Review of a new bone tumor therapy strategy based on bifunctional biomaterials
Jinfeng Liao,Ruxia Han,Yongzhi Wu and Zhiyong Qian
INTRODUCTION
Bone tumors involve the invasion of tumors into bone tissue and are classified as either primary tumors or metastatic tumors.Osteosarcoma is a well-known primary malignant bone tumor that often occurs in children and adolescents.It has been reported that this disease has become the second leading cause of tumorrelated death in young teenagers.1The majority of patients die from lung metastases.Its annual incidence worldwide is~1–3 cases per million.2The clinical signs of osteosarcoma are not obvious without spontaneous fracture or severe pain early on.Therefore,this disease is not easily diagnosed,but the tumors grow quickly.As a result,osteosarcoma causes a large bone defect and limitations in motion and can metastasize to the lungs.3The etiology of osteosarcoma is still not clear.4To date,the most common clinical treatment methods for bone tumors include chemotherapy,wide surgical resection,and radiotherapy.5However,osteosarcoma is not sensitive to radiotherapy and is prone to chemotherapy resistance.Surgical resection often fails to completely remove the tumor,which is the main cause of postoperative recurrence and metastasis.Moreover,osteosarcoma invades large areas of bone,which cannot repair itself,and has serious effects on the quality of life of patients.6The 5-year survival rate of patients with osteosarcoma is~60%.7Unfortunately,advances in osteosarcoma treatment have reached a plateau over the past 40 years.8
Metastatic bone tumors start somewhere else in the body and then spread to bone tissue at a later stage.Bone tissue is one of the most common metastatic sites,and certain cancers,such as breast,prostate,colon,and lung cancer,are closely related to bone metastasis.9–13Bone metastasis results from tumor cells migrating and adhering to the bone,thus interfering with the balance of bone formation and bone resorption.Osteosarcoma and bone metastasis share some similarities,14but metastatic bone tumors exist in the later stage of the tumor.The primary tumor is usually diagnosed before it metastasizes to the bone after treatment.In tumor-induced bone defects,metastatic bone tumors and osteosarcoma share similar tumor niches and microenvironments.Innovative and efficient therapeutic strategies are urgently needed to solve the problems in the treatment of bone tumors.15
Along with the development of bionanotechnology,new innovative treatment options have been designed for bone tumor therapy.Bone tumor therapy combines the complex issues of tumor therapy and bone regeneration,which demand functional biomaterials for treatment.It is challenging to design novel strategies with the dual capabilities of both preventing tumor recurrence and supporting bone formation,demanding an interdisciplinary research background.16–17Many researchers worldwide have focused their efforts on solving these bone tumor treatment problems.Although they are only in the early stages of development,new treatment methods have brought great hope to finding a cure for bone tumors.
Traditional postoperative bone tumor treatment is chemotherapy.However,these chemical drugs can lead to systemic side effects such as liver dysfunction,heart toxicity,and bone marrow suppression.The development of new supplementary or alternative tumor treatment methods based on biomaterials can avoid these side effects by selective delivery.18–26Specifically,photothermal therapy is an emerging treatment method that converts near-infrared(NIR)light into localized thermalenergy to destroy tumor tissue.27–35Photothermal therapy is based on nanomaterials with strong NIR absorption,such as gold nanoparticles,36–41carbon nanomaterials,42–43magnetic nanoparticles,44–48and copper nanomaterials.49–50Photothermal therapy is suitable for localized tumor therapy due to the concentrated irradiation region of the laser and its ability to limit the deep penetration of heat without damaging other organs or tissues.51–55With its rapid development,photothermal therapy is a potential supplement to preclinical and clinical tumor therapy.For example,photothermal therapy based on gold nanoshells has shown a great therapeutic effect in clinical trials for prostate tumor therapy.56–57Photothermal therapy is a suitable candidate method for bone tumor treatment,and related studies have focused on it.
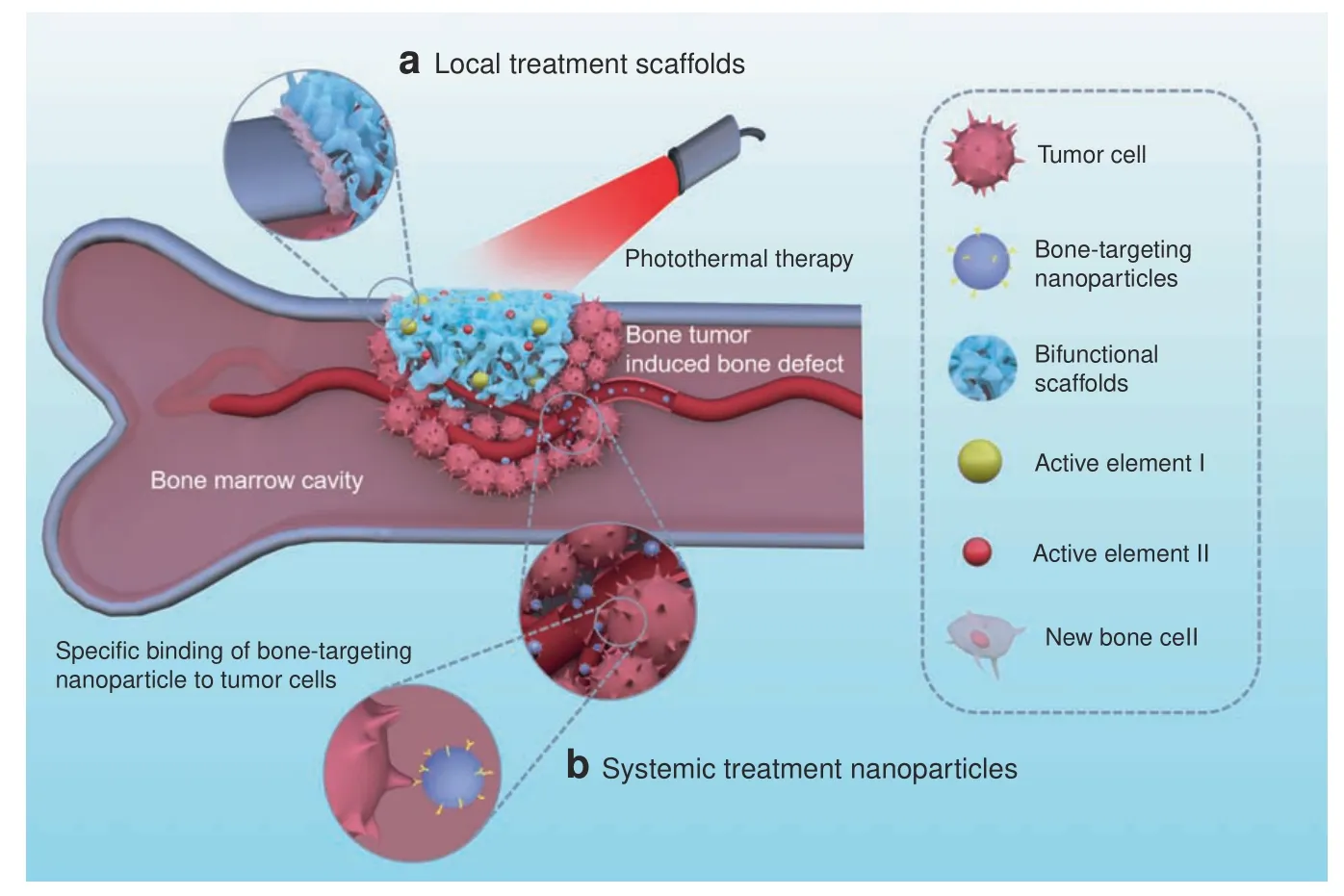
Fig.1 Bifunctional biomaterials include(a)local treatment scaffolds(such as 3D-printed scaffolds,nano/microparticle-containing scaffolds,and hydrogels)and(b)systemic treatment nanoparticles(such as bone-targeting nanoparticles)for tumor photothermal therapy and bone regeneration
Due to the offensive spreading of tumors into bone,bone metabolism becomes unbalanced.Healthy bone tissue is resorbed and invaded by the tumor,leading to bone defects.After tumor therapy,these bone defects become the next issue of concern.Bone tissue engineering is a fascinating field that gives hope to bone regeneration.The biomaterial scaffolds developed for bone tissue regeneration include nanofibers,3Dprinted scaffolds,hydrogels,microspheres,and nanoparticles.58–64Bioactivity,biocompatibility,and biodegradability are critical concerns in scaffold design,playing an important role in bone regeneration.65–68In particular,the key parameters of porosity,stiffness,and viscoelasticity can regulate cell adhesion,cell proliferation,and osteogenesis differentiation.69–76Scaffolds provide cells with sustainable regenerative factors,provide physical and biological support,and mobilize stem cells to regenerate the defect cavity.77–82Bifunctional scaffolds have been designed in recent years,thanks to the tireless work of researchers,for tumor photothermal therapy and bone repair.These scaffolds are capable of simultaneously providing tumor therapy and enhanced bone regeneration,a useful“two birds,one stone”strategy.Figure 1 shows a bone tumor that was killed by bifunctional biomaterials through either local or systemic administration.The locally administered bifunctional scaffolds(such as 3D-printed scaffolds,nano/microparticlecontaining scaffolds,and hydrogels)were inserted into the bone defect area for tumor photothermal therapy and,subsequently,improved bone repair.The systemically administered nanoparticles penetrated blood vessels to target the bone tissues for tumor treatment and to inhibit bone reabsorption.Some representative examples of bifunctional biomaterials were summerized and listed in Table 1.

Table 1.Examples of bifunctional biomaterials mainly include 3D-printed scaffolds,nano/microparticle-containing scaffolds,hydrogels,and bonetargeting nanomaterials in tumor therapy and bone regeneration
This review provides details of the recent developments in the use of bifunctional biomaterials to achieve bone tumor therapy.The new strategies in bifunctional biomaterial preparation and treatment methods are presented in the main text.Bone tumor therapy by bifunctional biomaterials is an important development direction for bone tissue engineering.83Moreover,bifunctional biomaterials will play a vital role in the therapy of complex diseases,which combine tumor therapy and tissue engineering(including bone tissue engineering,skin tissue engineering,adipose tissue engineering,etc.).
A NEW STRATEGY FOR TUMOR THERAPY AND BONE REGENERATION
The rapid proliferation and invasion of osteosarcoma cancer cells is still the main reason why the survival rate of osteosarcoma patients has not improved in decades.Therefore,there has become an urgent need to explore new ways to treat osteosarcoma.Biomaterials for bone tumor therapy need to possess two functions:killing tumor cells and helping bone regeneration.For administration,we divided the bifunctional biomaterials into local treatment and systemic treatment options.The local bifunctional biomaterials for bone tumor therapy concentrate mainly on 3D-printed scaffolds,nano/microparticlecontaining scaffolds and hydrogels.The representative systemic treatment biomaterial is bone-targeting nanoparticles for bone tumor therapy.Therefore,in this section,we present and discuss recent research on these strategies for tumor therapy and bone regeneration.
Local treatment
3D-printed scaffolds.The new,innovative technology of 3D printing was first proposed by Prof.Ely Sachs.84Through its rapid development,3D printing is now widely applied in the field of tissue engineering.85–88The bioactive ions in 3D-printed scaffolds,such as Ca2+,P5+,Si4+,Mg2+,Fe3+,and Mn4+,can improve osteogenic activity.89–95In only a few years,a series of3D-printed bifunctional ceramic scaffolds for tumor therapy and bone repair have been developed.Some outstanding work in this field has been done by Chengtie Wu’s group.96–97For example,a 3D-printed scaffold modified with a Ca-P/polydopamine nanolayer was formulated by their group.98The polydopamine nanoparticles used on the surface can cause hyperthermia to kill MDA-MB-231 tumors in nude mice.Additionally,this scaffold can release Ca and P in a sustainable manner to induce femoral defect regeneration.Moreover,a high-strength 3D bioscaffold with Fe-CaSiO3was designed and prepared for tumor therapy and bone repair(Fig.2).99The 3Dprinted Fe-CaSiO3scaffold possessed the high compressive strength of 126 MPa,contributing to the high inherent mechanical properties of Fe.The high mechanical strength of this scaffold meets the load-bearing application requirements of human bone.Fe nanoparticles not only can provide photothermal therapy due to localized surface plasmon resonance but also can promote H2O2decomposition to generate reactive oxygen species(ROS).Thus,synergistic photothermal and ROS therapies can enhance Saos-2 bone tumor inhibition.Furthermore,large bone defects in the legs of rabbits were repaired by an Fe-CaSiO3scaffold.Recently,β-tricalcium phosphate 3Dprinted scaffolds(TCP-PDLLA-LB)modified with LaB6micronanoparticles/poly(D,L-lactide)were fabricated for tumor photothermal therapy and bone repair.100Lanthanum and boron,as a“bone-seeking”element and a trace element,respectively,arebioactive,and their complex LaB6possesses NIR photothermal conversion properties.Therefore,the bone tumors were significantly suppressed by photothermal therapy.Regardless of NIR laser irradiation,TCP-PDLLA-LB 3D-printed scaffolds effectively assisted in new bone formation.

Fig.2 3D printing of Fe-CaSiO3 composite scaffolds for tumor therapy and bone regeneration.a The fabrication of Fe-CaSiO3 composite scaffolds for short-term tumor therapy and long-term bone regeneration.b Infrared(IR)radiation thermal images of tumor-bearing mice after irradiation with an 808 nm laser for 600 s.The photographs of the tumors from the six groups are from day 15.c Micro-CT images(a–c)and histological analysis(d–f)of the bone defects in the CaSiO3,Fe,and Fe-CaSiO3(30CS)groups postsurgery in a rabbit critical-sized femoral defect model.The statistical analysis of the defects(g,h)and histomorphometric measurements of in vivo osteogenesis(i)in the CaSiO3,Fe,and 30CS groups 8 weeks post surgery.Reprinted with permission from ref.99 © 2018,Nature Publishing Group
In a recent example,a larnite/C 3D-printed scaffold showed an excellent photothermal effect,killing MNNG/HOS human osteosarcoma cells and inhibiting tumor growth in nude mice.101Additionally,the multifunctional 3D-printed scaffold enhanced bone formation in a rat calvarial defect model.In another related study,an“all-in-one”3D-printed biomaterial coloaded with calcium peroxide(CaO2)and iron oxide(Fe3O4)nanoparticles was used to solve the abovementioned dilemma in osteosarcoma therapy.102The CaO2produced sufficient H2O2in the acidic tumor environment,and the Fe3O4nanoparticles generated toxic ROS via a Fenton-like catalytic reaction.Along with magnetic hyperthermia,these two agents can produce a synergistic effect in MNNG/HOS osteosarcoma tumor-bearing BALB/c in nude mice.Importantly,the CaO2nanoparticles released calcium ions to improve bone regeneration in SD rats cervical defects.
In the orthopedic field,there have been clinical trials in recent years in which 3D-printed personalized titanium plates were applied to bone defects.103Inspired by their utility and encouraging clinical outcomes,Mao et al.designed and fabricated titanium plates via computer-aided design and computer-aided manufacturing techniques customized and fixed to the patients’bone defects after the tumor was removed.104Twelve patients with osteosarcoma had their bone tumor tissues surgically removed and were then treated with microwave-induced hyperthermia to kill the residual tumor cells.Subsequently,allograft bone and poly(methyl methacrylate)(PMMA)cement were applied to fill the bone defect.Finally,the 3D-printed personalized plate was fixed to strengthen the bone segment.Hyperthermia and 3D plate therapy improved the clinical outcomes in terms of the mean maximum flexion of the affected knees and the Musculoskeletal Tumor Society score.
Nano/microparticle-containing scaffolds.Nano/microparticle-containing scaffolds usually refer to inorganic-organic hybrid scaffolds.Particle-containing bifunctional hybrid scaffolds are the desired design for bone tumor therapy.105–107Microspheres composed of calcium phosphate-phosphorylated adenosine were prepared with high doxorubicin(DOX)loading for bone tumor therapy.108The pH-sensitive properties of microspheres presented a positive therapeutic effect on subcutaneous 143B osteosarcoma tumors in rats.Additionally,the hybrid microspheres can release active molecules to promote osteogenic differentiation in vitro.The study showed the potential application of calcium phosphatephosphorylated adenosine microspheres for tumor inhibition and bone repair.
Additionally,a multifunctional magnetic mesoporous calcium silicate/chitosan(MCSC)porous scaffold that consisted of M-type ferrite particles(SrFe12O19),mesoporous calcium silicate(CaSiO3),and chitosan was prepared.109The SrFe12O19particles improved the photothermal efficacy with DOX-induced chemotherapy to reduce bone tumors.The MCSC hybrid scaffold upregulated indicators for osteogenesis.The data indicated that the MCSC hybrid scaffold promoted human bone marrow stromal cells to differentiate into osteogenic cells.In another study by the same author,110fabricated SrFe12O19nanoparticles containing bioglass/chitosan scaffolds also showed good bone repair of calvarial defects in rats.
Organic and inorganic materials are typically combined for complex disease in bone tumor treatment.Inorganic biomaterials,including nHA,TCP,bioglass,and bioceramics supply nutrients for tumor-defective bone repair.In a recent study,111the surface of beta-tricalcium phosphate bioceramic(β-TCP)materials was coated with carbon aerogel,which was developed for MNNG/HOS osteosarcoma tumor therapy.The carbon aerogel coating particularly enhanced the roughness and surface area of β-TCP,resulting in good bone regeneration in a calvarial defect model.
Breast cancer-induced bone metastasis is shown to cause cancer recurrence and local bone defects.A multifunctional magnetic chitosan matrix incorporating Fe3O4nanoparticles and GdPO4nanorods was utilized for breast tumor therapy and bone defect regeneration.112The Fe3O4nanoparticles in the scaffold supplied a high temperature through photothermal effects every other day for 14 days to avoid postoperative cancer recurrence in MDA-MB-231 tumor-bearing mice.Additionally,the GdPO4nanorods became orderly arranged in the scaffold and acted as a new bioactive component to induce M2 polarization of macrophages for enhanced stabilizing angiogenesis in the calvarial defect.
Another scaffold was developed from Fe3O4magnetic nanoparticles containing PMMA bone cement with mechanical support,magnetic photothermal ablation,and bone repair features(Fig.3).113The liquid phase of these PMMA-Fe3O4scaffolds can be accurately injected into the bone defect area.Once PMMA-Fe3O4solidifies,an alternating magnetic field was used for the thermal ablation of the bone tumor.The fast phase transition of the PMMA-Fe3O4scaffold prevented the leakage of Fe3O4nanoparticles,which were nonbiodegradable during the long recovery period.Fortunately,good mechanical support is useful for physical function reconstruction.To simulate the clinical characteristics of the bone tumor,the therapeutic efficacy of the PMMA-Fe3O4scaffold was evaluated in the tibia a tumor-bearing rabbit.The excellent heating performance provided a good VX2 tibial plateau tumor ablation outcome.The PMMA-Fe3O4scaffold was shown to be a promising and minimally invasive agent with great clinical translation potential for the treatment of bone tumors.
Another very smart strategy is to simultaneously integrate photothermal therapy and bioactivity for bone regeneration into a single material.Bismuth(Bi)-doped bioglass provides photoinduced hyperthermia and enhanced remineralized bone tissue.114The high photothermal conversion of Bi was first reported in this study.The photothermal effects were controlled by managing the radiative and nonradiative processes.Under NIR light,Bi hybrid bioglass can efficiently kill bone tumors.Moreover,Bi promotes the proliferation,differentiation,and mineralization of osteogenic cells.
当室内环境温度高于设定温度时,开启定频压缩机制冷对环境进行降温;当室内环境温度达到设定温度时,压缩机关闭。
Titanium is widely used in the clinical application of dental implants and is also a good choice for bone tumor therapy applications.Zheng et al.115prepared a hydrogenated black TiO2(abbreviated as H-TiO2)coating with a hierarchical porous topography on a titanium implant.The H-TiO2coating surface has photothermal abilities and can induce necrosis of Saos-2 bone tumor cells.Considering that the micro/nanostructures on the implant improved the osteogenic differentiation of BMSCs,it is promising to hypothesize that BMSCs can migrate to the implant surface for bone defect regeneration.Further in vivo demonstrations of the of defect repair results are needed.
Hydrogels.Hydrogels are very large meshes that can contain water and have similar properties to the extracellular matrix.Hydrogels possess a highly porous structure,good biocompatibility,biodegradability,and a capability to load growth factors,leading to good bone defect repair.116–119Thus,hydrogels are good candidates for bone repair.Several studies have shown potential for the use of hydrogels in bone tissue regeneration.For bone tumor therapy,the hydrogel needs to also be capable of treating tumors.It is highly advised to administer drugs or ingredients into the resected tumor area.120–121Hydrogels can provide sustainable drug release for tumor illumination.122–125Some hydrogels integrate interior antitumor activity with localized delivery in one system.126Localized cancer treatment by hydrogels can replace the need for systemic chemotherapy administered intravenously or orally.127–130With the development of multifunctional hydrogels,their applications are not limited to tissue repair but also extend to tumor cure and bone repair.
The ideal hydrogel system requires favorable parameters with good biocompatibility,a porous structure,adhesion to the cavity,good mechanical properties,and injectability.131Among them,an injectable hydrogel can fill or match irregular defects with a mild gelation process in a minimally invasive manner.132–135Recently,an injectable hydrogel was formed via a Schiff base reaction between the amino group of chitosan and the aldehyde groups of oxidized sodium alginate.136As shown in Fig.4,a hydrogel was mixed with nanohydroxyapatite(n-HA)to induce bone repair in the joint bone of a rabbit.Moreover,n-HA was decorated with polydopamine and cisplatin,which can supply photothermal therapy and chemotherapy to treat 4T1 breast tumor-bearing mice.
In another study,an in situ UV-crosslinked gelatin methacryloyl hydrogel-encapsulated liposome was formed for the local release of gemcitabine.137Drug release lasts for 4 days in vitro,resulting in excellent inhibition of osteosarcoma in BALB/c mice bearing MG63 tumors.Thermosensitive hydrogels are also popular for localized drug release.For example,thermosensitive poly(Llactide-co-glycolide)-poly(ethylene glycol)-poly(L-lactide-co-glycolide)(abbreviated as PLGA-PEG-PLGA)hydrogels were used to load DOX,methotrexate and cisplatin for localized drug delivery.138Synergistic cytotoxic effects were found in the multiple drug-loaded hydrogels against osteosarcoma in vitro and in vivo.Furthermore,localized treatment caused no obvious harm to normal tissues.
A nanohydroxyapatite hybrid reduced graphene oxide(nHArGO)hydrogel was developed for tumor-related bone defects.105The nHA-rGO hydrogel killed almost all MG-63 osteosarcoma cells via photothermal therapy.Additionally,this hydrogel promoted bone regeneration by stimulating osteoblast mineralization and collagen deposition in a rat cranial defect model.
Systemic treatment
In recent years,there has been strong growth in nanotechnology in the fields of biology,medicine,and pharmaceuticals.Nanosized drug-based delivery platforms have been extensively studied and used for the treatment of osteosarcoma.Various nanoparticles have emerged as effective drug delivery systems in osteosarcoma treatment.Osteosarcoma tumor-invaded bone destruction contributes to an imbalance between bone reabsorption by osteoclasts and bone reconstruction by osteoblasts.Bone reabsorption promotes bone destruction and tumor metastasis processes.139–140Moreover,a vicious cycle exists in osteolytic metastasis with bidirectional interactions between osteoclasts and tumor cells.9Due to the low blood flow in the bone(0.05–0.20 mL·min-1per gram)141and blood–bone marrow barrier,targeted delivery of anticancer agents is highly recommended for bone tumor therapy.Moreover,a targeted delivery strategy shows great potential to solve systemic toxic effects and multidrug resistance,which are longstanding problems with the standard cancer chemotherapy treatment.142–143
Bone-modifying agents with a high affinity for bone are used for active bone targeting,including alendronate,144–146zoledronic acid,147–149aspartic acid,150denosumab,151and aptamers.152–153Nanomaterials and their drug delivery systems have unique advantages for the treatment of bone tumors.154–158Because bone is composed of organic matrices and inorganic minerals that are assembled at the nanoscale,and nanomaterials can assimilate into the bone microenvironment to heal diseased bone.159–160Furthermore,targeted delivery systems based on nanotechnology can improve the treatment efficiency of bone tumors.161–162
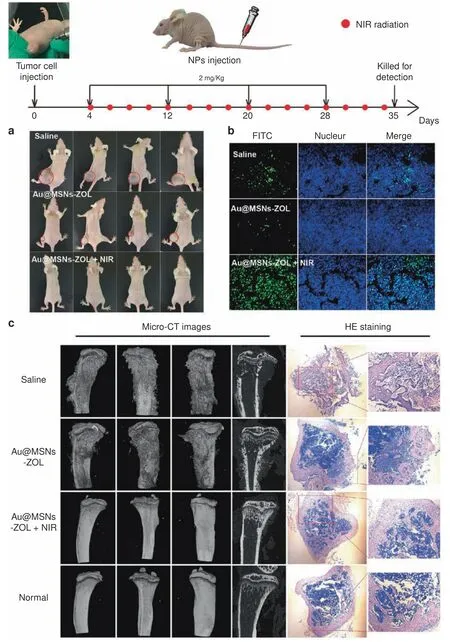
Fig.5 Bone-targeted nanoplatform combining zoledronate and photothermal therapy to treat breast cancer bone metastasis.Top:Timeline of the treatment schedule.A breast cancer bone metastasis mouse model was established by direct injection of MDA-MB-231 cells into the left hindlimbs of nude mice.Nanoparticles and NIR irradiation were administered as indicated.a Images of tumor-bearing nude mice recorded at the end of treatment(day 35).b TUNEL fluorescence detected in the tumor slices.c CT images of the tibias from different angles and micrographs of H&E-stained tibias(right).Reprinted with permission from ref.167 © 2019,ACS Publications
Although bisphosphonates are widely used as clinical drugs in metastatic bone tumor treatment,they may cause adverse effects such as atypical femoral fractures and esophageal cancer after long-term use.168In a recent study by Cheng Yiyun’s group,169phytic acid(PA)-capped platinum(Pt)nanoparticles were developed for bone-targeting therapy.PA is a natural compound that contains six phosphate groups,indicating its high bone-targeting capability.In addition,PA shows an inherent anticancer ability,which can be combined with the Pt nanoparticle photothermal therapy.An in situ bone tumor model was established by engrafting PC-9-Luc cells in the tibias of nude mice,which can be detected by imaging the luminescence of the tumor regions.After PA/Pt nanosystem treatment,PA led to an enhancement of the PA/Pt nanoparticles at the tumor site.Additionally,PA/Pt nanoparticleassociated cotherapy inhibited tumor invasion.
DISCUSSION AND PERSPECTIVE
From the review on the recent development of bifunctional biomaterials in bone tumor therapy,a promising new strategy was introduced.According to the method of administration,bifunctional biomaterials can be divided into those delivered by local or systemic administration.Locally administered biomaterials mainly include 3D-printed scaffolds,nano/microparticlecontaining scaffolds,and hydrogels.The representative systemically administered biomaterial is bone-targeting nanoparticles.A similarity and difference exist between these two types of biomaterials.The similarity is that a localized photothermal effect is used to kill tumor cells to prevent recurrence early on.The difference lies in the mechanism of bone repair.Locally administered scaffolds can be designed to match the bone defect area,and active molecules can be carried into the scaffold to stimulate bone regeneration.Systemically administered nanoparticles target bone tissues to inhibit bone reabsorption.The former is an example of positive regulation of bone regeneration,and the latter represents negative regulation of bone reabsorption.Although their mechanisms of bone repair are different,their outcomes in bone tumor therapy are similar.
For further development,there are three possible directions for bifunctional biomaterials for tumor therapy,and bone repair may arise.First,NIR-II window-responsive biomaterials for photothermal therapy have been developed for deep tumor treatment,as mild photothermal effects can effectively protect bone tissue.For example,a recent study reported the use of bifunctional CDs combined with WS2to cure osteosarcoma under laser irradiation at 1 064 nm.170Even when covered with a chicken breast with a thickness of 10 mm,the deep bone tumor was able to be killed.Moreover,mild photothermal effects(~43°C)reported in recent studies can not only greatly enhance the proliferation of MSCs but also promote osteogenesis.171–175This is good news for bone tumor therapy.The mild photothermal effect was shown to stimulate and accelerate in vitro and in vivo osteogenesis.170,176Second,future treatment strategies for bone tumor therapy may not be limited to photothermal therapy and chemotherapy combined with biomaterials,and other therapeutic strategies,such as radiochemotherapy and gas therapy,may also be potential methods to treat malignant bone tumors.171–172Third,scaffolds developed in the future may need to be multifunctional,considering infection along with tumor therapy and bone regeneration.177–178During tumor surgery,bleeding and soft tissue defects need to be considered.As shown in Fig.6,a nanohydroxyapatite/graphene oxide/chitosan(nHA/GO/CS)scaffold was designed to inhibit osteosarcoma growth with mild photothermal therapy and mildly high temperature(~42°C)to promote the osteogenesis of hBMSCs.179Furthermore,this scaffold showed a good hemostatic effect that can improve soft tissue regeneration.
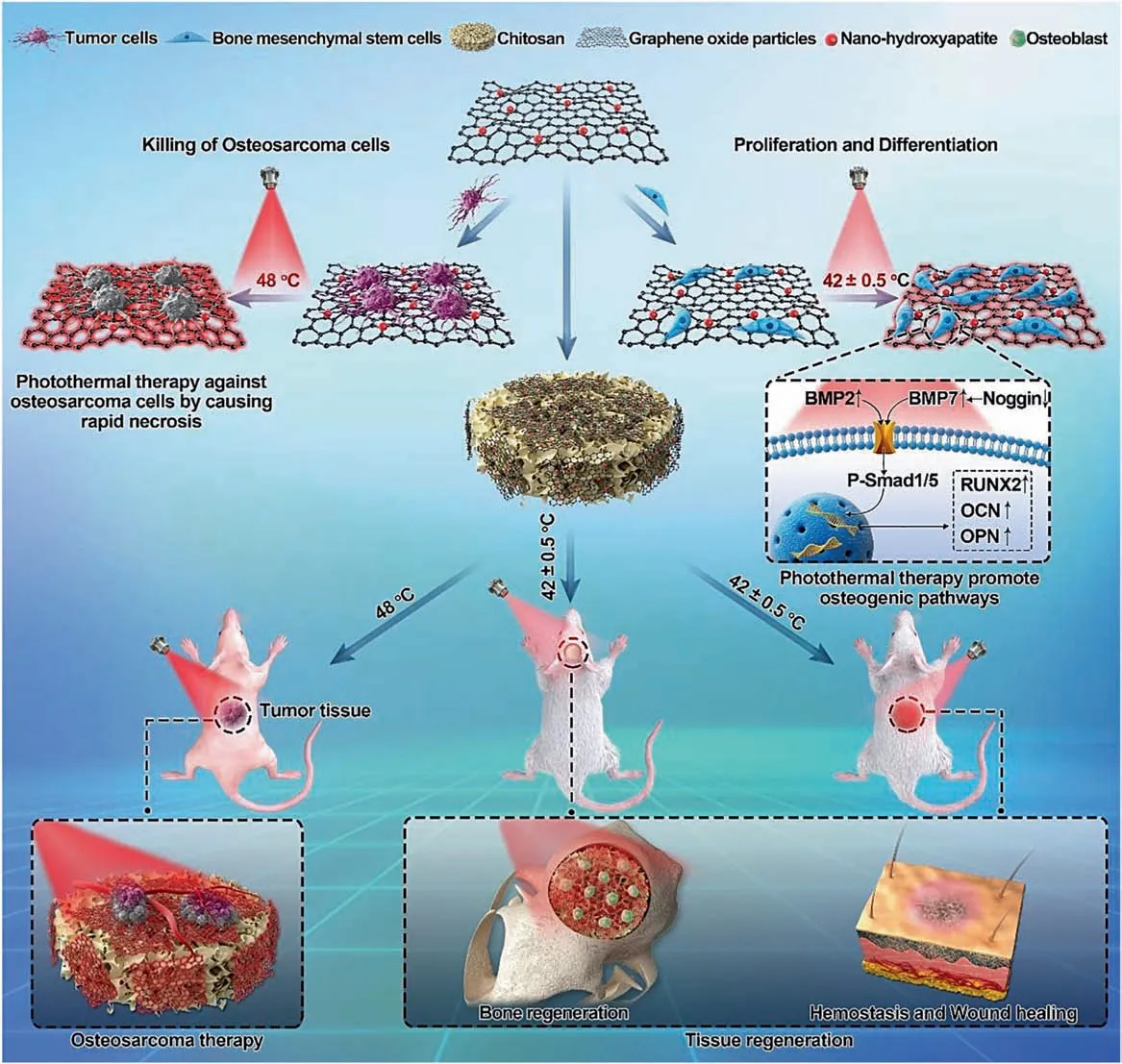
Fig.6 Schematic illustration of the nanohydroxyapatite/graphene oxide/chitosan(nHA/GO/CS)scaffold for osteosarcoma treatment under photothermal therapy and the promotion of tissue regeneration.Reprinted with permission from ref.179 © 2020,RSC Publishing
New strategies based on bifunctional biomaterials for bone tumor therapy have the potential to extend to the treatment of other types of tumors(such as melanoma,oral tumors,and breast tumors),as well as damaged neighboring normal tissues.Melanoma treatment requires the complicated removal of tumor tissue and cutaneous defect repair.173–175Oral tumors simultaneously destroy the facial bone.In clinical treatment,doctors remove oral tumor tissues along with the surrounding jawbone then collect the patient’s fibula and use it to replace the jawbone with vascular anastomosis.Breast tumor treatment involves curing the residual tumor after surgical resection and breast defect regeneration.180Thus,the treatment of breast tumors relates to tumor therapy and adipose tissue engineering.In the latest study by Huang’s group,a bifunctional 3D-printed dopamine-modified alginate scaffold was used to kill breast tumors and fill the defective breast area.23Researchers found a favorable photothermal effect from this scaffold to illuminate breast tumors.Additionally,the modulus of the scaffold was similar to that of normal breast tissue,and the scaffold enhanced the proliferation of breast epithelial cells.This method design can be used as a potential strategy for the prevention of breast tumor recurrence after surgery and adipose tissue repair.Future research should be focused on tumor therapy and tissue engineering for other complex diseases.
Although the use of bifunctional biomaterials for bone tumor therapy is developing rapidly,there is still a long way to go.The bifunctional biomaterial strategy further shows great potential for the treatment of complex diseases combined with tumor and tissue defects.Great confidence in researchers and clinicians will push this new strategy forward to solve clinical problems.
CONCLUSION
This review highlights the recent development of bifunctional biomaterials for bone tumor therapy.A new strategy based on bifunctional biomaterials can inhibit tumor growth in the early treatment period and enhance bone repair in the late treatment period.Photothermal therapy for tumor treatment has a short duration,but bone regeneration takes a long time.With the benefits of locally administered(3D-printed scaffolds,nano/microparticle-containing scaffolds,and hydrogels)and systemically administered(bone-targeting nanoparticles)bifunctional biomaterials,the survival rate of bone tumor patients has great potential to increase.Bifunctional biomaterial treatment may provide new hope for future clinical bone tumor therapy while improving patient quality of life and decreasing mortality.
ACKNOWLEDGEMENTS
This work was financially supported by the National Key Research and Development Program of China(2017YFC1103500,2017YFC1103502),the National Natural Science Foundation(31972925,31771096,31930067,31525009),the 1·3·5 project for disciplines of excellence,West China Hospital,Sichuan University(ZYGD18002),the Sichuan Science and Technology Program(2020YJ0065),the Sichuan University Spark Project(2018SCUH0029),and the State Key Laboratory of Oral Diseases Foundation(SKLOD202016).
ADDITIONAL INFORMATION
Competing interests:The authors declare no competing interests.
- Bone Research的其它文章
- Reply to“Can femoral head necrosis induced by steroid therapy in patients infected with coronaviruses be reversed?”
- Can femoral head necrosis induced by steroid therapy in patients infected with coronaviruses be reversed?
- Tumor-and osteoclast-derived NRP2 in prostate cancer bone metastases
- Peptidomimetic inhibitor of L-plastin reduces osteoclastic bone resorption in aging female mice
- Piezo1 channel activation in response to mechanobiological acoustic radiation force in osteoblastic cells
- Ultra-processed food targets bone quality via endochondral ossification

