Endoscopic treatment of periampullary duodenal duplication cysts in children:Case series and literature review
Anna Lavinia Bulotta,Maria Vittoria Stern,Dario Moneghini,Filippo Parolini,Maria Pia Bondioni,Guido Missale,Giovanni Boroni,Daniele Alberti
Anna Lavinia Bulotta,Maria Vittoria Stern,Filippo Parolini,Giovanni Boroni,Daniele Alberti,Department of Pediatric Surgery,Azienda Socio Sanitaria Territoriale degli Spedali Civili di Brescia,Brescia 25123,Italy
Dario Moneghini,Guido Missale,Department of Digestive Endoscopy,Azienda Socio Sanitaria Territoriale degli Spedali Civili di Brescia,Brescia 25123,Italy
Maria Pia Bondioni,Department of Pediatric Radiology,Azienda Socio Sanitaria Territoriale degli Spedali Civili di Brescia,Brescia 25123,Italy
Abstract BACKGROUND Duodenal duplications are rare congenital anomalies of the gastrointestinal tract.As the periampullary variant is much rarer,literature is scant and only few authors have reported their experience in diagnosis and treatment,particularly with operative endoscopy.AIM To report our experience with the endoscopic treatment in a series of children with periampullary duodenal duplication cysts,focusing on the importance of obtaining an accurate preoperative anatomic assessment of the malformations.The pediatric periampullary duodenal duplication cyst literature is reviewed.METHODS We conducted a systematic review according to the PRISMA guidelines.The PubMed database was searched for original studies on “duodenal duplication,”“periampullary duplication” or “endoscopic management” published since 1990,involving patients younger than 18 years of age.Eligible study designs were case report,case series and reviews.We analyzed the data and reported the results in table and text.RESULTS Fifteen eligible articles met the inclusion criteria with 16 patients,and analysis was extended to our additional 4 cases.Median age at diagnosis was 13.5 years.Endoscopic treatment was performed in 10 patients (50%),with only 2 registered complications.CONCLUSION Periampullary duodenal duplication cysts in pediatric patients are very rare.Our experience suggests that an accurate preoperative assessment is critical.In the presence of sludge or stones inside the duplication,endoscopic retrograde cholangio-pancreatography is mandatory to demonstrate a communication with the biliary tree.Endoscopic treatment resulted in a safe,minimally invasive and effective treatment.In periampullary duodenal duplication cyst endoscopically treated children,long-term follow-up is still necessary considering the potential malignant transformation at the duplication site.
Key Words:Periampullary duodenal duplication cyst;Duodenal duplication;Endoscopic ultrasound;Endoscopic treatment;Double wall sign
INTRODUCTION
Duodenal duplications (DD) are rare congenital anomalies of the gastrointestinal tract,which usually arise during the first decade of life[1-3].Due to variability of location and size,DD do not display pathognomonic clinical presentation,but they can manifest with a variety of complications including pancreatitis,bleeding,perforation and duodenal obstruction[1].Unfortunately,little is reported about the anatomical details of DD,which can be divided into two groups:periampullary and non-periampullary duplication cyst.Periampullary duodenal duplication cysts (PADDC) are defined as cysts located near the major papilla and the biliary-pancreatic ampulla,sometimes with a small aberrant pancreatic duct drained into the cyst[4].As the periampullary variant is much rarer,literature is scant and only few authors have reported their experience in diagnosis and treatment.Moreover,the recent introduction of operative endoscopy for DD treatment in adults has also been extended to the pediatric population with promising results[5-10].
The aim of this paper is to report our experience with the endoscopic treatment (ET)in a series of children with PADDC,focusing on the importance of obtaining an accurate preoperative anatomic assessment of the malformations.The pediatric PADDC literature is reviewed.
MATERIALS AND METHODS
All consecutive children with PADDC managed at our tertiary-level institution from 2015 to 2020 were retrospectively reviewed.A written consent was obtained from all patients.All data were retrospectively collected and recorded according to the Declaration of Helsinki.
Case 1
Case presentation:A 14-year-old boy was admitted with a 1-year history of recurrent pancreatitis.The abdominal computed tomography (CT) scan,previously performed at another center,showed a cyst within the duodenal lumen.On inspection,the abdomen was distended with tenderness in epigastrium upon superficial and deep palpation.Laboratory values revealed an increased serum levels of lipase (1077 UI/L;normal value (n.v.) 70-280 UI/L),amylase 514 UI/L (n.v.15-53 UI/L) and C-reactive protein 168 mg/dL (n.v.<5 mg/L),while gamma glutamyl transferase 69 U/L (n.v.6-42 UI/L),count of blood cells,white cell count,total and conjugated bilirubin,alkaline phosphatase level,aspartate aminotransferase and alanine aminotransferase were normal.The radiological workup first included an abdominal ultrasound (US) that showed a heterogeneous hyperechogenicity of the whole pancreas and an intraluminal duodenal cyst (5.8 cm x 4.5 cm x 4.0 cm in size) near the pancreas head.An 8.5 mm dilatation of the main common bile duct (CBD) was also detected.Intrahepatic biliary ducts and gallbladder were normal.
A magnetic resonance imaging (MRI) on HASTE T2-w sequence showed a homogeneously hyperintense cyst below the pancreatic head,located within a partially occluded duodenum (Figure 1A).On cholangiographic reconstruction the intrahepatic bile ducts were normal,the cystic duct appeared dilated with a tortuous course and the common hepatic duct presented saccular dilation.CBD had a caliber at the upper limits of the normal range with a regular course;the Wirsung duct was normal (Figure 1B).

Figure 1 Magnetic resonance imaging on HASTE T2 w sequence.
Final diagnosis:Endoscopic ultrasound (EUS) showed a bulging in the second duodenal portion,covered with normal mucosa,next to the Vater’s papilla and filled with biliary sludge (Figure 2).The lesion preserved a five layer wall consisting with the typical echoendoscopic feature for the gastrointestinal wall consistent with a PADDC,and ET was proposed to parents.
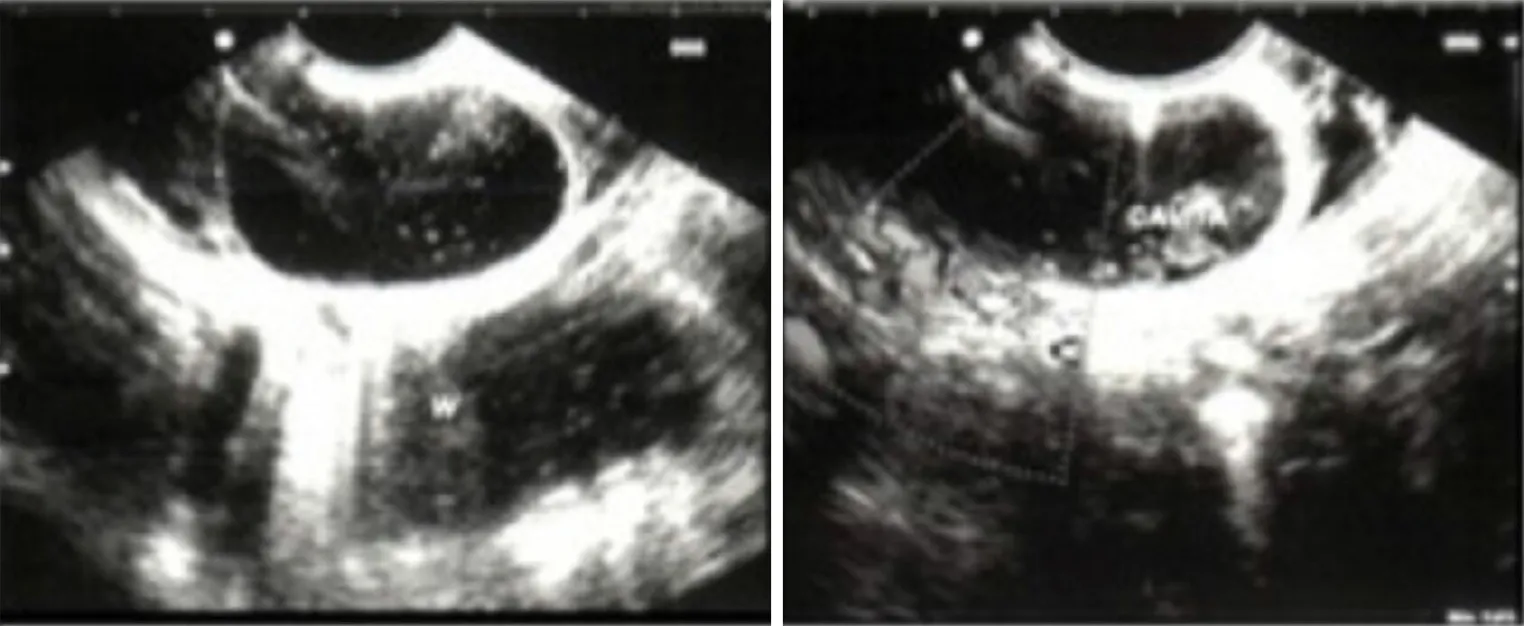
Figure 2 Endoscopic ultrasound.
Treatment:Upon endoscopic retrograde cholangio-pancreatography (ERCP),elective cannulation of the CBD showed a direct communication with the cyst and multiple stones in its lumen.A sphincterotome incision of the wall cyst,laterally to the papilla,was performed,and the stones were removed.
Follow-up:The patient had an uneventful postoperative course and was discharged home 8 d later with a quick resolution of the abdominal pain and normalization of serum pancreatic enzymes.Ursodeoxycholic acid therapy and a hypolipic diet were continued until the next follow-up.At the 3 mo follow-up,magnetic resonance cholangiopancreatography (MRCP) control after ET,PADDC was no longer detected(Figure 3).At the 10-year follow-up the patient is doing well,without any therapy or further episodes of pancreatitis.
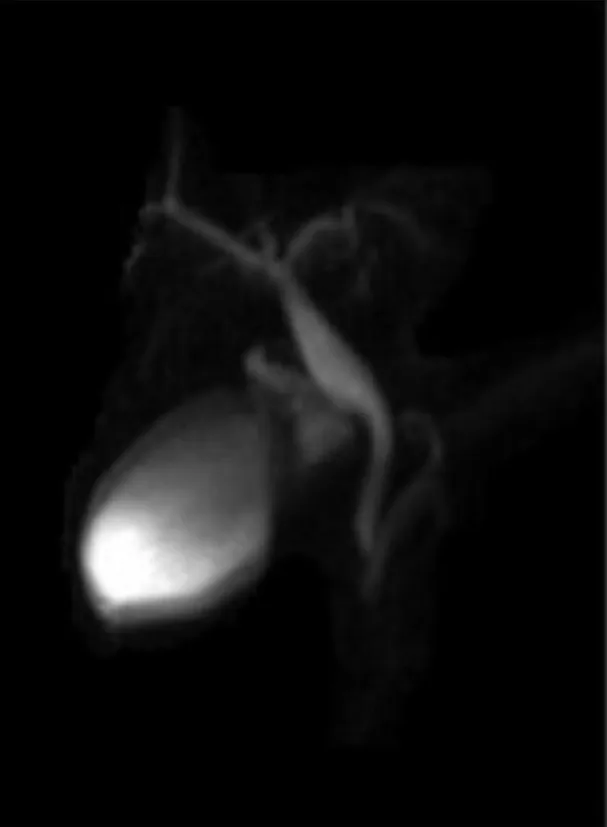
Figure 3 Magnetic resonance cholangiopancreatography performed 3 mo after the endoscopic treatment did not show periampullary duodenal duplication cysts.
Case 2
Case presentation:A 16-year-old girl was admitted to our emergency room with abdominal pain.In the past 2 years she had suffered from recurrent abdominal pain due to pancreatitis.An abdominal CT scan,performed in another hospital,detected a pancreatic pseudocyst.
Physical examination at admission showed a mild distended abdomen and diffuse tenderness upon superficial and deep palpation.Blood samples revealed increased serum levels of lipase (2365 UI/L;n.v.70-280 UI/L);the full panel of liver tests including cholestasis indexes were normal.US showed the presence of an anechoic cystic lesion within the pancreatic head.Intrahepatic and extrahepatic biliary ducts were normal.
An MRI on HASTE T2 w sequence revealed (Figure 4A) a round homogeneous hyperintense lesion on the pancreas uncinate process,determining a major compression of the second portion of the duodenum.At cholangiographic reconstruction,the intra- and extrahepatic biliary tree along with the pancreatic ductal system were normal (Figure 4B).

Figure 4 Magnetic resonance imaging of case 2.
Final diagnosis:An EUS showed an anechoic cystic lesion within the second duodenal portion,characterized by normal echographic bowel wall stratification and containing multiple hyperechoic stones;the cyst was not in communication with the CBD,and thereby PADDC was diagnosed.
Treatment:Upon ERCP,a small orifice on the lateral surface of the cyst was cannulated;a contrast injection failed to demonstrate any communication with the CBD.Intracystic stones were confirmed.The DD wall was incised with sphincterotome,,and stones were removed.
Follow-up:The patient had an uneventful recovery and was discharged home 2 d after the procedure with low fat meals.The 9 mo follow-up MRCP did not show any residual duplication (Figure 5),and at 8 years follow-up no further pancreatitis episodes were reported.
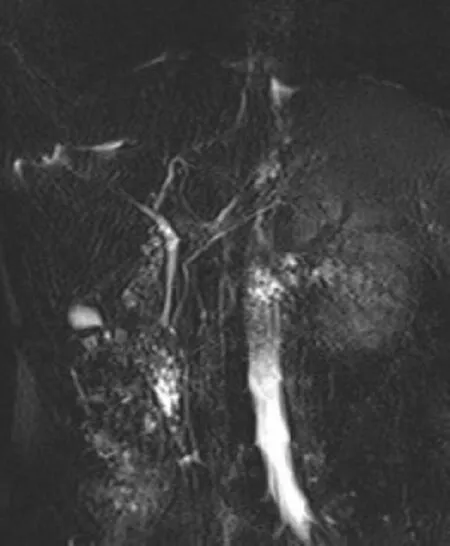
Figure 5 Magnetic resonance cholangiopancreatography performed after 9 mo endoscopic treatment did not show periampullary duodenal duplication cysts.
Case 3
Case presentation:A Chinese 11-year-old girl was admitted for 1-year history of epigastric pain with vomiting and weight loss.The girl was previously examined in her country,and a CT scan showed a cyst in the second part of the duodenum.Physical examination showed mild diffuse abdominal tenderness upon superficial and deep palpation.Laboratory values revealed increased serum levels of lipase (43440 UI/L;n.v.70-280 UI/L).The full panel of liver tests was normal.
An MRI on HASTE T2 w sequence showed an oval heterogeneous hyperintense lesion,measuring 4.5 cm x 3.5 cm,containing multiple stones and located in the second part of the duodenum.Cholangiographic reconstruction indicated a normal/physiologic gallbladder as well as intra- and extrahepatic bile ducts.The lesion,irregularly hyperintense,was located below the gallbladder and laterally to the CBD and pancreatic duct (Figures 6 and 7).
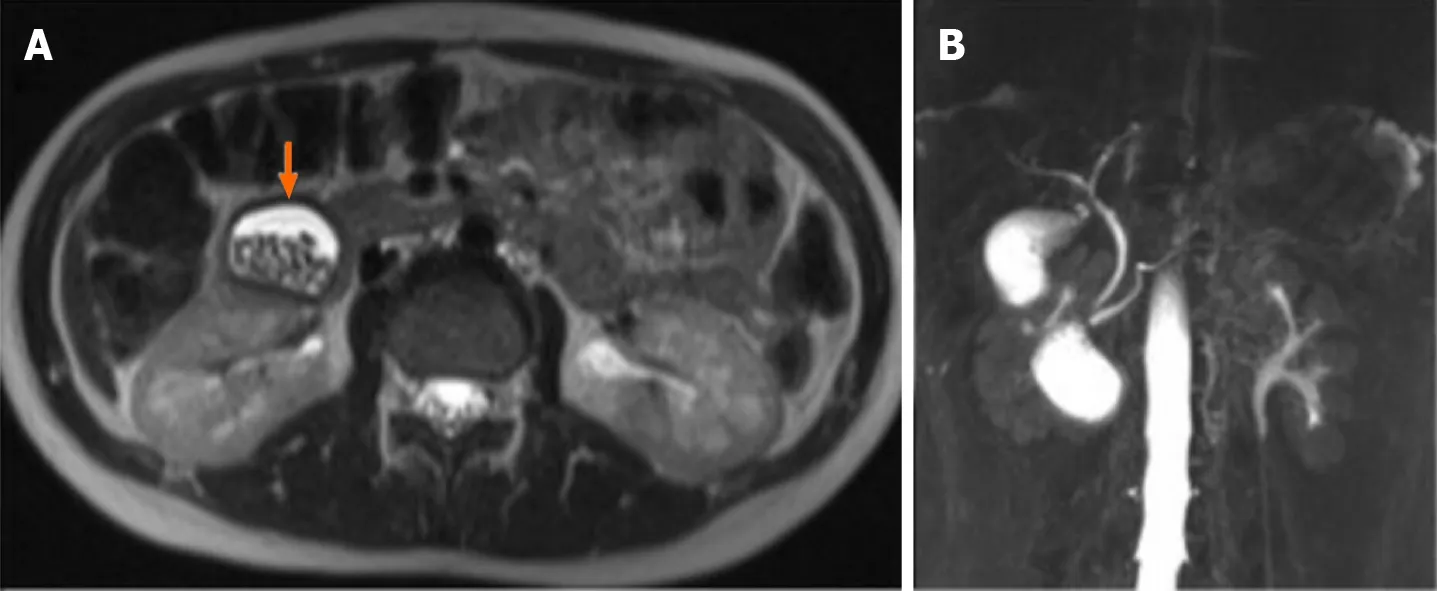
Figure 6 Magnetic resonance imaging of case 3.

Figure 7 Magnetic resonance imaging showed periampullary duodenal duplication cysts filled with stones.
Final diagnosis:An EUS revealed an anechoic cystic lesion characterized by a normal echographic bowel wall stratification and containing biliary sludge.
Treatment:ERCP showed a regular main pancreatic duct;after distal papillotomy,contrast was injected,and it filled the PADDC (Figure 8).Marsupialization of the cyst with sphincterotome was then performed.

Figure 8 Endoscopic retrograde cholangio-pancreatography.
Follow-up:The postoperative course was complicated by severe melena on day 3,which required packed red cell transfusion.Esophagogastroduodenoscopy detected bleeding at the cyst section site.Endoscopic metallic clip placement was effective for bleeding control.The patient showed a progressive normalization of the serum lipase,and she was discharged home with ursodeoxycholic acid therapy and a low-fat diet.MRCP,done 2 mo later,did not show any duodenal cyst or intra- or extrahepatic bile and pancreatic duct dilatation.At the 4-year follow-up,she was well,and no further episodes of abdominal pain were reported.
Case 4
Case presentation:An 11-year-old girl was admitted to our unit with abdominal pain and vomiting.Physical examination showed severe tenderness upon superficial and deep palpation of the upper abdomen.Biochemical investigation revealed hyperlipasemia (5497 UI/L;n.v.70-280 UI/L) and increased levels of aspartate aminotransferase (5.3 x n.v.),alanine aminotransferase (9.2 x n.v.) and gamma-glutamyl transferase (169 UI/L,n.v.6-42).
US examination found a cyst (2.5 cm×2.5 cm×1.6 cm) sharing bowel wall stratification with the second part of the duodenum and full of hyperechogenic debris.
An MRI on HASTE T2 sequence detected an oval mass,located below the gallbladder and laterally to the CBD and pancreatic duct (Figure 9),adjacent to the pancreatic head.The cyst was filled with fluid and multiple stones.Cholangiographic reconstruction indicated a normal gallbladder and intra- and extrahepatic bile ducts.

Figure 9 Magnetic resonance imaging.
Final diagnosis:Duodenoscopy revealed an intraduodenal cyst,next to the papilla of Vater and not in communication with the duodenal lumen.
Treatment:ERCP showed a normal pancreatic duct,dilation of CBD (20 mm diameter)without a detectable communication with the cyst.Cyst marsupialization was performed with subsequent extraction of biliary microstones (Figure 10).
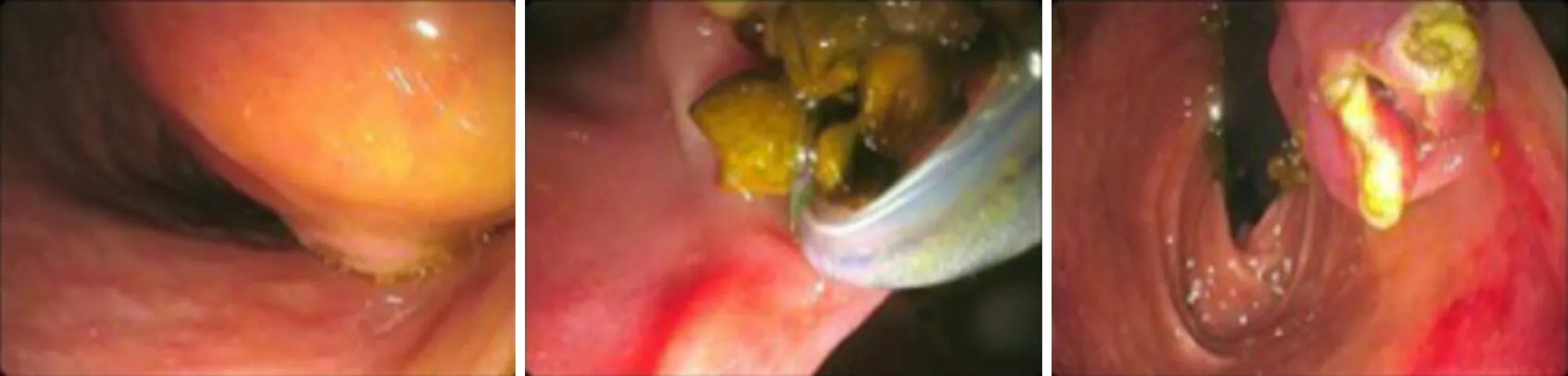
Figure 10 Cyst marsupialization was performed with subsequent extraction of biliary microstones.
Follow-up:The patient had an uneventful recovery and was discharged home 10 d after the procedure,with an ursodeoxycholic acid therapy and low-fat meals for 3 mo.
At the 2-year follow-up,she was totally asymptomatic,abdominal US was normal,and she eats a free diet.
Literature search
This literature review was performed according to preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines[11] (Figure 11).The PubMed database was searched for original studies on “duodenal duplication,”“periampullary duplication” or “endoscopic management” published since 1990,involving patients younger than 18 years of age.Eligible study designs were case reports,case series and reviews.We omitted reports in which abstracts indicated an adult population (>18 years) and improper reporting of the diagnosis and treatment methods.We then evaluated the full text of the selected articles and consider PADDC only where that diagnosis was confirmed by authors.

Figure 11 PRISMA 2009 flow diagram.
According to Tröbset al[4],PADDC were defined as cysts located near the major papilla and the biliary-pancreatic ampulla that can have a small aberrant pancreatic duct drained into the cyst.We excluded all patients with a diagnosis of biliary/gallbladder disease (including acute acalculous cholecystitis) or with a diagnosis of duodenal duplication not located near the major papilla.
The date of the last search was December 2020.For each study,data were extracted for two primary outcomes (diagnostic assessment and type of treatment) and several secondary outcomes (including sex and age at presentation,clinical presentation,pathological examination and outcome).Analysis was extended to our additional 4 cases.
RESULTS
The initial PubMed search yielded 42 potentially relevant studies.Eventually,16 eligible articles met the inclusion criteria,involving a total of 17 children with PADDC[1,3,4,6-9,12-20] (Table 1 and Figure 11).All selected studies were case reports (class of evidence Ⅲ and rating scale of evidence E) and clearly reported the two primaryoutcomes.
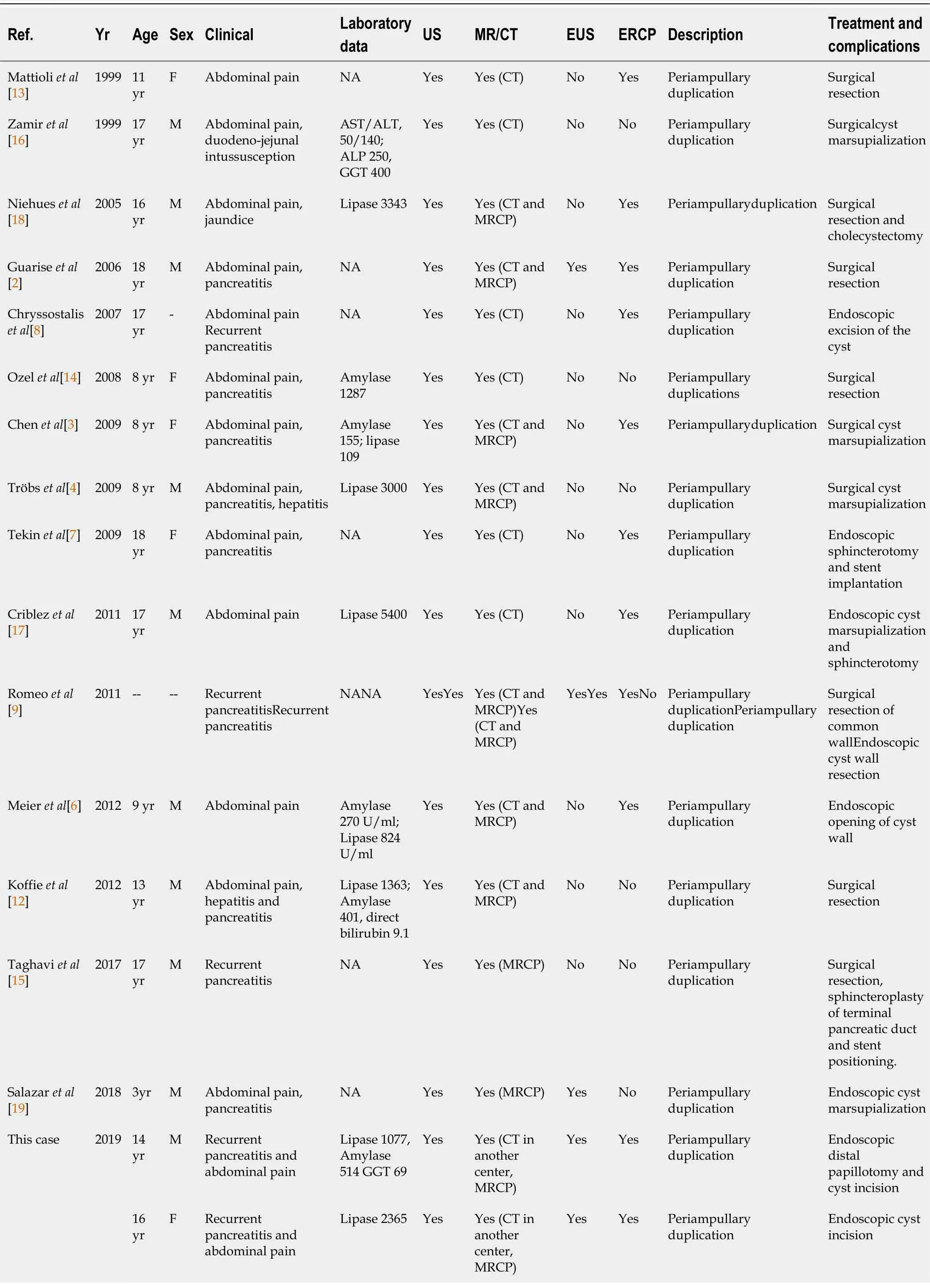
Table 1 Data of included studies
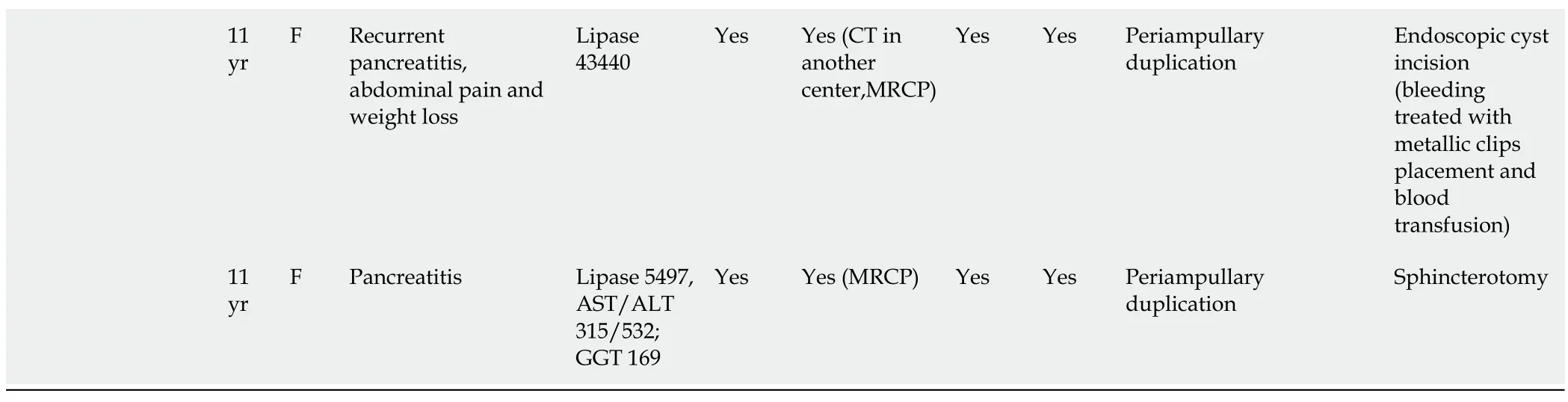
Unit used were as follows:Amylase (UI/L),lipase (UI/L),bilirubin (mg/dL),alkaline phosphatase (UI/L),aspartate aminotransferase (UI/L),alanine aminotransferase (UI/L) and gamma-glutamyl transferase (UI/L).US:Ultrasound;CT:Computed tomography;EUS:Endoscopic ultrasound;ERCP:Endoscopic retrograde cholangio-pancreatography;NA:Not available;ALP:Alkaline phosphatase level;AST:Aspartate aminotransferase;ALT:Alanine aminotransferase;MRCP:Magnetic resonance cholangiopancreatography;GGT:Gamma-glutamyl transpeptidase;MR:Magnetic resonance;F:Female;M:Male.
The patients’ median age at diagnosis was 14 years (range:3-18 years),and PADDC was reported in 10 males and 8 females.For 3 patients,data were not available.Clinical presentation was unspecific,with abdominal pain reported in all cases.Recurrent pancreatitis was the most common complication and was observed in 14 cases (70%),followed by cholestasis,jaundice and intussusception.
All patients underwent abdominal ultrasound,followed by abdominal CT scan in 18 cases (90%),ERCP in 13 (65%),MRCP in 7 (35%) and EUS in 8 (4%);1 patient was only examined with ERCP (5%) (Table 1).
Endoscopic treatment was performed in 10 patients (50%),with two reported complications,namely bleeding at the duplication incision site,which were treated with packed red cell transfusion and endoscopic clipping of the bleeding site in one case and with local injection of epinephrine in the other case (Table 1)[9].The median follow-up was 22.5 mo (range:4-108 mo);all endoscopically treated patients are doing well with disappearance of the duplication on imaging.No case of malignancy was reported.
DISCUSSION
Duodenal duplications are uncommon congenital anomalies of the gastrointestinal tract,which usually present during the first decade of life[4,5].They represent 5%-7%of all gastrointestinal duplications and result from disturbances in the embryonic development,probably due to duodenal epithelial pinching during the outgrowth of the dorsal pancreatic bud or secondary to an epithelial sequestration[4].The majority of them are cystic,adherent and located on the mesenteric side of the second or third portion of the duodenum,with an epithelial mucosal lining and a smooth muscle layer[10,21].A communication with the duodenal lumen has been reported in up to 25% of cases[1],and some authors have also described the possibility of a pancreato-biliary involvement in 30% of patients,although this cannot always be the only explanation of pancreatitis[5,6].
Three different mechanisms have been reported as responsible for pancreatitis:(1)External papilla obstruction by duplication enlargement;(2) Presence of an aberrant pancreatic duct within the duplication,which can become obstructed by mucus and debris;and (3) Migration of biliary sludge and/or microstones from the cyst into the bilio-pancreatic duct[3,4].Migration of biliary sludge and/or microstones from the cyst to the bilio-pancreatic duct is possible only due to a communication between the duplication and the bilio-pancreatic duct with stone formation due to the bile stasis within the duplication seeing as its peristalsis is intermittent[2].For this reason,the presence of stones or biliary sludge inside a duodenal mass do not ruled out the possibility of a DD.
DD can be divided into two subgroups:periampullary (PADDC) and non-periampullary duplication cyst.According to Tröbset al[4] periampullary duodenal duplication is defined as a duplication cyst located near the major papilla and the biliary-pancreatic ampulla,sometimes with a small aberrant pancreatic duct drained into the cyst[4].
Our experience suggests the possibility of communication between PADDC and the CBD and pancreatic duct,which explains both the possibility of observing sludge or calculi in the cyst and the pancreatitis.Unfortunately,detailed descriptions of the relationships between duplication and major papilla and/or pancreatic ampulla are lacking,and our review found that only 17 out of 49 pediatric patients reported a detailed description of the DD that can be classified as periampullary type (Table 1).
PADDC cases have been reported in childhood with a median age of diagnosis of 14 years (range:3-18 years);this was consistent also in our series (Table 1).
The first radiological tool for diagnosis was US,which is highly suggestive for a DD when peristalsis and pathognomonic “double wall sign,” consisting of an outer hypoechoic muscular layer,an internal echogenic mucosal layer and corpuscular fluid inside the lesion,are found[22].However,this finding should be confirmed with a more exhaustive radiological work-up by abdominal CT scan or preferably by MRCP[23],which provides more information about the location,size,enhancement and multilayered duplication cyst wall as well as anatomical details of the biliary and pancreatic ductal system.Furthermore,ionizing radiation should be limited as much as possible in childhood.
Moreover,we suggest performing an EUS in children with a cystic lesion next to the papilla.In our experience,EUS offered two major advantages:(1) Endoscopic vision allowed a better definition of the intraluminal duodenal lesion and an accurate localization of the papilla;and (2) US vision highlighted the presence of an anechoic structure surrounded by a five layer wall,consisting with the typical echo-endoscopic feature for the gastrointestinal wall,distinguishing DD from the other cystic and neoplastic duodenal or pancreatic masses,including cystic dystrophy of the duodenal wall,pseudocysts,cystic lymphangiomas,mesenteric cysts and choledochocele[4,24].
In particular,the performance of EUS to identify the presence of normal echographic bowel wall stratification at the DD allowed us to make differential diagnosis with choledochocele,where that hallmark is absent,but which represents the most frequent and challenging differential diagnosis.Furthermore,although many authors consider biopsy as the gold standard for the differential diagnosis between DD and choledochocele,duodenal type mucosa has been reported in choledochocele[25-27].Sarris and Tsang reported 15 cases of choledochocele with duodenal mucosa at pathological examination[27,28].
Eventually,EUS can well indicate the relationships between the duplication and biliary-pancreatic duct.Therefore,when a PADDC is suspected,we suggest considering radiological (EUS) and anatomic criteria appropriate to confirm the diagnosis.Only 4 out of the 16 patients (25%) that were included in our literature review,underwent a preoperative EUS evaluation (Table 1),but this is partly explainable by the recent EUS availability in pediatrics.
Despite having carried out the EUS,before proceeding with the endoscopic duplication unroofing,ERCP would have to be mandatory in order to obtain a detailed anatomic view of the bilio-pancreatic system and to detect a possible communication between the duplication and the biliary and/or pancreatic duct,particularly in patients with stones or sludge inside the cyst.
Endoscopic treatment of children with PADDC was first described in 2007[8],and a later meta-analysis of the pediatric population confirmed the safety,feasibility and effectiveness of this approach in this population[10].Our review revealed that 10/20 patients with PADDC (50%) underwent ET[6-9,17,19].
Two postoperative complications occurred (bleeding) and were both endoscopically treated;this point stresses the importance of ensuring a careful coagulation of the severed edges of the duplication.When planning an ET we thereby advise that a thorough preoperative radiological imaging encompassing EUS be mandatory,and our experience suggests that the real incidence of PADDC is underestimated because of incomplete preoperative imaging.
The anatomic location of the PADD and the possible communication with the biliary and/or pancreatic ductal system makes an open surgical approach highly demanding and not necessarily safer than ET.Furthermore,surgery has several disadvantages over ET,including worse postoperative pain,higher risk of postoperative complications,visible scars and longer hospitalization time.
Endoscopic cyst marsupialization was highly effective in relieving symptoms and cyst disappearance even at long-term follow-up.
Undoubtedly endoscopic management of PADDC requires a skilled multidisciplinary team,and the still limited use of the endoscopic strategy in a pediatric setting is probably explained,other than the rarity of PADDC,by the unavailability of a trained ERCP endoscopic team.
We suggest considering ET as a first line approach after a complete EUS study and reserving a surgical approach only when it is impossible to understand the relationship between PADDC and the pancreato-biliary tree.
ET provides marsupialization or incision of PADDC,therefore it is rare,but possible,to leave ectopic gastric or pancreatic tissue with potential risk of malignant degeneration.
Eventually,although DD (PADDC included) are generally benign lesions and only a few cases of malignant transformation have been reported in literature[5,29,30],a longterm follow up is mandatory in endoscopically treated patients,even in asymptomatic ones.
CONCLUSION
PADDC in pediatric patients are very rare.Our experience suggests that an accurate preoperative assessment with EUS is essential to differentiating the duplication from other duodenal lesions.In the presence of sludge or stones inside the duplication,ERCP is mandatory to demonstrate a communication with the biliary tree.ET is a safe,minimally invasive and effective treatment in children with PADDC.Long-term follow-up of this population throughout adulthood is mandatory and necessary considering that malignant degeneration of duodenal duplication has been described[5,29,30].
ARTICLE HIGHLIGHTS
Research background
The aim of the paper is to report a literature review of periampullary duodenal duplication cyst in the pediatric population and describe our four new cases.Data were extracted for two primary outcomes (preoperative assessment and treatment).The authors focused on the role of an endoscopic approach that recently was introduced for treatment in adults and has been extended to the pediatric population with promising results.
Research motivation
The authors reviewed the literature analyzing in particular the anatomical and radiological details and the type of treatment in pediatric patients.The authors have been able to demonstrate that PADDC is more frequent than the authors think and that endoscopic treatment may be the treatment of first choice due to being less invasive and having excellent efficacy.Furthermore,the authors have ascertained that endoscopic ultrasound makes a strong contribution in the diagnosis and above all in the understanding of the anatomy of the malformation.Therefore,its use in pediatric diagnosis should be encouraged.
Research objectives
Analyzing the case reports,the authors realized that the anatomy of the duodenal duplication was different from what is reported in the literature.For this reason the authors wanted to give our scientific contribution to stimulate a more accurate future description and to broaden the anatomical knowledge of this malformation.
Research methods
All consecutive children with periampullary duodenal duplication cyst managed at our tertiary-level institution from 2015 to 2020 were retrospectively reviewed.A written consent was obtained from all patients.All data were retrospectively collected and recorded according to the Declaration of Helsinki.The authors also performed a literature review according to preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines.
Research results
The experience suggeststhat an accurate preoperative assessment with EUS is essential to differentiating the duplication from other duodenal lesions.In the presence of sludge or stones inside the duplication,ERCP is mandatory to demonstrate a communication with the biliary tree.ET is a safe,minimally invasive and effective treatment in children with PADDC.Long-term follow-up of this population throughout adulthood is mandatory and necessary considering that malignant degeneration of duodenal duplication has been described.
Research conclusions
Through this review the authors have shown that duodenal duplication can have a communication with the biliary tract,and this explains why it is possible to find stones within the malformation.The authors have also given our contribution on which tools to use to diagnose,in particular with endoscopic ultrasound,and on how the minimally invasive approach with duodenal duplication can be effective and safe.Our work can also be a warning to provide more anatomical details of the malformation and therefore draw up a new and broader anatomical classification in the future.
Research perspectives
The message the authors want to give through this review is that it is necessary to study anatomically in detail the patients who present a malformation compatible with duodenal duplication,also by endoscopic ultrasound.Furthermore,endoscopic treatment in children is safe and effective.It will be necessary to follow the patients at a distance to understand the risks of relapse or cancer.
 World Journal of Gastrointestinal Endoscopy2021年9期
World Journal of Gastrointestinal Endoscopy2021年9期
- World Journal of Gastrointestinal Endoscopy的其它文章
- Ectopic pancreas at the ampulla of Vater diagnosed with endoscopic snare papillectomy:A case report and review of literature
- Enlarged folds on endoscopic gastritis as a predictor for submucosal invasion of gastric cancers
- COVID-19 in the endoscopy unit:How likely is transmission of infection? Results from an international,multicenter study
- Clinical characteristics and prognosis of patients with ulcerative colitis that shows rectal sparing at initial diagnosis
- Gastrointestinal hemorrhage in the setting of gastrointestinal cancer:Anatomical prevalence,predictors,and interventions
- Endoscopic balloon dilation for management of stricturing Crohn’s disease in children
