蚯蚓毒物兴奋效应研究进展
顾浩天 张天澍 滕海媛 常晓丽 王冬生 袁永达

摘要 近年来,大量研究发现以低剂量刺激、高剂量抑制为表征的双相剂量效应关系(毒物兴奋效应,hormesis)在动植物及微生物中广泛存在。根据测试终点的不同,hormesis可被绘制成倒“U”型或“J”型曲线,主要表现为生物体因内稳态受到干扰而引起过度补偿的生物学效应。蚯蚓作为“生态系统的工程师”,对改良土壤结构功能、提高土壤生物肥力均有积极有益影响。同时,蚯蚓作为毒理学研究中的指示生物和模式生物,被广泛应用于评价污染物的环境风险。针对蚯蚓hormesis的研究进展进行综述,以期为未来研究提供理论依据和参考。
關键词 毒物兴奋效应;污染物;蚯蚓;剂量-反应关系;测试终点
中图分类号 X 171.5 文献标识码 A 文章编号 0517-6611(2021)15-0018-03
Abstract In recent years, a large number of studies have found that the biphasic doseeffect relationship (hormesis) characterized by lowdose stimulation and highdose inhibition is widespread in animals, plants and microorganisms.According to the different test endpoints, hormesis can be drawn as an inverted "U" or "J" curve, which is mainly manifested as the biological effect of overcompensation caused by the disturbance of the homeostasis of the organism.As the "engineer of the ecosystem", earthworms have a positive and beneficial effect on improving soil structure and function and improving soil biological fertility. At the same time, earthworms, as indicator organisms and model organisms in toxicology research, are widely used to assess the environmental risks of pollutants.This article reviewed the research progress of earthworm hormesis in order to provide theoretical basis and reference for future research.
Key words Hormesis;Pollutants;Earthworm;Doseresponse relationship;Test endpoint
基金项目 上海市科技兴农重点推广项目“稻田秸秆蚯蚓原位处理模式与循环产业技术集成示范”(沪农科推字(2018)第4-14号)。
作者简介 顾浩天(1993—),男,辽宁阜新人,研究实习员,硕士,从事农药生态毒理效应及环境风险、农业害虫生理分子调控机理研究。*通信作者,副研究员,硕士,从事农作物有害昆虫的生理特性及综合治理研究。
收稿日期 2020-12-05
剂量效应关系是污染物毒性风险评价中最基本、最核心的概念之一。环境中污染物通常以极低剂量存在,而大多数研究仅仅关注污染物的效应剂量,而忽略了其在低于未观察到损害作用剂量(noobserved adverse effect level,NOAEL)下所产生的环境影响。研究发现亚效应剂量(subeffective dose)与高剂量下所诱导的生物效应截然相反,即毒物兴奋效应(hormesis)[1-2]。根据所研究的测试终点,毒物兴奋效应表现为倒“U”型或“J”型曲线的剂量-时间-效应关系[3-4],其机理为毒物在极低剂量水平下打破生物体生理内稳态,刺激生物体产生过度补偿或应激适应性响应来重建生理内稳态平衡[2]。毒物兴奋效应现象在植物、动物、微生物中普遍存在[5],可由有机或无机污染物、离子辐射、温度、pH、盐度等胁迫诱导产生[6-7]。毒物兴奋剂量模型提示了污染物风险评价的阈剂量[8],在毒理学研究中较阈值模型、线性非阈值模型更为常见[9]。因此探讨毒物兴奋效应的环境影响具有重要意义。
蚯蚓是土壤生态系统中最大的动物区系,通过掘穴、排泄、取食等生理活动改善土壤透气性和孔隙度,维持土壤理化结构,促进土壤养分循环、团聚体形成、有机质降解矿化,提高土壤微生物多样性、活性及生物肥力[10]。蚯蚓对土壤中低剂量污染物敏感,且能通过表皮被动吸收、主动取食、肠道消化等途径与多种类型的环境污染物暴露接触,因而被用作评价污染物环境风险及化学品毒性的指示生物[11]。近年来,已有大量关于蚯蚓响应污染物胁迫的hormesis现象报道,笔者针对此进行梳理和总结,以期为未来研究提供参考。
1 蚯蚓中的hormesis现象
毒物兴奋效应是暴露于低浓度污染物条件下,生物内稳态产生的过度补偿响应机制[9,12],仅出现在低于NOAEL的较窄低剂量带(hormetic zone)[12]。研究表明兴奋效应具有普遍性,农药、重金属、抗生素、环境压力等均可诱导蚯蚓形成低剂量促进-高剂量抑制的兴奋效应,且该现象可能与蚯蚓受胁迫后体内氧化应激压力产生活性氧簇(ROS)的动态变化及作用机制相关[13-14]。当环境污染物浓度较低时低剂量ROS可诱导生物体产生积极的响应[15],促进体内抗氧化酶系如过氧化氢酶(SOD)、超氧化物歧化酶(CAT)、愈创木酚过氧化物酶(POD)及解毒酶系如细胞色素P450(CYP450)、乙酰胆碱酯酶(AChE)、胆碱酯酶(ChE)、羧酸酯酶(CES)、谷胱甘肽硫转移酶(GST)等活性增加;而当污染物浓度持续增加,过量ROS的积累破坏机体正常的氧化还原动态平衡,从而对生理代谢活动产生毒害作用[16]。
Hackenberger等[17-18]研究发现双硫磷和马拉硫磷浓度分别为0.12、0.10 ng/cm2处理2 h后 Eisenia fetida 胆碱酯酶(ChE)活性显著增加,相比对照组(0 ng/cm2)分别提高3628%、30.30%。低剂量重金属镉(Cd)诱导 E.fetida 的 SOD、CAT活性增加且SOD(34.498%)的最大响应效应较CAT(27.637%)高,暗示SOD对Cd胁迫响应更敏感,毒物兴奋效应产生可能与蚯蚓体内适应性通路的激活有关且不同受试终点的hormesis反应幅度不同[19]。同理,Cao等[20]报道 E.fetida 在滤纸中染毒48 h后,Cd诱导细胞色素P450(CYP3A4)酶活出现hormesis,可能与维持细胞和生理内稳态的适应性通路被激活相关;CYP450的hormesis在受毒死蜱胁迫的 Aporrectodea caliginosa [21]及苯并芘胁迫的 E.fetida [22]中也被发现。蚯蚓暴露于添加低剂量镧元素的天然土中,其体内SOD和POD酶活、蛋白质含量等均表现hormesis[23]。0.01 mg/mL福尔马林暴露2 h后诱导 E.andrei 的AChE活性增加12%(与对照组相比)[24]。 E.fetida 暴露于环丙沙星污染的土壤中,POD、抗坏血酸过氧化物酶(APX)活性表现hormesis[25]。Li等[26]研究发现恩诺沙星污染的人工土中, E.fetida CYP450 基因表达显示低剂量(1 mg/kg)促进、高剂量(10~500 mg/kg)抑制的毒物兴奋效应,表明毒物也可在分子水平上诱导蚯蚓的hormesis[12]。
污染物暴露也会诱导蚯蚓的存活、繁殖、生长、生物量等测试终点的hormesis。如添加Cd或Cu的人工土壤诱导 E.andrei 繁殖力产生hormesis,即低浓度刺激产茧量而高浓度抑制产茧量[27]。在 E.fetida 中,产茧量随暴露草甘膦的浓度升高而增加[28]。Spurgeon等[29]研究发现Cu诱导 Lumbricus rubellus 幼蚓的生长发育产生hormesis。同理,Bustos等[30]研究也发现采矿区土壤砷(As)浓度低于45 mg/kg时诱导 E.fetida 幼蚓产量出现类似现象。Zhu等[31]研究发现低剂量氯化汞(0.780~3.125 mg/kg)暴露11~17 d后诱导 E.fetida 存活率增加114%~132%,推测可能与蚯蚓体内代谢解毒酶、抗氧化酶酶活的补偿响应有关,并总结蚯蚓的存活率在hormesis研究中是敏感稳定的测试终点。 E.andrei 暴露于添加62.5 mg/kg氟虫腈的人工土中,孵育28 d后存留生物量相比对照組高,暗示低剂量氟虫腈诱导了hormesis[32],也可能由于对照组蚯蚓将更多能量和营养物质用于交配及产茧过程,从而造成体重下降[33]。
此外,环境压力也会诱导蚯蚓产生毒物兴奋效应,如Wu等[15]报道土壤酸胁迫(pH 3.0~6.3)28 d后,诱导 E.fetida 的 CAT酶活性、金属硫蛋白(MT)及总蛋白(TP)含量产生hormesis。低剂量辐射诱导蚯蚓体内成熟卵母细胞增加,产生有性繁殖,而高剂量辐射后无性繁殖显著增加,暗示低剂量辐射也可诱导蚯蚓产生hormesis[34]。
2 蚯蚓hormesis的研究方法
近年来在蚯蚓hormesis研究中常用的方法包括滤纸接触法、人工土壤法、微宇宙及中宇宙试验(图1)。人工土壤法、滤纸接触法是经济合作与发展组织(organization of economic cooperation and development,OECD)和国际标准化组织(international standardization organization,ISO)推荐的蚯蚓毒理试验标准方法,主要以 E.fetida、E.andrei 这2种表栖蚓种为研究对象,在实验室模拟条件下开展急性、亚急性毒性试验[35-37]。Velki等[38-39]采用OECD法研究乐果对 E.andrei 的生态毒理效应,在滤纸接触法中发现低剂量乐果诱导AChE和CES酶活性产生hormesis,而该现象在人工土壤法中并未发现[40],这可能是由于在土壤试验中选取的浓度过高,因此并未在较窄的低剂量带观察到毒物兴奋效应。Domínguez等[33]采用ISO人工土壤法评估氨甲基磷酸(草甘膦代谢产物,AMPA)对蚯蚓 E.andrei 的毒理效应,结果发现浓度为1 000~2 500 μg/kg暴露56 d后,与对照组相比,AMPA诱导产茧量和幼蚓数量发生hormesis而显著增加。
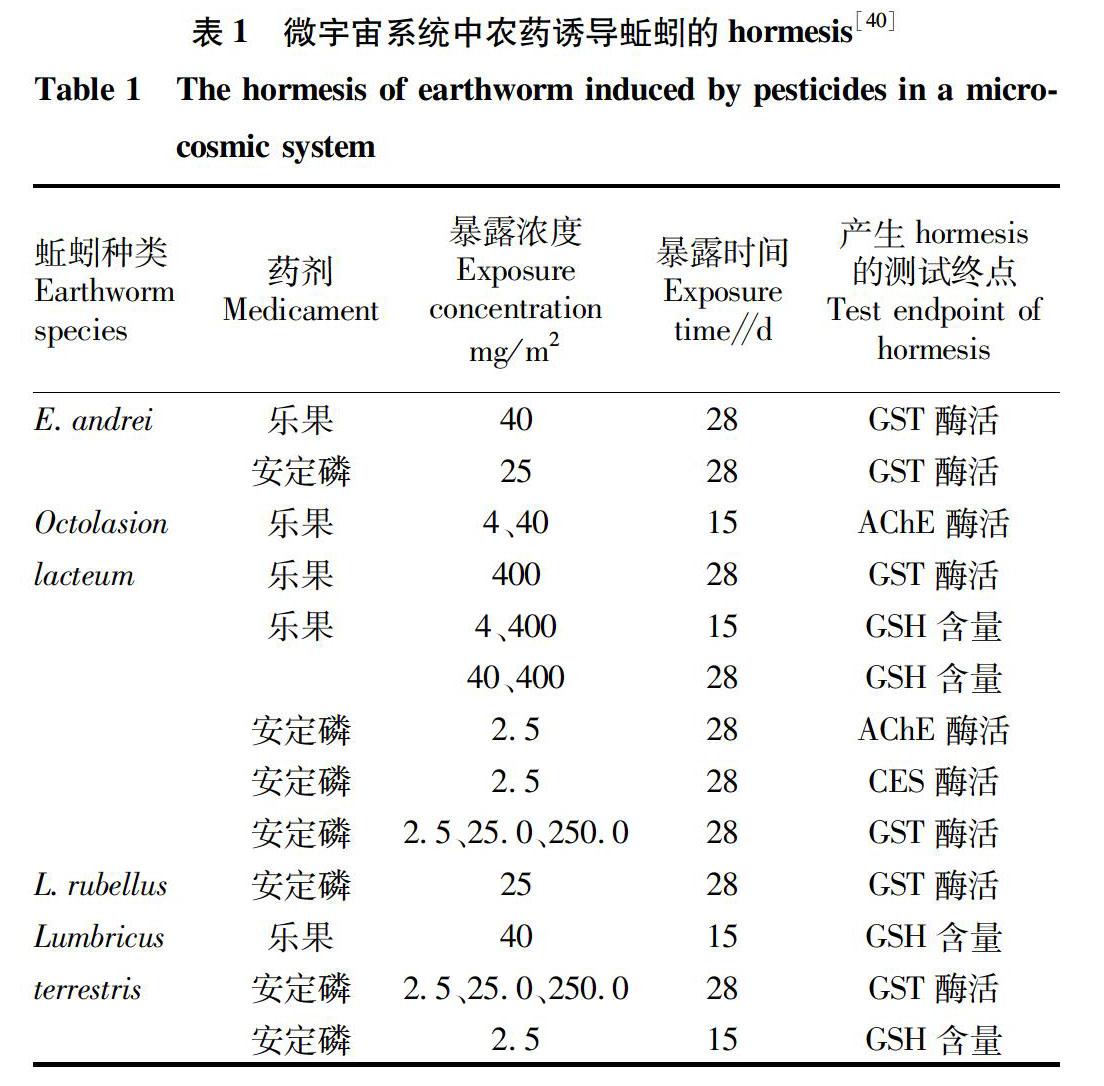
土壤微宇宙和中宇宙试验是人工模拟的半野外多物种试验系统,用以评估污染物对蚯蚓的生态毒性。将蚯蚓引入一个土壤单元(收集的、过筛的、完整的土样)中,分析污染物在分子、细胞、组织、个体、种群、群落等水平上产生的生物学效应[41]。为接近真实环境试验条件,污染物通常仅添加在土壤表面,且需要较多生物样本容量以降低个体差异性[40]。Bundy等[42]采用半野外中宇宙试验结合cDNA微阵列的转录组分析表栖蚓种 L.rubellus 对亚致死剂量Cu(10~480 mg/kg)的代谢响应,结果发现,40 mg/kg Cu处理后70 d其能量代谢及氧化应激相关基因和通路显著上调,暗示Cu在分子水平上诱导蚯蚓hormesis。Velki等[40]采用微宇宙法将表栖蚓种、内栖蚓种、深栖蚓种暴露于3种杀虫剂的田间推荐剂量,与对照相比,低剂量有机磷农药暴露不同时间显著诱导蚯蚓体内AChE、CAT、CES、GST酶活性及谷胱甘肽(GSH)含量的hormesis,结果详见表1。
在真实环境中,土壤污染物常以极低的浓度存在。尽管OECD标准方法具有简单快速、易操作、结果重现性好等优势,但采用半野外微宇宙、中宇宙试验可以最大程度模拟真实的自然条件,较为准确反映污染物环境相关的毒理风险数据,因此是环境污染及监测研究中的有力工具[3,40]。
3 小结与展望
在环境监测中,毒物兴奋效应被视为生物暴露于污染物亚效应剂量的标志,因此建立毒物兴奋模型对污染物毒性评价、化学品风险评估具有重要价值[3,9]。蚯蚓的毒物兴奋效应可能与污染物诱导产生的体内氧化压力相关[19,43],因此在土壤生态毒理学研究中应注意污染物低剂量长期暴露引起的氧化压力与hormesis之间的关联性。针对蚯蚓毒物兴奋效应的研究较为复杂,受试验条件、土壤环境、污染物浓度、污染物类型、污染物的生物有效性、蚯蚓生态类群、暴露时间、受试终点等多重因素的影响。未来研究应选用合适的试验方法、试验浓度及高特异性、高灵敏的受试终点来检测蚯蚓在低于NOAEL浓度下的胁迫响应,以解析不同污染物诱导蚯蚓产生毒物兴奋效应的内在分子机理。此外,当土壤中多种有毒物兴奋效应的污染物共存时,其联合暴露引起蚯蚓的hormesis表现为相加、拮抗还是协同作用有待进一步探索。同时研究污染物剂量低于hormesis剂量时对蚯蚓的毒理学效应也是未来研究的难点与挑战。
参考文献
[1] AGATHOKLEOUS E,CALABRESE E J.Hormesis:The dose response for the 21st century:The future has arrived[J/OL].Toxicology,2019,425[2020-04-25].https://doi.org/10.1016/j.tox.2019.152249.
[2] CALABRESE E J.Hormetic mechanisms[J].Critical reviews in toxicology,2013,43(7):580-606.
[3] VELKI M,ECˇIMOVIC' S.Important issues in ecotoxicological investigations using earthworms[M]//DE VOOGT P.Reviews of environmental contamination and toxicology:Volume 239.Cham:Springer,2016:157-184.
[4] SUN H,CALABRESE E J,LIN Z,et al.Similarities between the Yin/Yang doctrine and hormesis in toxicology and pharmacology[J].Trends in pharmacological sciences,2020,41(8):544-556.
[5] JIA L,LIU Z L,CHEN W,et al.Hormesis effects induced by cadmium on growth and photosynthetic performance in a hyperaccumulator, Lonicera japonica Thunb.[J].Journal of plant growth regulation,2015,34(1):13-21.
[6] LINDSAY D G.Nutrition,hormetic stress and health[J].Nutrition research reviews,2005,18(2):249-258.
[7] CALABRESE E J,BLAIN R B.Hormesis and plant biology[J].Environmental pollution,2009,157(1):42-48.
[8] QIN L T,LIU S S,LIU H L,et al.Support vector regression and least squares support vector regression for hormetic doseresponse curves fitting[J].Chemosphere,2010,78(3):327-334.
[9] CALABRESE E J,BALDWIN L A.The hormetic doseresponse model is more common than the threshold model in toxicology[J].Toxicological sciences,2003,71(2):246-250.
[10] 陈钰琪,张虹,周垂帆.蚯蚓生态功能研究进展[J].内蒙古林业调查设计,2019,42(6):101-104.
[11] 邵將,张宗鹏,谢宇震,等.蚯蚓在生态毒理试验中的应用研究[J].农业与技术,2020,40(16):24-26.
[12] CALABRESE E J.Paradigm lost,paradigm found:The reemergence of hormesis as a fundamental dose response model in the toxicological sciences[J].Environmental pollution,2005,138(3):379-411.
[13] GUO B,LIANG Y C,ZHU Y G,et al.Role of salicylic acid in alleviating oxidative damage in rice roots( Oryza sativa )subjected to cadmium stress[J].Environmental pollution,2007,147(3):743-749.
[14] RAZINGER J,DERMASTIA M,KOCE J D,et al.Oxidative stress in duckweed( Lemna minor L.)caused by shortterm cadmium exposure[J].Environmental pollution,2008,153(3):687-694.
[15] WU J L,REN Z L,ZHANG C,et al.Effects of soil acid stress on the survival,growth,reproduction,antioxidant enzyme activities,and protein contents in earthworm( Eisenia fetida )[J].Environmental science and pollution research,2020,27:33419-33428.
[16] LIU T,WANG X G,CHEN D,et al.Growth,reproduction and biochemical toxicity of chlorantraniliprole in soil on earthworms( Eisenia fetida )[J].Ecotoxicology and environmental safety,2018,150:18-25.
[17] HACKENBERGER B K,JARIC'PERKUIC' D,STEPIC' S.Effect of temephos on cholinesterase activity in the earthworm Eisenia fetida (Oligochaeta,Lumbricidae)[J].Ecotoxicology & environmental safety,2008,71(2):583-589.
[18] VELKI M.Hormetic response of earthworm( Eisenia fetida )to subeffect exposure to malathion[C]//International Life Sciences Students Conference 2008.Hrvatska znanstvena bibliografija i MZOSSvibor,2008.
[19] ZHANG Y,SHEN G,YU Y,et al.The hormetic effect of cadmium on the activity of antioxidant enzymes in the earthworm Eisenia fetida [J].Environmental pollution,2009,157(11):3064-3068.
[20] CAO X,SONG Y,KAI J,et al.Evaluation of EROD and CYP3A4 activities in earthworm Eisenia fetida as biomarkers for soil heavy metal contamination[J].Journal of hazardous materials,2012,243:146-151.
[21] SANCHEZHERNANDEZ J C,NARVAEZ C,SABAT P,et al.Integrated biomarker analysis of chlorpyrifos metabolism and toxicity in the earthworm Aporrectodea caliginosa [J].Science of the total environment,2014,490:445-455.
[22] ZHANG W,SONG Y F,GONG P,et al.Earthworm cytochrome P450 determination and application as a biomarker for diagnosing PAH exposure[J].Journal of environmental monitoring,2006,8(9):963-967.
[23] 杜宇,王應军,武阳,等.镧胁迫对蚯蚓几种重要酶活性的影响[J].中国稀土学报,2014,32(1):84-93.
[24] HACKENBERGER B K,VELKI M,STEPIC' S,et al.The effect of formalin on acetylcholinesterase and catalase activities,and on the concentration of oximes,in the earthworm species Eisenia andrei [J].European journal of soil biology,2012,50:137-143.
[25] WANG C R,RONG H,LIU H T,et al.Detoxification mechanisms,defense responses,and toxicity threshold in the earthworm Eisenia foetida exposed to ciprofloxacinpolluted soils[J].Science of the total environment,2018,612:442-449.
[26] LI Y S,ZHAO C,LU X X,et al.Identification of a cytochrome P450 gene in the earthworm Eisenia fetida and its mRNA expression under enrofloxacin stress[J].Ecotoxicology and environmental safety,2018,150:70-75.
[27] KILPIKOSKI J,PENTTINEN O P,VISNEN A O,et al.Toxicity of binary mixtures of Cu,Cr and As to the earthworm Eisenia andrei [J].Ecotoxicology,2020,29(7):900-911.
[28] SANTADINO M,COVIELLA C,MOMO F.Glyphosate sublethal effects on the population dynamics of the earthworm Eisenia fetida (Savigny,1826)[J].Water,air,& soil pollution,2014,225(12):1-8.
[29] SPURGEON D J,SVENDSEN C,KILLE P,et al.Responses of earthworms( Lumbricus rubellus )to copper and cadmium as determined by measurement of juvenile traits in a specifically designed test system[J].Ecotoxicology and environmental safety,2004,57(1):54-64.
[30] BUSTOS V,MONDACA P,VERDEJO J,et al.Thresholds of arsenic toxicity to Eisenia fetida in fieldcollected agricultural soils exposed to copper mining activities in Chile[J].Ecotoxicology and environmental safety,2015,122:448-454.
[31] ZHU J,YANG D L,FU R B,et al.Hormetic effects of mercury on survival of Eisenia fetida (Oligochaeta)[M] //International conference civil engineering and urban planning 2012.Reston,VA,USA:American Society of Civil Engineers,2012:299-307.
[32] ALVES P R L,CARDOSO E J B N,MARTINES A M,et al.Earthworm ecotoxicological assessments of pesticides used to treat seeds under tropical conditions[J].Chemosphere,2013,90(11):2674-2682.
[33] DOMNGUEZ A,BROWN G G,SAUTTER K D,et al.Toxicity of AMPA to the earthworm Eisenia andrei Bouché,1972 in tropical artificial soil[J].Scientific reports,2016,6:1-8.
[34] MIYACHI Y,KANAO T,OKAMOTO T.Low dose βemitter source induces sexual reproduction instead of fragmentation in an earthworm, Enchytraeus japonensis [J].J Environ Radioact,2005,79(1):1-5.
[35] OECD.OECD Guideline for Testing of Chemicals,No.207,Earthworm Acute Toxicity[R].Paris,France:OECD,1984.
[36] OECD.OECD Guideline for Testing of Chemicals,No.222,Earthworm Reproduction Test( Eisenia fetida/Eisenia andrei )[R].Paris,France:OECD,2004.
[37] ISOInternational Organization for Standardization.Soil qualityEffects of pollutants on earthworms - Part 1:Determination of acute toxicity to Eisenia fetida/Eisenia Andrei and Part 2:Determination of effects on reproduction of Eisenia fetida/Eisenia andrei.ISO 112681 and ISO 112682[S].Geneva,Switzerland:International Organization for Standardization,2012,2015.
[38] VELKI M,HACKENBERGER B K.Speciesspecific differences in biomarker responses in two ecologically different earthworms exposed to the insecticide dimethoate[J].Comparative biochemistry and physiology part C:Toxicology & pharmacology,2012,156(2):104-112.
[39] VELKI M,HACKENBERGER B K.Inhibition and recovery of molecular biomarkers of earthworm Eisenia andrei after exposure to organophosphate dimethoate[J].Soil biology and biochemistry,2013,57:100-108.
[40] VELKI M,HACKENBERGER B K,LONCˇARIC' ,et al.Application of microcosmic system for assessment of insecticide effects on biomarker responses in ecologically different earthworm species[J].Ecotoxicology and environmental safety,2014,104:110-119.
[41] BURROWS L A,EDWARDS C A.The use of integrated soil microcosms to predict effects of pesticides on soil ecosystems[J].European journal of soil biology,2002,38(3/4):245-249.
[42] BUNDY J G,SIDHU J K,RANA F,et al.‘Systems toxicology approach identifies coordinated metabolic responses to copper in a terrestrial nonmodel invertebrate,the earthworm Lumbricus rubellus [J].BMC biology,2008,6(1):1-21.
[43] YANG P,HE X Q,PENG L,et al.The role of oxidative stress in hormesis induced by sodium arsenite in human embryo lung fibroblast(HELF)cellular proliferation model[J].Journal of toxicology and environmental health:Part A,2007,70(11):976-983.

