Discussion on the Application of Real-World Study in the Inheritance and Development of Empirical Prescriptions of Traditional Chinese Medicine
Wang Yuzhuo,Li Wanting,Fan Guangwei,Chen Yuwen*
(1.School of Business Administration,Shenyang Pharmaceutical University,Shenyang 110016,China;2.Research Institute of Drug Regulatory Science,Shenyang Pharmaceutical University,Shenyang 110016,China)
Abstract Objective To study the research on real-world data and to provide new ideas and methods for the inheritance and development of empirical prescriptions of traditional Chinese medicine (TCM).Methods The disadvantages of using randomized controlled trials for empirical prescriptions of TCM and the advantages of using real-world study (RWS) were analyzed by summarizing the previous RWS and the empirical prescriptions.Meanwhile,the methods for marketing of new TCMs derived from empirical prescriptions of TCM,the data source and trial design of the RWS were discussed.Results and Conclusion RWS can provide new ideas for the listing of new TCMs.With the improvement of relevant laws and regulations,RWS will promote the development of TCM greatly.To promote the application of RWS in the inheritance and development of empirical prescriptions of TCM,the government should improve laws and regulations as soon as possible,and enterprises and research institutions should strengthen patient privacy protection and clarify the responsible parties.
Keywords:real-world study;empirical prescriptions;traditional Chinese medicine;real-world data
Traditional Chinese medicine (TCM) is an important part of China’s pharmaceutical industry.In the long development of TCM industry,many empirical prescriptions of TCM have been handed down.These prescriptions,summarized by TCM practitioners in their long-term clinical practice,are effective and safe.These prescriptions reflect the traditional Chinese doctors’ understanding of the law of disease occurrence and development,their precious experience of prescription and medication,which represents the highest level of Chinese medicine in clinical diagnosis and treatment[1].Some of these prescriptions are still only circulated and used among the people,some of them have become national certified famous prescriptions,and some of them have been improved to be in-hospital preparations that are only circulated and used within hospitals.But for a long time,due to the different thinking mode and clinical evaluation of TCM and Western medicine,the recognition of TCM in western world is not high.In early 2020,Convid-19 broke out globally.TCM played a great role in fighting the pandemic.Its importance has been gradually accepted and recognized by everyone,which confirms the necessity of inheritance and development of TCM,especially the empirical prescriptions.The best way to develop TCM is to make it registered and listed as a national brand medicine.In recent years,the empirical prescriptions of TCM have become the source of new drug research and development (R&D).In December 2019,the State Council issued “Opinions on Promoting the Inheritance and Innovation of TCM”,pointing out that it is necessary to optimize the technical requirements for the review of new TCM based on ancient empirical prescriptions of TCM and to speed up their approval[2].
With the advent of the era of big data,real-world study (RWS),which has been widely applied in the field of medicine,provides new ideas and methods for the inheritance and development of empirical prescriptions of TCM.RWS refers to the research process of collecting health-related data (realworld data,RWD) or aggregate data derived from these data in real-world settings for preset clinical problems and obtaining clinical evidence (real-world evidence,RWE) of drug use and potential benefit and risk through analysis[3].In 1993,Professor Kaplan and others proposed RWS for the first time in a paper on prospective efficacy evaluation of ramipril intervention in patients with hypertension.From then on,RWS has attracted the attention of researchers[4].Since 2016,the US Food and Drug Administration(FDA) has successively issued the “21st Century Cure Act” “Using Real-World Evidence to Support Medical Device Regulatory Decisions” “Real-World Evidence Programme Framework” and the “Industry Guidelines for Submitting Documents Using Real-World Data and Real-World Evidence to FDA for Pharmaceutical and Biological Products”,etc.,continuously conveying to pharmaceutical enterprises the importance they attach to RWE in the clinical evaluation stage[5-8].In 2017,Trans Enterix submitted the robotically assisted surgical device (RASD) to FDA,the results of 150 patients undergoing gynecological surgery and 45 patients undergoing colorectal surgery in realworld environment were used to apply for the listing of Senhance system.FDA believed that RWE and simulation application data could prove that Senhance system and Da Vinci Si IS3000 were equivalent in gynecological and colorectal surgery and approved the listing of Senhance system[9].In 2015,the FDA accelerated the approval of Osimertinib as a secondline treatment for advanced EGFR T790M+nonsmall cell lung cancer.But AstraZeneca,the drug R&D company,was required to provide data on the overall response rate of at least 100 patients in one or more real-world cohort studies.Then,AstraZeneca used electronic health record (HER) to collect data on the efficacy and safety of Octetinib in the real world and updated the overall survival data on the treatment of non-small cell lung cancer patients with Octetinib in 2018[10].In 2019,FDA approved a supplementary application of Pfizer’s Palbociclib(trade name:Ibrance) combined with aromatase inhibitor or Fulvestrant for the treatment of male HR+,HER2-advanced or metastatic breast cancer based on RWS[11].With the deepening of RWS,China’s drug regulatory authorities have actively explored it.In April 2019,to give full play to the pilot role of Boao Lecheng Pilot Zone of International Medical Tourism,the RWS was first carried out in Hainan.In October 2019,Boao Lecheng Pilot Zone of International Medical Tourism began to select the first batch of clinical products based on RWS.In November 2019,Aierjian’s glaucoma drainage tube became the first medical device to start RWS in Lecheng,Hainan.In March 2020,with the approval of the National Medical Products Administration (NMPA),the registration of glaucoma drainage tube was successful,which were the results of RWS helping approval and listing of pharmaceutical devices[11].At the beginning of 2020,China also issuedthe “Guiding Principles for Drug R&D and Evaluation Supported by Real-World Evidence (Trial)” to regulate and guide RWS[3].
1 Limitations of randomized controlled trials (RCTs) on clinical research of TCM empirical prescriptions
At present,RCTs are widely used in clinical drug research,and RCTs are also considered as the gold standard for evaluating the safety and effectiveness of drugs in the pharmaceutical industry.First,RCT is conducted in the ideal test environment with the strict inclusion and exclusion criteria.For example,RCT will generally limit the disease classification and staging,consider the patient’s gender,age,whether suffering from other diseases and minimize the interference of other factors on the test.Therefore,the research conclusion is more definite and reliable.However,this will undoubtedly increase the difficulty and workload of the test.Since the test is carried out in an ideal environment,the extrapolation of the test conclusion is poor.Secondly,RCT mostly evaluates the single outcome index,which is different from the overall treatment outcome of TCM.Finally,empirical prescriptions have a lot of human experience in clinical practice,and their safety and effectiveness have been confirmed to a certain extent.To conduct RCT for pharmacological,toxicological,pharmacokinetic,animal experimental and clinical trials on them not only prolongs the time to market,but also wastes a lot of manpower,materials and financial resources.
2 The advantages of using RWS to inherit and develop the empirical prescriptions of TCM
2.1 Policy advantages
In recent years,China has made great efforts in exploring new drug development with the characteristics of TCM.Relevant policies have been issued to encourage and support the new drug development based on empirical prescriptions of TCM.Relevant policies are shown in Table 1.
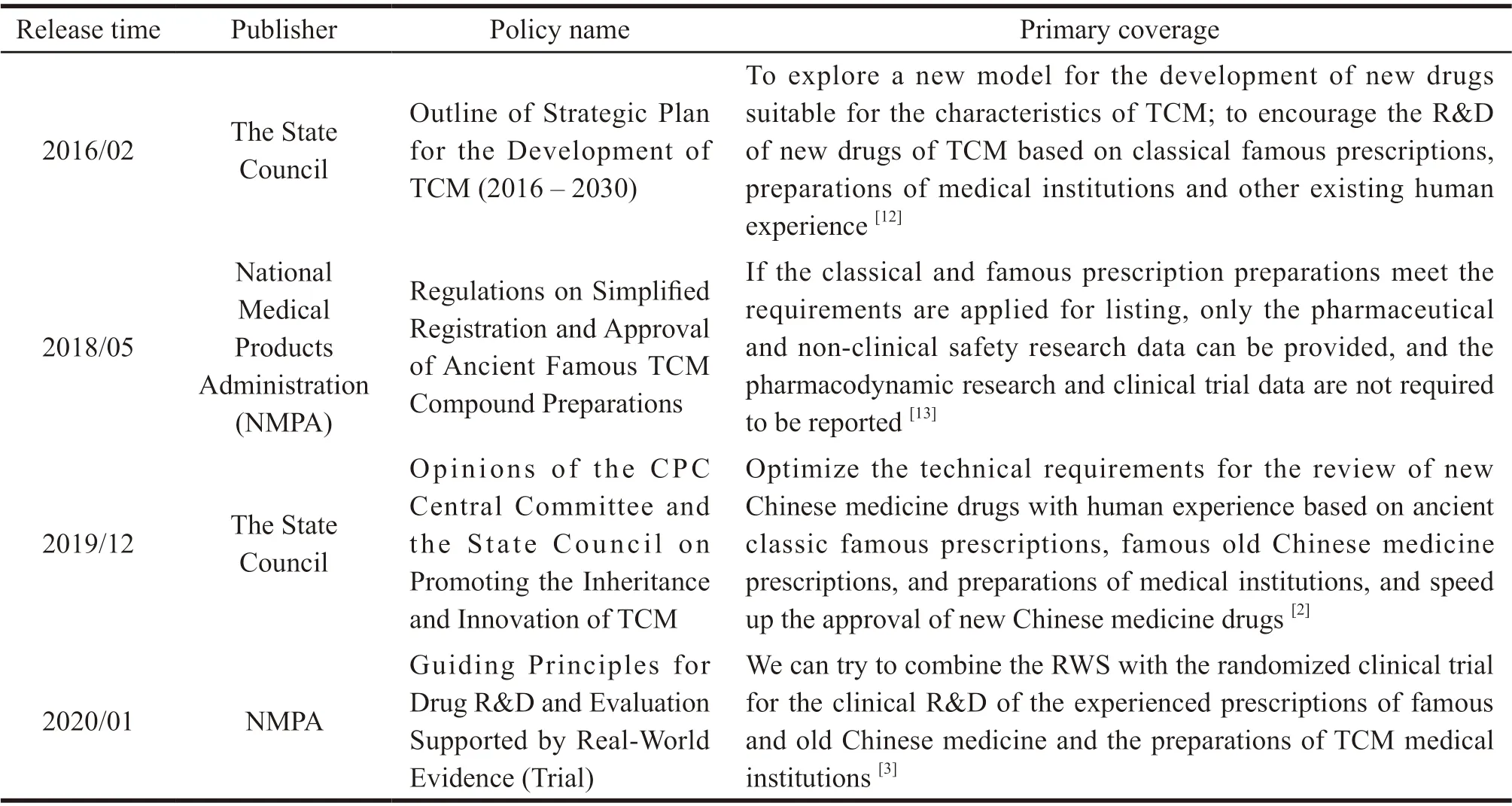
Table 1 New drug research and development (R&D) policies related to TCM empirical prescriptions in the past 5 years
2.2 Theoretical and ideological advantages
RWS has a broad standard of inclusion and exclusion,which determines the treatment plan according to the conditions of patients and the hospital,limiting the complex drug use,and the result is an index with broad significance,rather than a specific symptom or feature,which coincides with the holistic philosophy of TCM[14].
2.3 Extrapolation advantage
RWS is carried out in the real clinical environment,and the conclusion about drug safety and effectiveness has better extrapolation.
2.4 Data advantage
The empirical prescriptions of TCM have a long history of use in China,with a large amount of RWD accumulation,which has great advantages for their combination with RWS.In particular,with the use of hospital information system (HIS) in all major hospitals,some hospital preparations provide great convenience for the traceability of RWD.
3 RWS supports the registration and listing of empirical prescriptions of TCM
The “Guiding Principles for Drug R&D and Evaluation Supported by Real-World Evidence(Trial)” points out that there are two ways to register and market new drugs of TCM empirical prescriptions combined with RWS.Firstly,if the RWD is directly used as the basis for supporting the drug marketing,and the RWE formed through well-designed observational studies is scientific and sufficient after evaluation,it can communicate with the drug regulatory department and apply for being directly used as the basis for supporting the product marketing[2].Secondly,clinical research is carried out by combining observational study with pragmatic RCT(pRCT) to help TCM empirical prescriptions to be registered and listed as new Chinese medicine drugs[2].RWS is like traditional clinical research in general.The difference is that the data of traditional clinical research is obtained after the trial,while the data in RWS is the starting point of research.The design framework of RWS is to put forward research questions and to conduct observational study based on existing data.Meanwhile,it also conducts pRCTs based on data framework system to draw experimental conclusions.
4 Data source and experimental design of RWS for the registration and listing of empirical prescriptions of TCM
4.1 Data sources
Data is the premise of RWS.According to the existing research and resources,the RWD of TCM empirical prescriptions mainly comes from the following four platforms.HIS is the first platform that covers the patient’s orders,nursing,outpatient,hospitalization,and other information[15].TCM hospital is an important area to produce RWD.TCM inheritance support system (TCMISS) is the second platform,which was jointly developed by the Institute of Chinese Medicine of China Academy of Chinese Medical Science (CACMS),and Institute of Automation,Chinese Academy of Sciences.It has been applied in summarizing empirical prescriptions,application regulations,new drug R&D,prescription screening and so on[16].The system provides a platform for the famous TCM doctors to summarize their clinical medication experience.Some scholars have used the system to summarize the clinical medication experience of Professor Yan Zhenghua and Huang Chunlin,the famous TCM doctors.TCM clinical research sharing information system is the third platform.Supported by the national science and technology program of the 11th Five Year Plan and other projects,it was developed by CACMS and related software companies.It integrated with the HIS to develop a structured clinical diagnosis and treatment information collection system,formulating the dynamic structured data entry specification for the electronic medical records of TCM[17].At present,the system has collected clinical diagnosis and treatment data of more than 5 famous TCM doctors and 40 000 patients,developing 5 data analysis methods,which are used for comprehensive analysis of syndrome differentiation experience and medication of these doctors[18].The ancient and modern medical case cloud platform is the fourth one which is developed by the Department of Clinical Application and Health Management,Institute of Chinese Medicine Information,CACMS.This software includes frequency statistics,association analysis,chi-square test,Bayesian,community analysis,cluster analysis,complex network,and other algorithms,which can carry out prescription,syndrome differentiation,medicine and other multidimensional research.Its data mining algorithm has more advantages than other software[17].Among them,platform 2 and 3 are data mining software and system of TCM,which are based on the inheritance of TCM empirical descriptions.With the development of RWS,the government and pharmaceutical enterprises can try to establish new software or system to track and collect the medication of famous traditional Chinese doctors,and to summarize RWD of the empirical descriptions of TCM.
4.2 Experimental design
4.2.1 Observational study design
Observational study means that under natural conditions,researchers do not intervene in the research,but observe the research only.It can fully respect the diagnosis and treatment of famous TCM doctors[19].Observational studies used by researchers include cohort studies,case-control studies,etc.
Cohort study is an observational study method to divide a specific population into different subgroups according to their exposure to a certain suspicious factor.Then,the outcomes or disease occurrence of members are observed in each group,and the differences in the incidence of outcomes among groups are compared to determine whether there is a causal correlation and the degree of correlation between a certain suspicious factor and the outcome[20].Cohort studies can be divided into prospective cohort studies,historical cohort studies and two-way cohort studies according to the time of trial and the time of patients entering and leaving the cohort.The advantage of cohort study is that the data source is reliable,which is helpful to understand the history of the disease.Besides,it can confirm the etiological relationship.However,incidence rate bias is also high in cohort studies,which is costly and not suitable for low incidence diseases.
Case control study,also known as retrospective study,is to compare people who have certain diseases with other people without such diseases,investigating whether they have been exposed to suspected pathogenic factors and their degree in the past.Then the statistical connection and degree between research factors and diseases are determined through comparison,and the connection between exposure factors and diseases are determined as well[21].Case control study is more suitable for rare diseases research,which not only can reduce workload,but also obtain results in a short time.Due to the absence of external intervention measures,it will not harm the research object.Case control studies have some shortcomings,such as bias and recall bias.In addition,it cannot calculate the incidence rate directly.In the whole research framework,observational study is mainly used to explore the clinical efficacy and safety of TCM empirical prescriptions preliminarily and determine whether they have potential benefits in clinical application.
4.2.2 pRCT design
pRCT refers to the study of comparing the treatment results (including actual effect,comparative effect,safety or/and cost-effectiveness,etc.) of different interventions in clinical practice by using a randomized controlled design in a real or near real medical environment[22].PCT will set the subjects’standards and intervention control standards as few as possible to reflect the differences between patients in the real clinical practice,which can help solve the problem of choosing different treatment measures in clinical practice.Because the experimental setting is closer to the real clinical environment,the conclusions obtained by PCT are better RWE in most cases.While analyzing data,we should also communicate with famous TCM doctors to discuss whether the analysis results can prove the effectiveness and safety of the empirical prescriptions.
5 Challenges faced by RWS in the inheritance and development of TCM empirical prescriptions
The advent of the era of big data and the wide application of RWS provide new ideas and methods for the registration and listing of new TCM,but also it faces many challenges.
5.1 Imperfect laws and regulations related to RWS supporting TCM empirical prescriptions to develop new TCM
Since 2016,FDA has continuously issued relevant laws and guidelines to regulate and support the use of RWD to speed up the registration and marketing of new drugs or increase the new indications of marketed drugs.However,it was not until 2018 that the first “A Guide to Real-World Study” appeared in China,which systematically and scientifically introduced RWS to scholars and the public.It was not until 2020 that the “Guiding Principles for Drug R&D and Evaluation Supported by Real-World Evidence (Trial)” was issued by NMPA to regulate the application of RWE in the registration and listing of new drugs.However,the above guidelines and principles are for all drugs.Based on the characteristics of non-unitary indicators such as the experience and outcome of the empirical prescriptions of TCM,it is necessary for the government and relevant departments to issue corresponding regulations timely to standardize how to use RWS to speed up the registration and listing of new Chinese medicine.
5.2 Privacy protection of patients
In the era of big data,it is easy to obtain patients’information,but we should protect the privacy of patients when collecting and using RWD.Once privacy leakage occurs,it may lead to huge economic losses or ethical problems.When the study involves the personal information of patients,it needs to be explained in the study plan and approved by the ethics committee.Besides,some strict and precise measures should be taken to protect individual privacy,such as setting access rights,desensitization methods,data security protections and so on[22].
5.3 Insufficient exploration of empirical prescriptions of TCM
Compared with Japan,South Korea and other countries,China’s efforts for the exploring TCM empirical descriptions are not enough.In 2018,Center for Drug Evaluation of NMPA accepted a total of 157 applications for the registration of class one innovative chemical drug,while there were only 37 applications for the registration of class 1-6 new TCM[23].Although the application for new drug registration of TCM has increased compared with that in 2017,there is still a big gap with chemical drugs.Pharmaceutical enterprises should make greater efforts to research and register the empirical prescriptions of TCM,so that they can benefit the people to the greatest extent while inheriting and developing these empirical prescriptions.
5.4 Clarifying the subject of responsibility for new TCM drugs based on TCM empirical prescriptions
The empirical prescriptions of TCM include not only the classical prescriptions left by ancient TCM doctors,but also the effective prescriptions explored by modern TCM doctors in clinical treatment.In the new TCM’s R&D,the subject that can be the applicant,holding the certificate of drug marketing license,and be responsible for the whole life cycle of drugs should be clarified.For the classical prescriptions left by ancient doctors,the relevant national institutions can be the applicants.For the modern prescriptions,the drug marketing licensors who first proposed the prescription can be the applicants.Besides,these doctors can transfer the certificate of the marketing licensors of the prescription to the pharmaceutical enterprises or research institutions after negotiation.
5.5 The conflict between profitability and inheritance and development
There is a long history of using TCM empirical prescriptions in China and many of them have been popular among the people.We don’t know if there is enough profit space for pharmaceutical enterprises to develop new drugs and inherit them.Therefore,pharmaceutical enterprises should conduct market research before developing the new TCM.Meanwhile,pharmaceutical enterprises should not only pay more attention to the domestic market but foreign market as well,thus they can get sufficient profit.
- 亚洲社会药学杂志的其它文章
- Research on Retrospective Studies of Real-World Study and Its Selection Bias
- Research on the Pragmatic Clinical Trial Design Based on Real-World Study
- How Real-World Evidence Supports Healthcare Decisions in EU and Its Enlightment to China
- Application of Real-World Evidence in Regulatory Decision-Making for Medical Devices
- Research on Real-World Evidence and Application in EU and Its Enlightment to China
- EU Real-World Evidence Supports the Expansion Indications for Drugs and Its Enlightenment to China

