0.95;为开尔文温度,、、、为开尔文温度150~450K条件下常数,数据来源于文献[37-38].
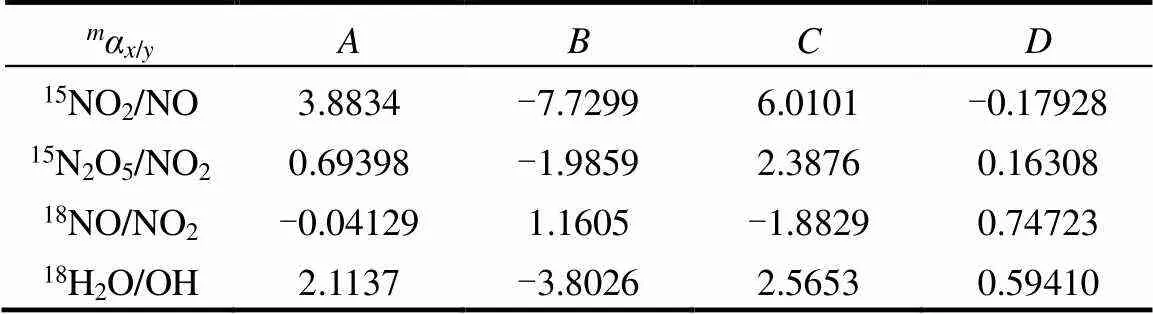
表1 开尔文温度150~450K条件下常数A、B、C、D取值
2 结果与讨论
2.1 基于化学组成分析降水中NO3-来源
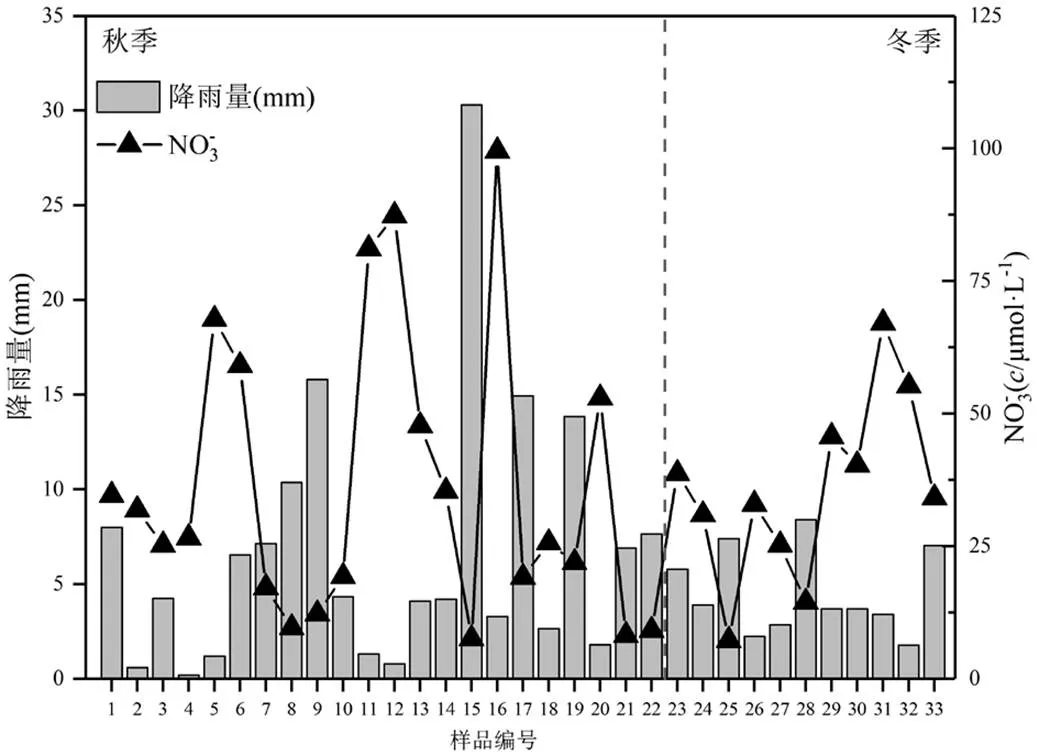
图1 采样期间降雨量和NO3-离子浓度变化
秋季:1~22号,冬季:23~33号
如图1和表2所示,南昌市秋冬季降水中NO3-浓度变化范围为7.3~99.5μmol/L,平均值为36.1μmol/L,变化范围较大.研究表明降雨量对硝酸盐浓度有较大影响[39],如图1所示,在本研究中,秋季降水量波动较大, NO3-浓度变化范围更大,而冬季降水较平均,NO3-浓度变化范围更小,且NO3-浓度与降雨量呈显著负相关(=-0.52,<0.01),说明硝酸盐浓度变化受降雨量影响.与其他城市相比,南昌市降水中硝酸盐浓度明显低于长江三角洲、珠江三角洲[1,40-41]等经济发达区域以及沈阳、北京、天津等[9,42-43]北方燃煤城市降水中硝酸盐浓度,但远高于百慕大、永兴岛、拉萨等[32,44-45]受人为活动影响较小区域降水中硝酸盐浓度(表2),这表明南昌地区降水中硝酸盐受人为活动影响仍较为严重.
通过对降水的化学组成进一步分析发现, NO3-与SO42-和K+呈显著正相关(=0.39,<0.05;=0.63,<0.01),这表明NO3-与SO42-和K+可能存在相似来源[3,8,35,46].在大气中,SO2是形成SO42-前体物质,主要来源于煤燃烧[1];而K+主要来源于生物质燃烧,是生物质燃烧的指示性离子[8,46],这表明煤燃烧和生物质燃烧产生的NO可能是南昌市秋冬季降水中硝酸盐的重要来源.
2.2 基于同位素分析降水中硝酸盐来源
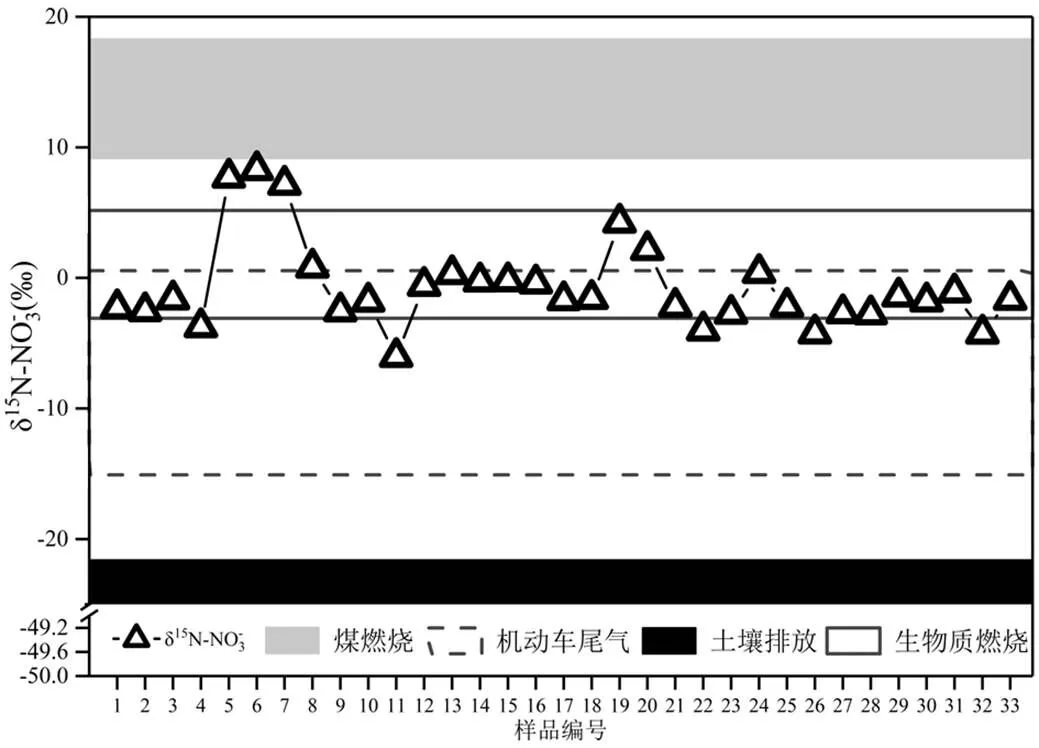
图2 南昌秋、冬季大气降水中δ15N-NO3-组成
秋季:1~22号,冬季:23~33号
如图2所示,南昌市秋季降水15N-NO3-变化范围为-6.0~+8.3‰,平均值为0.0‰,主要分布在煤燃烧、机动车尾气和生物质燃烧范围内;冬季15N- NO3-变化范围为-4.3~+0.4‰,平均值为-2.2‰,均分布在生物质燃烧和机动车尾气15N值范围内,说明秋季降水中硝酸盐可能主要来源于煤燃烧、生物质燃烧和机动车尾气排放,而冬季主要来源于生物质燃烧和机动车尾气排放.与其他城市相比,南昌市秋、冬季降水中硝酸盐15N值较为偏负,均远低于沈阳、西安、北京等秋冬季存在燃煤供暖的北方城市和广州、湛江等东南沿海燃煤量较高城市降水中硝酸盐15N值(表2)[1-2,9,47],说明煤燃烧源可能对南昌市秋冬季降水中硝酸盐贡献占比较小.就秋季而言,降水中硝酸盐15N值与杭州和湖州等[3]城市降水中硝酸盐15N值相接近,表明南昌可能与杭州、湖州存在着相似来源构成.在杭州和湖州降水中,硝酸盐受机动车尾气排放和北方煤燃烧传输影响[3].在冬季降水中硝酸盐15N值与贵阳等[29]地区降水中15N-NO3-值相接近.在贵阳降水中,机动车尾气排放和生物质燃烧是硝酸盐最主要来源[29],说明机动车尾气排放和生物质燃烧源可能也是南昌市冬季降水中硝酸盐主要来源.

表2 不同地区大气降水中δ15N-NO3-值
注:冷季为10月至次年3月;—表示无数据;*表示该采样点为秋冬季数据.
2.3 利用贝叶斯混合模型评估不同来源硝酸盐贡献

图3 南昌秋、冬季大气降水中NO3−来源贡献占比
近年来,贝叶斯混合模型被广泛用于计算大气中硝酸盐不同来源的贡献[2,4-5,8-9,29,35].为量化南昌市降水中硝酸盐各来源贡献,本文利用贝叶斯混合模型对南昌市秋季、冬季及整个采样期间硝酸盐来源贡献进行计算.如图3所示,在四种潜在来源中,生物质燃烧占比最高,约占32.5%,说明生物质燃烧对秋冬季降水中硝酸盐来源具有重要贡献,这可能与秋冬季南昌市周边野外生物质燃烧有关[50].美国航空航天局MODIS卫星火点地图显示,在采样期间,采样点周围野外火点分布密集(图4).机动车尾气排放是仅次于生物质燃烧的第二大来源,贡献占比超过30%(图3),说明机动车尾气排放已成为秋冬季降水中硝酸盐的主要来源,这可能与南昌市机动车保有量快速增加有关,且《江西省环境统计年报》数据显示南昌市NO排放组成中机动车尾气排放约占75%[51].煤炭是江西省的主要能源,在能源结构中占比超过60%[52-53],但在南昌市秋冬季降水中煤燃烧贡献占比相对较小,约占23.1%,这可能与近年来我国实施的减排措施有关.在南昌市NO排放组成中,煤燃烧排放的NO仅占22%[51].在四种潜在来源中,土壤排放源占比最小,约占13.5%±8.4%,这可能与秋冬季温度较低,土壤中微生物活动减少有关[4-5,9].
就季节变化而言,机动车尾气排放对秋季降水贡献占比为27.6%±18.9%,对冬季贡献占比为34.7%±20.4%(图3),呈现明显冬季高秋季低趋势,说明冬季机动车尾气污染更为严重.与机动车尾气排放不同,生物质燃烧在秋季贡献占比为33.5%± 21.9%,冬季贡献占比为30.7%±21.8%,表现为秋季高冬季低的趋势,说明秋季生物质燃烧现象更为严重.美国航空航天局MODIS卫星火点地图显示,秋季采样点周围野外火点分布更为密集(图4),也进一步表明南昌市秋季降水中NO3-可能受生物质燃烧影响更大.此外,煤燃烧对南昌市降水贡献也存在季节性差异,表现为秋季高冬季低的趋势.在秋季,其贡献占比为25.0%±14.8%,冬季贡献占比为22.0%±14.0%.结合采样期间气团后向轨迹分析推测,这能与外源输入有关.在秋季,气团主要起源于北方和东部沿海发达城市,而冬季气团主要起源于省内西南区域及中部区域(图4).在东部城市及长三角区域,如:武汉、上海、南京等城市,其大气降水或气溶胶中NO3-来源中煤燃烧源占比较高[3,5,54-55],而北方存在燃煤供暖影响.
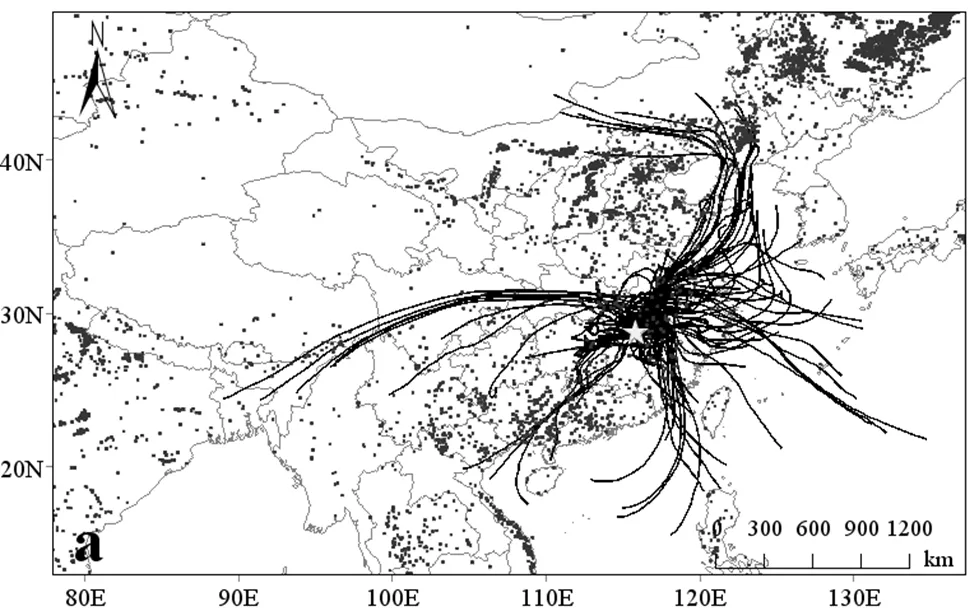
a:秋季,b:冬季
3 结论
3.1 采样期间,降水的NO3-浓度变化范围为7.3~ 99.5μmol/L,平均值为36.1μmol/L,低于经济发达城市和北方同时期降水中硝酸盐浓度,但远高于边远地区降水中硝酸盐浓度,表明南昌受人为活动影响仍较为严重.
3.2 南昌市秋季δ15N-NO3-平均值为0.0‰,冬季δ15N-NO3-平均值为-2.2‰,呈秋季高冬季低的趋势,存在明显季节性变化.引起这一变化的原因可能是冬季降水中机动车尾气排放偏高和秋季降水中煤燃烧来源偏高双重因素作用的结果.
3.3 同位素及贝叶斯模型源解析结果表明南昌秋冬季降水中硝酸盐主要来源于生物质燃烧、机动车尾气排放和煤燃烧,三者贡献超过86%;而机动车尾气排放和生物质燃烧是最主要来源,二者贡献均超过30%.煤燃烧虽然也是重要来源,但相对生物质燃烧和机动车尾气排放较小,约占23%,这可能与近年我国减排措施有关.因此,政府应在对煤燃烧管控的同时,进一步加强生物质燃烧和机动车尾气管控,从而减少NO排放.
[1] Fang Y T, Koba K, Wang X M, et al. Anthropogenic imprints on nitrogen and oxygen isotopic composition of precipitation nitrate in a nitrogen-polluted city in southern China [J]. Atmos. Chem. Phys., 2011,11(3):1313-1325.
[2] Chen F, Lao Q, Jia G, et al. Seasonal variations of nitrate dual isotopes in wet deposition in a tropical city in China [J]. Atmospheric Environment, 2019,196:1-9.
[3] Jin Z, Wang Y, Qian L, et al. Combining chemical components with stable isotopes to determine nitrate sources of precipitation in Hangzhou and Huzhou, SE China [J]. Atmospheric Pollution Research, 2019,10(2):386-394.
[4] Zhao Z-Y, Cao F, Fan M-Y, et al. Coal and biomass burning as major emissions of NOin Northeast China: Implication from dual isotopes analysis of fine nitrate aerosols [J]. Atmospheric Environment, 2020.
[5] Zong Z, Tan Y, Wang X, et al. Dual-modelling-based source apportionment of NOin five Chinese megacities: Providing the isotopic footprint from 2013 to 2014 [J]. Environ Int, 2020,137: 105592.
[6] Zhang W, Zhang Y. Oxygen isotope anomaly (Δ < sup> 17</sup> O) in atmospheric nitrate: A review [J]. Chinese Science Bulletin, 2019,64(7):649-662.
[7] Walters W W, Simonini D S, Michalski G. Nitrogen isotope exchange between NO and NO2and its implications for δ15N variations in tropospheric NOand atmospheric nitrate [J]. 2016,43(1):440-448.
[8] Zong Z, Wang X, Tian C, et al. First Assessment of NOSources at a regional background site in North China using isotopic analysis linked with modeling [J]. Environmental Science & Technology, 2017,51(11): 5923-5931.
[9] Li Z, Walters W W, Hastings M G, et al. Nitrate isotopic composition in precipitation at a Chinese megacity: Seasonal variations, atmospheric processes, and implications for sources [J]. Earth and Space Science, 2019,6(11):2200-2213.
[10] Itahashi S, Yumimoto K, Uno I, et al. A 15-year record (2001~2015) of the ratio of nitrate to non-sea-salt sulfate in precipitation over East Asia [J]. Atmospheric Chemistry and Physics, 2018,18(4):2835-2852.
[11] 中华人民共和国生态环境部.中国生态环境状况公报[M]. 2013: 23-24.
Ministry of Ecology and Environment of the People’s Repulic of China. Report on the State of the Ecology and Environment in China [M]. 2013:23-24.
[12] 中华人民共和国生态环境部.中国生态环境状况公报[R]. 2014: 27-28.
Ministry of Ecology and Environment of the People’s Repulic of China. Report on the state of the ecology and environment in China [R]. 2014:27-28.
[13] 中华人民共和国生态环境部.中国生态环境状况公报[R]. 2015:14-15.
Ministry of Ecology and Environment of the People’s Repulic of China. Report on the state of the ecology and environment in China [M]. 2015:14-15.
[14] 中华人民共和国生态环境部.中国生态环境状况公报[R]. 2016: 15-16.
Ministry of Ecology and Environment of the People’s Repulic of China. Report on the state of the ecology and environment in China [R]. 2016:15-16.
[15] 中华人民共和国生态环境部.中国生态环境状况公报[R]. 2017: 15-16.
Ministry of Ecology and Environment of the People’s Repulic of China. Report on the state of the ecology and environment in China [R]. 2017:15-16.
[16] Wang Y, Gao W, Wang S, et al. Contrasting trends of PM2.5and surface-ozone concentrations in China from 2013 to 2017 [J]. National Science Review, 2020.
[17] Felix J D, Elliott E M, Avery G B, et al. Isotopic composition of nitrate in sequential Hurricane Irene precipitation samples: Implications for changing NOsources [J]. Atmospheric Environment, 2015,106:191- 195.
[18] Felix J D, Elliott E M, Shaw S L. Nitrogen isotopic composition of coal-fired power plant NO: influence of emission controls and implications for global emission inventories [J]. Environ. Sci. Technol., 2012,46(6):3528-35.
[19] Walters W W, Tharp B D, Fang H, et al. Nitrogen isotope composition of thermally produced NOfrom various fossil-fuel combustion sources [J]. Environ. Sci. Technol., 2015,49(19):11363-71.
[20] Felix J D, Elliott E M. Isotopic composition of passively collected nitrogen dioxide emissions: Vehicle, soil and livestock source signatures [J]. Atmospheric Environment, 2014,92:359-366.
[21] Heaton T H, Spiro B, Robertson S M. Potential canopy influences on the isotopic composition of nitrogen and sulphur in atmospheric deposition [J]. Oecologia, 1997,109(4):600-607.
[22] Walters W W, Goodwin S R, Michalski G. Nitrogen stable isotope composition (δ15N) of vehicle-emitted NO[J]. Environmental Science & Technology, 2015,49(4):2278-2285.
[23] Felix J D, Elliott E M. The agricultural history of human-nitrogen interactions as recorded in ice core δ15N-NO3−[J]. Geophysical Research Letters, 2013,40(8):1642-1646.
[24] Fibiger D L, Hastings M G. First Measurements of the nitrogen isotopic composition of NOfrom biomass burning [J]. Environ Sci Technol, 2016,50(21):11569-11574.
[25] Hastings M G, Jarvis J C, Steig E J. Anthropogenic impacts on nitrogen isotopes of ice-core nitrate [J]. Science, 2009,324(5932): 1288.
[26] Li D, Wang X. Nitrogen isotopic signature of soil-released nitric oxide (NO) after fertilizer application [J]. Atmospheric Environment, 2008, 42(19):4747-4754.
[27] Liu X-Y, Yin Y-M, Song W. Nitrogen isotope differences between major atmospheric NOspecies: Implications for transformation and deposition processes [J]. Environmental Science & Technology Letters, 2020,7(4):227-233.
[28] Xiao H W, Zhu R G, Pan Y Y, et al. Differentiation between nitrate aerosol formation pathways in a Southeast Chinese city by dual isotope and modeling studies [J]. Journal of Geophysical Research: Atmospheres, 2020,125(13).
[29] Liu X Y, Xiao H W, Xiao H Y, et al. Stable isotope analyses of precipitation nitrogen sources in Guiyang, southwestern China [J]. Environ Pollut, 2017,230:486-494.
[30] Luo L, Kao S J, Bao H, et al. Sources of reactive nitrogen in marine aerosol over the Northwest Pacific Ocean in spring [J]. Atmos. Chem. Phys., 2018,18(9):6207-6222.
[31] Altieri K E, Hastings M G, Gobel A R, et al. Isotopic composition of rainwater nitrate at Bermuda: The influence of air mass source and chemistry in the marine boundary layer [J]. Journal of Geophysical Research: Atmospheres, 2013,118(19):11,304-11,316.
[32] Hastings M G, Sigman D M, Lipschultz F. Isotopic evidence for source changes of nitrate in rain at Bermuda [J]. Journal of Geophysical Research: Atmospheres, 2003,108(D24):n/a-n/a.
[33] Buffam I, Mcglathery K. Effect of ultraviolet light on dissolved nitrogen transformations in coastal lagoon water [J]. Limnology and Oceanography, 2003,48:723-734.
[34] Gobel A R, Altieri K E, Peters A J, et al. Insights into anthropogenic nitrogen deposition to the North Atlantic investigated using the isotopic composition of aerosol and rainwater nitrate [J]. Geophysical Research Letters, 2013,40(22):5977-5982.
[35] Luo L, Wu Y, Xiao H, et al. Origins of aerosol nitrate in Beijing during late winter through spring [J]. Science of The Total Environment, 2019, 653:776-782.
[36] Parnell A C, Phillips D L, Bearhop S, et al. Bayesian stable isotope mixing models [J]. Environmetrics, 2013:n/a-n/a.
[37] Walters W W, Michalski G. Theoretical calculation of nitrogen isotope equilibrium exchange fractionation factors for various NOmolecules [J]. Geochimica et Cosmochimica Acta, 2015,164:284-297.
[38] Walters W W, Michalski G. Theoretical calculation of oxygen equilibrium isotope fractionation factors involving various NOmolecules, OH, and H2O and its implications for isotope variations in atmospheric nitrate [J]. Geochimica et Cosmochimica Acta, 2016,191: 89-101.
[39] 肖红伟,肖化云,龙爱民,等.贵阳地区大气降水中δ15N-NO3组成及来源分析[J]. 环境科学学报, 2012,32(4):940-945.
Xiao H W, Xiao H Y, Long A M, et al. Nitrogen isotopic composition and source of nitrate in precipitation at Guiyang [J]. Acta Scientiae Circumstantiae, 2012,32(4):940-945.
[40] Jia G, Chen F. Monthly variations in nitrogen isotopes of ammonium and nitrate in wet deposition at Guangzhou, south China [J]. Atmospheric Environment, 2010,44(19):2309-2315.
[41] Zhao X, Yan X, Xiong Z, et al. Spatial and temporal variation of inorganic nitrogen wet deposition to the Yangtze River Delta Region, China [J]. Water, Air, and Soil Pollution, 2009,203(1-4):277-289.
[42] 肖致美,李 鹏,陈 魁,等.天津市大气降水化学组成特征及来源分析[J]. 环境科学研究, 2015,28(7):1025-1030.
Xiao Z M, Li P, Chen K, et al. Characteristics and sources of chemical composition of atmospheric precipitation in Tianjin [J]. Research of Environmental Sciences, 2015,28(7):1025-1030.
[43] Xu Z, Han G. Chemical and strontium isotope characterization of rainwater in Beijing, China [J]. Atmospheric Environment, 2009, 43(12):1954-1961.
[44] 肖红伟,肖化云,张忠义,等.西沙永兴岛大气降水化学特征及来源分析[J]. 2016,36(11):3237-3244.
Xiao H W, Xiao H Y, Zhang Z Y, et al. Chemical characteristics and source apportionment of atmospheric precipitation in Yongxing Island [J]. China Environmental Science, 2016,36(11):3237-3244.
[45] Zhang D D, Peart M, Jim C Y, et al. Precipitation chemistry of Lhasa and other remote towns, Tibet [J]. Atmospheric Environment, 2003, 37(2):231-240.
[46] Chen W, Yan L, Zhao H. Seasonal Variations of Atmospheric Pollution and Air Quality in Beijing [J]. Atmosphere, 2015,6(11):1753-1770.
[47] Xing M, Liu W. Variations in the concentration and isotopic composition of nitrate nitrogen in wet deposition and their relation with meteorological conditions in Xi’an city, Northwest China [J]. Applied Geochemistry, 2012,27(4):831-840.
[48] Li C, Li S L, Yue F J, et al. Nitrate sources and formation of rainwater constrained by dual isotopes in Southeast Asia: Example from Singapore [J]. Chemosphere, 2020,241:125024.
[49] Zhang Y, Liu X J, Fangmeier A, et al. Nitrogen inputs and isotopes in precipitation in the North China Plain [J]. Atmospheric Environment, 2008,42(7):1436-1448.
[50] Xiao H-W, Wu J-F, Luo L, et al. Enhanced biomass burning as a source of aerosol ammonium over cities in central China in autumn [J]. Environmental Pollution, 2020,266:115278.
[51] 江西省生态环境厅.江西省环境统计年报[M]. 2016:21-32.
Department of Ecology and Environment of JiangXi Province. Environmental statistics annual report of JiangXi Province [M]. 2016:21-32.
[52] 江西省统计局.江西统计年鉴[M]. 中国统计出版社, 2017.
Jiangxi Provincial Bureau of Statistics. Jiangxi statistical yearbook [M]. 2017
[53] 江西省统计局.江西统计年鉴[M]. 中国统计出版社, 2018.
Jiangxi Provincial Bureau of Statistics. Jiangxi statistical yearbook [M]. 2018.
[54] Chang Y, Zhang Y, Tian C, et al. Nitrogen isotope fractionation during gas-to-particle conversion of NOto NO3−in the atmosphere – implications for isotope-based NOsource apportionment [J]. Atmospheric Chemistry and Physics, 2018,18(16):11647-11661.
[55] Shen J, Zhao Q, Cheng Z, et al. Insights into source origins and formation mechanisms of nitrate during winter haze episodes in the Yangtze River Delta [J]. Sci. Total Environ., 2020,741:140187.
Tracking the source and contribution of NO3-in precipitation in Nanchang in autumn and winter by dual isotope.
AI Wen-qiang1,2, XIAO Hong-wei1,2*, SUN Qi-bin3, ZHANG Yong-yun3, ZHANG Zei-yu2, LI Jing-wen2
(1.Jiangxi Province Key Laboratory of the Causes and Control of Atmospheric Pollution, Nanchang 330013, China;2.School of Water Resources and Environmental Engineering, East China University of Technology, Nanchang 330013, China;3.School of Atmospheric Sciences, Sun Yat-sen University, Zhuhai 519082, China)., 2021,41(9):4043~4050
Rainwater was collected from September 1, 2016 to February 28, 2017 in Nanchang, as well as stable isotope of nitrate (δ15N) and chemical compositions of rainwater were analyzed. The results showed that the concentration of NO3-ranged from 7.3 to 99.5μmol/L, and the mean is 36.1μmol/L; the δ15N of NO3-ranged from -6.0‰ to +8.3‰, and the mean is -0.8‰. Combined with chemical composition and isotope analysis, it is shown that NO3-was mainly affected by the region and mainly comes from biomass burning, traffic, coal combustion while biological soil is the secondary source in the sampling period. Bayesian mixing model (MixSIA) which took account of the isotope fractionation wes used to make precise estimations of the contribution of different sources. The results showed that the contribution of biomass burning emissions, traffic and coal combustion was more than 86%. However, in this case, the contribution of biomass burning emissions and traffic was more than 63%, and the coal combustion was only 23.1%, which indicated that a necessity to control NOfrom traffic and biomass burning strictly to reduce air pollution.
Nanchang;nitrate;precipitation;nitrogen isotope;Bayesian mixing model (MixSIA)
X571
A
1000-6923(2021)09-4043-08
艾文强(1994-),男,江西南昌人,硕士研究生,主要研究方向为同位素地球环境化学.发表论文1篇.
2021-02-03
国家自然科学基金(42063001,41663003)
*责任作者, 副研究员, xiaohw@ecut.edu.cn

