Role of PAI-1 in adipose tissue senescence and inflammation in obesity
XU Jin,GAO Qian,WU Jian-bo
1.Department of Gastroenterology,the Affiliated Hospital of Southwest Medical University, Luzhou 646000, China;2.Drug Discovery Research Center,Southwest Medical University,Luzhou 646000,China;3.Laboratory for Cardiovascular Pharmacology, Department of Pharmacology, School of Pharmacy, Southwest Medical University, Luzhou 646000,China
【Abstract】Plasminogen activator inhibitor-1(PAI-1)is a member of the evolutionarily conserved serine protease inhibitor family.The increased expression of PAI-1 leads to pathological diseases such as vascular diseases,obesity,and metabolic syndrome.Senescence-associated secretory phenotype (SASP) mediates tissue damage and plays a role in adipose tissue dysfunction.Chronic inflammation in adipose tissue is associated with the development of metabolic diseases,including obesity,insulin resistance,and type 2 diabetes.PAI-1 plays a vital role in adipose tissue physiology,glucose metabolism,insulin secretion and sensitivity,inflammation,and macrophage chemotaxis.This article reviews the possible role of PAI-1,as one of the SASP markers,and speculates the potential role of PAI-1 in adipose tissue senescence and inflammatory macrophage infiltration in obesity.
【Key words】PAI-1 Adipose tissue Macrophage Senescence
Introduction
In many countries,overweight and obesity pose an increasing threat to population health[1].Clinical and epidemiological studies have shown that obesity is associated with an increased risk of developing cardiovascular disease,insulin resistance,and type 2 diabetes(T2D)[2-3].Most T2D patients are obese or overweight.Many longitudinal studies have linked obesity with insulin resistance,which is a common precursor of diabetes.As a major endocrine and secretory organ,adipose tissue releases a number of cytokines and hormones,such as free fatty acids (FFA),tumor necrosis factor(TNF-α),interleukin 6 (IL-6),plasminogen activator inhibitor (PAI-1),leptin,adiponectin,and resistin[4-5].The inflammatory process is activated in the early stages of fat.During the occurrence and development of chronic obesity,the immune system is permanently inclined to a pro-inflammatory phenotype,so this article begins to describe the interaction between obesity and inflammation.Diet-induced obesity causes Adipose Tissue Macrophage(ATM) in lean animals to switch from M2 activated or "replacement activated" macrophage polarization state,thereby protecting fat cells from inflammation to M1 or "regularly activated" macrophage state,leading to insulin resistance.The increased anti-inflammatory cytokine IL-10 in the ATM of lean mice protects adipocytes from TNF-α-induced insulin resistance[6].The discovery of islet inflammation and its involvement in T2D cell dysfunction further emphasizes the importance of inflammation in metabolic diseases.M1 polarized macrophages recruit more inflammatory phenotypes and secrete cytokines such as TNF-α[6-7].Macrophages are the effectors of the complex immune program induced by obesity.It is well known that macrophages play an essential role in insulin secretion and insulin-sensitive tissues.
PAI-1,encoded by the SERPINE1 gene,is the primary inhibitor of endogenous plasminogen activator and is synthesized in the liver and adipose tissue.Many studies have shown that the increase in PAI-1 is related to thrombosis,fibrosis,obesity and insulin resistance[6,8].The expression of PAI-1 mRNA and PAI-1 protein in normal adipose tissue indicates that adipose tissue may be the active site of PAI-1[9-10].In obesity,adipose tissue is the primary source of circulating PAI-1[4-5]and usually causes obesity complications.PAI-1 derived from adipocytes is mainly expressed in visceral fat[11-12],released into the circulation parallel with the increased fat mass.The endogenous TNF-α produced by adipose tissue may play an essential role in PAI-1.The expression of PAI-1 in human adipose tissue causes insulin resistance,which may be the clinical link between insulin resistance syndrome and elevated plasma levels of PAI-1[10].
This review will focus on the potential connection between obesity-induced adipose tissue inflammation and adipose tissue aging to reveal the role of PAI-1 as an aging-related secreted protein in this process during obesity.
PAI-1-LRP1 signaling in insulin resistance
PAI-1 may play a variety of obesity roles to affect insulin signal transduction indirectly,thereby affecting adipocyte differentiation and regulating the recruitment of inflammatory cells in adipose tissue[13].The production of inflammatory reactions increases oxidative stress and inflammatory cytokines.Oxidative stress causes high expression of PAI-1 in various tissues,including adipose tissue,especially in aged mice[14].
Low-density lipoprotein receptor-related protein 1(LRP1)is a critical signal protein belonging to the LDL receptor(LDLR) superfamily that is widely expressed in multiple cell types[15].PAI-1 can interact with LRP1 on adipocytes.LRP1 receptor controls adipogenesis and is up-regulated in obese adipose tissue[16].However,the pharmacological PAI-1 inhibition has no effects on visceral white adipose tissue LRP1 content[17].
LRP1 deficient adipocytes are more likely to be inflamed and more likely to cause mononuclear macrophage infiltration and high expression of inflammatory cytokines[18].The migration of macrophages depends on the adhesion/exfoliation between the macrophage surface components and the extracellular matrix and the role of many inflammatory diseases.PAI-1 affects macrophage motility through a different effect from its classical regulation of the plasmin-based fibrinolytic process.Studies have shown that PAI-1 promotes monocyte/macrophage recruitment and M2 polarization through other domains.Its LRP1 interaction domain regulates macrophage migration,and its c-terminal uPA interaction domain activates p38MAPK and NF-κB,induces the autocrine IL-6/STAT3 activation pathway,and promotes M2 macrophage polarization[19-20].The small molecule PAI-1 inhibitors,as a new class of anti-inflammatory drugs,can target macrophage migration by inhibiting the interaction of PAI-1 and low-density lipoprotein receptor-related proteins[21].
Both insulin receptor (IR) and insulin-like growth factor 1 receptor (IGF1R) are expressed in adipocytes[22-23].Previous in vitro and in vivo studies showed that PAI-1 is implicated in adipose tissue development and the control of insulin signaling in adipocytes[24-25].PAI-1 deficiency enhanced basal and insulin-stimulated glucose uptake in adipose cells in vitro[26].PAI-1 inhibitor prevented high-fat diet (HFD)-induced systemic insulin resistance and restored HFD-induced impaired JNK and Akt phosphorylation,suggesting that inhibition of PAI-1 prevents the insulin resistance of adipocytes[27].These findings indicate that PAI-1 plays an essential role in the control of insulin resistance in adipose tissue.
Role of PAI-1 in ATMs
PAI-1 is involved in adipose tissue inflammation[24].Obesity is a chronic,systemic inflammatory disease[27-29],leading to insulin resistance[26].Chronic inflammation is related to various pathological conditions.The production of inflammation increases oxidative stress and inflammatory cytokines.Studies have shown that PAI-1 gene deletion or pharmacological PAI-1 inhibition (inhibitor PAI-039) can promote the transformation of macrophages from M1 to M2 phenotype,characterized by the decrease of pro-inflammatory factors CD11c,IL-6 and MCP-1,and the increase of anti-inflammatory factors CD206 and IL-10[11,24].TNF-α has previously been involved in adipose tissue inflammation and insulin resistance during obesity[30].We observed a significant reduction in the level of TNF-α mRNA in the white adipose tissue (WAT) of PAI-1 deficiency or PAI-039 treated high fat diet-fed mice[24].Interestingly,drugs such as thiazolidinediones,metformin and receptor antagonists have been found to reduce PAI-1 expression in fat[31].However,the specific molecular regulatory details and mechanisms of PAI-1 in adipose tissue need to be further studied.
Adipose tissue is the primary source of inflammatory mediators related to diabetes and other chronic diseases[32-33].There are abundant pre-adipocytes in the interstitial blood vessels of adipose tissue,closely related to macrophages.Pro-inflammatory cytokines act in an autocrine and paracrine manner to interfere with insulin signaling in peripheral tissues or induce β-cell dysfunction and subsequent insulin deficiency[33].
The study of pathway triggers can provide effective methods to inhibit inflammatory pathways and have important clinical significance for treating obesity and its complications.In stromal cells,ATM plays a crucial role in the pathogenesis of inflammation and metabolic complications caused by obesity.The altered environment and inflammatory phenomena coordinate the polarization pattern of macrophages,which is generally conducive to obtaining a metabolically activated state.As an inflammatory factor of adipose tissue,PAI-1 can respond to obesity and insulin resistance,reduce insulin sensitivity,and participate in aging-related adipose tissue inflammation and its complications.We summarized the relationship between PAI-1 and macrophage infiltration and insulin resistance in adipose tissues from this perspective.These latest findings highlight the relationship between PAI-1 and adipose tissue mediate insulin resistance and metabolic syndrome(Figure 1).
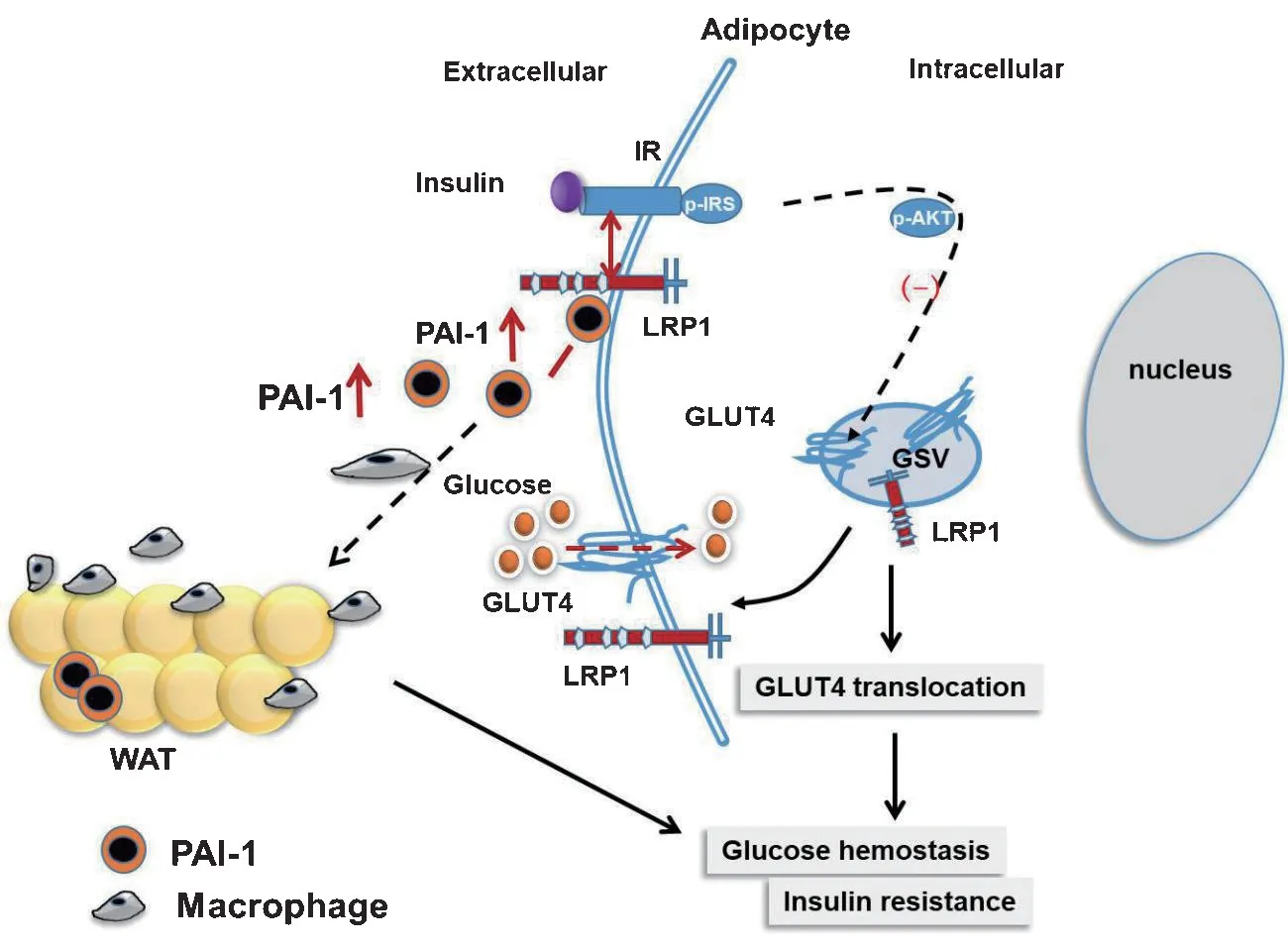
Figure 1.Schematic illustration of PAI-1 in adipose tissue inflammation and metabolic syndrome GLUT4,glucose transporter 4;GSV,GLUT4 storage vesicle;IR,insulin receptor;LRP1,LDL receptor associated protein 1;WAT,white adipose tissue
PAI-1 is a key mediator of adipose tissue senescence
Cellular senescence is a continuous cell cycle exit state[34],and its molecular driving factors mainly include telomere shortening,oxidative stress,oncogene activation,and DNA damage[35].Senescence-associated secretory phenotype (SASP) is associated with the chronic inflammation caused by the increased PAI-1 as a part of SASP[36-37].Elimination of senescent cells through senolytic treatment could alleviate obesity related metabolic complications[38].However,the role of SASP,especially PAI-1,in the human lifespan is uncertain.
SASP could promote the pro-inflammatory process of adipose tissue,inhibit differentiation,and induce the infiltration of immune cells[39-40].PAI-1 is a critical downstream target for p53 to induce replicative senescence and directly promote cell senescence[41-42].Recent genetic studies have shown that PAI-1 is a crucial component of the senescence-related secretion group and a direct mediator of cell senescence and lifespan[43].PAI-1 is a crucial senescence mediator and a potential therapeutic target for preventing and improving vascular aging and aging-related diseases caused by hyperhomocysteinemia[44].Accelerated aging induced by time and stress is related to cellular aging and is accompanied by a significant increase in the expression of PAI-1 in tissues.Interestingly,the flow cytometry analysis of PAI-1 positive cells found a significant positive correlation between the increase of PAI-1 level and aging-related β-galactosidase (SA-β-gal)positive cells.It is verified that PAI-1 is a substantial sign of endothelial cell senescence[45].In adipose tissue,PAI-1 is significantly higher than nonsenescent in p16INK4a-positive senescent cells,which clearly shows a correlation with the expression of senescence-associated galactosidase SA-β-gal is up-regulated in preadipocytes under the condition of inflammatory macrophage culture medium and is positively related with the expression of PAI-1,CC chemokine ligand 2 (CCL2) and IL-6[33].Here,we review that PAI-1,as one of the significant factors of SASP in adipose tissue,is a marker of cellular senescence and a key mediator of cell-level senescence,and a substantial contributor to physiological senescence.We discuss recent studies suggesting that the role of PAI-1 as a critical aging mediator,such as genetic defects or pharmacological inhibition of PAI-1,is sufficient to prevent cellular replicative aging in mammals,preventing age-related pathology and morbidity(Figure 2).
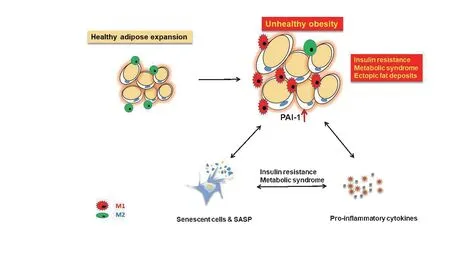
Figure 2.Synergistic effects of adipose tissue dysfunction cellular senescence,and pro-inflammatory state leads to insulin resistance and metabolic syndrome SASP,senescence-associated secretory phenotype.
Conclusion
PAI-1 can be used as a marker for disease activity and targeted clinical drug development.The plasticity mechanism,polarization activation,and molecular recognition mechanisms of macrophages provide the basis for diagnosis and treatment strategies centered on macrophages.In summary,understanding how obesity changes ATMs function and the molecular mechanisms that support inflammation may provide new treatment strategies for preventing or treating obesity caused by inflammation.We hope that the targeting of PAI-1 in adipose tissues can provide a new and reasonable method for controlling adipose tissue inflammation,cell aging and age-related pathologies,including obesity,diabetes,thrombosis,organ fibrosis and metabolic syndrome.

