Primary extra-pancreatic pancreatic-type acinar cell carcinoma in the right perinephric space: A case report and review of literature
Yi-Yuan Wei, Ying Li, Yan-Jie Shi, Xiao-Ting Li, Ying-Shi Sun
Yi-Yuan Wei, Ying Li, Yan-Jie Shi, Xiao-Ting Li, Ying-Shi Sun, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Department of Radiology, Peking University Cancer Hospital & Institute, Beijing 100142, China
Abstract BACKGROUND Primary extra-pancreatic pancreatic-type acinar cell carcinoma (ACC) is a rare malignancy, and has only been reported in the gastrointestinal tract, liver, and lymph nodes until now. Extra-pancreatic pancreatic-type ACC in the perinephric space has not been reported. Herein, we report the first case of ACC in the perinephric space and describe its clinical and imaging features, which should be considered when differentiating perinephric space neoplasms.CASE SUMMARY A 48-year-old man with a 5-year history of hypertension was incidentally found to have an asymptomatic right retroperitoneal mass during a routine health check-up. Laboratory tests were normal. Abdominal computed tomography and magnetic resonance imaging showed an oval hypervascular mass with a central scar and enhanced capsule in the right perinephric space. After surgical resection of the neoplasm, the diagnosis was primary extra-pancreatic pancreatic-type ACC. The patient was alive without recurrence or metastasis during a 15-mo follow-up.CONCLUSION This is the first report of an extra-pancreatic ACC in right perinephric space,which should be considered as a possible diagnosis in perinephric tumors.
Key Words: Acinar cell carcinoma; Retroperitoneal space; Tomography; X-ray; Magnetic resonance imaging; Case report
INTRODUCTION
Acinar cell carcinoma (ACC) is a rare malignant pancreatic exocrine tumor arising from pancreatic acinar cells, and accounting for less than 1% of all primary pancreatic tumors[1,2]. The incidence of extra-pancreatic pancreatic-type ACC is extremely rare,and few cases have been reported. These tumors can arise in extra-pancreatic tissue such as the gastrointestinal tract, liver, and lymph nodes[3-21], and their radiological findings have not been well described. To the best of our knowledge, extra-pancreatic pancreatic-type ACC arising in the perinephric space has not been reported. Herein,we report a case of primary extra-pancreatic pancreatic-type ACC in the right perinephric space and discuss the imaging findings on computed tomography (CT)and magnetic resonance imaging (MRI).
CASE PRESENTATION
Chief complaints
A 48-year-old asymptomatic man with a history of hypertension was incidentally found to have a right retroperitoneal tumor during a routine health check-up.
History of present illness
The patient had been suffering from hypertension (up to 180/100 mmHg) for 5 years and was regularly taking an antihypertensive drug (telmisartan) to maintain the blood pressure at 155/90 mmHg.
History of past illness
Apart from hypertension, the patient had no relevant previous illnesses.
Personal and family history
No aberrant family history was reported.
Physical examination
The patient was normal and healthy, with no suspicious finding on physical examination.
Laboratory examination
Laboratory tests were normal, including carbohydrate antigen 72-4 (CA72-4), alphafetoprotein, carcinoembryonic antigen, CA19-9, CA12-5, and neuron-specific enolase.
Imaging examination
CT of the abdomen and pelvis was performed with a 64-row helical scanner (Discover CT750 HD, General Electrical Medical Systems, Milwaukee, WI, United States) before and after the injection of nonionic contrast medium (Iohexol; Omnipaque 300, GE healthcare)viathe median cubital vein. CT showed an oval 8.0 cm × 7.0 cm × 5.0 cm mass with homogeneous isodensity and a clear margin in the right perinephric space.The tumor showed intense heterogeneous enhancement in the arterial phase and rapid homogeneous washout enhancement in the portal vein and delayed phases (Figure 1A-D). Although the mass was close to the right kidney, no remarkable renal invasion was seen. The mass compressed the duodenum. On multiplanar reconstruction, the blood supply of the tumor was derived from the right testicular artery (Figure 1E).Examination of the abdomen was performed in the supine position with a 1.5T MRI scanner (Brivo MR355; GE healthcare, Waukesha, WI, United States) using a phasedarray body coil. A respiration-triggered fast spin echo T2-weighted images (TR/TE,6667/101) and spin echo T1-weighted images (TR/TE, 180/4.3) showed a right perinephric mass with a heterogeneous signal (low signal on T1WI, slightly high signal on T2WI, Figure 2A and B) and diffusion restriction with high signal on diffusion-weighted imaging (Figure 2C). On dynamic gadolinium-enhanced phases,fat-suppressed spin echo T1-weighted images (TR/TE, 3.7/1.7) showed the same contrast pattern as on the CT scan, but revealed a more sensitive, complete capsule(Figure 2D-F). Owing to high soft tissue contrast on MRI, another characteristic was the presence of a hyperintense central scar on T2-weighted images (Figure 2A), which manifested as late enhancement in the portal vein and delayed phases (Figure 2E and F) that was more sensitive on MRI.

Figure 1 Computed tomography. A: Pre-contrast image in the transverse plane showed that the density of mass was 40 HU (arrow); B-D: On a contrastenhanced scan, the tumor presented uneven high enhancement with a stellate central scar in arterial phase (B, 111 HU, orange star) and withdrawal enhancement uniformly in portal vein (C, 95 HU) and delayed phases (D, 79 HU); E: On multiplanar reconstruction, the blood supply of the tumor derives from the right testicular artery (orange arrowhead).

Figure 2 Magnetic resonance imaging. A and B: A mass near the right kidney had a hyperintense signal on fat suppression T2WI (A) and a hypointense signal on T1WI (B); there was a stellate area with hyperintense signal on T2WI within the tumor (A, orange arrow); C: Diffusion-weighted imaging showed restricted diffusion with high signal; D-F: Gadolinium-enhanced imaging showed the internal stellate area (central scar) and hypo-enhancement in arterial phase (D, orange arrow) and further hyper-enhancement in the portal vein (E, orange arrow) and delayed phases (F, orange arrow). MRI of the abdomen in the transverse plane obtained during the portal vein and delayed phases showed tumor-enhanced encapsulation (F, orange arrowhead).
FINAL DIAGNOSIS
The preoperative differential diagnosis was suspected paraganglioma, solitary fibrous tumor (SFT) or renal oncocytoma. There was no definite clinical or radiological abnormality in the pancreas body, head, or neck. Consistent with the histological features and immunohistochemical staining, the final diagnosis was considered as primary extra-pancreatic pancreatic-type ACC of perinephric space.
TREATMENT
The patient underwent a complete surgical resection. During the operation, a tumor was found in the right perirenal space. It had tortuous and dilated reproductive vessels on the medial side, and was adjacent to the right renal capsule on the rear side.The tumor was completely removed without any complications.
Histopathological findings
The gross tumor appeared as a near-spherical mass with a distinct border, mediumsize, gray cross sections, and an intact capsule. Histomorphologically, the growth pattern of the proliferative tumor cells had an acinar appearance. Immunohistochemical staining revealed tumor cells that were negative for chromogranin A,synaptophysin, and CD56, which suggested the absence of neuroendocrine differentiation (Figure 3). Immunohistochemical staining also found Ki-67-positive (10%+)tumor cells; other indicators were negative.
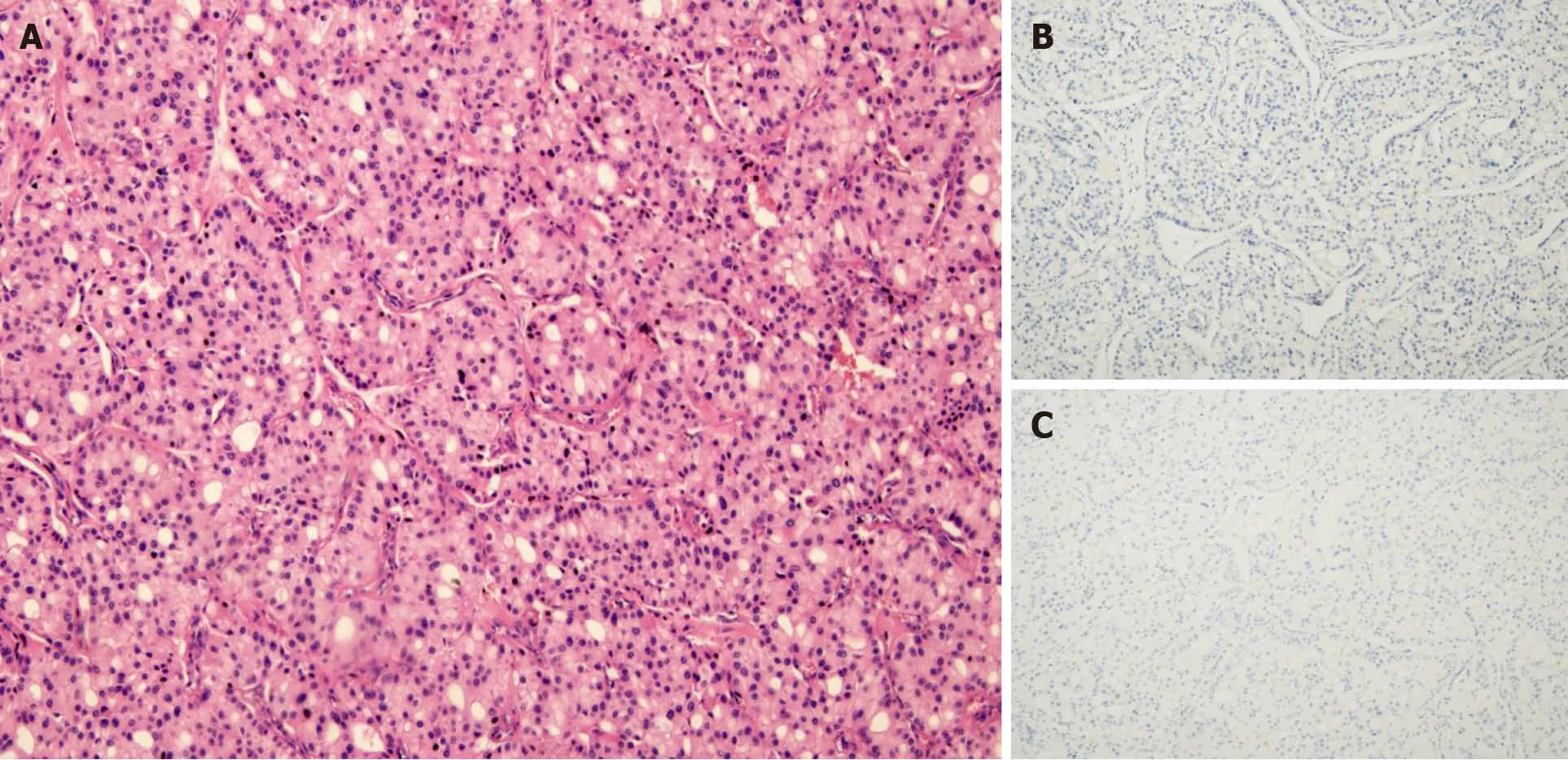
Figure 3 Microscopic features of acinar cell carcinoma. A: Acinar pattern of tumor cell structure; B and C: Most tumor cells were negative for chromogranin A (B) and synaptophysin (C).
OUTCOME AND FOLLOW-UP
The patient had no complications and was discharged from our hospital 7 d after the resection. The patient did not receive any adjuvant therapy and had no recurrence or metastasis at the 15 mo follow-up evaluation.
DISCUSSION
Primary extra-pancreatic pancreatic-type ACC is rare. The first case of primary extrapancreatic pancreatic-type ACC was traced back to 1746 and mentioned by Hamburger in a 1932 article by Bookman[10]. Since then, 21 cases of extra-pancreatic pancreatic-type ACC have been reported in PubMed. The causes of extra-pancreatic pancreatic-type ACC remain unclear. There are two hypotheses about the possible mechanism of extra-pancreatic pancreatic-type ACC. The first is that malignant transformation occurs in tissue metaplasia or in ectopic pancreatic tissue, which is common in the gastrointestinal tract[5,6,13,14,17,21]. This hypothesis is supported by three cases with definite evidence of residual metaplasia or ectopic pancreatic tissue in the resected specimens[6,14,21]. The investigators speculated that the tumor might have completely destroyed the original structure, without any residue of benign ectopic pancreatic tissue remaining[14]. Another hypothesis that multipotential progenitor cells acquire acinar pancreatic features is supported by Terriset al[16] and Agaimyet al[22], who reported three cases of extra-pancreatic pancreatic-type ACC in peripancreatic lymph nodes, and in lymph nodes along the biliary tract, hepatic hilum,colon, and retroperitoneum[16].
The characteristic microscopic architecture of ACC includes acinar units, with neoplastic cells arranged in small acinar units, and in solid nests of neoplastic cells lacking luminal formations[18]. In our patient, the tumor had the characteristic acinar growth pattern, neuroendocrine marker-negativity and acinar structures, and tumor cells were arranged as pancreatic acini (Figure 3). Lesions were not detected either by imaging (CT, MRI, endoscopic ultrasonography) or during the surgical exploration.There was no confirmation of a macroscopic intrapancreatic tumor. We also considered the differentiation of salivary gland ACC, which most often occurs in the parotid gland, and its histological characteristics show serous acinar differentiation[19]with frequent expression of cytokeratin and partial expression of S-100[20]. As the head and neck workup did not identify a primary salivary gland tumor, and the histological and immunohistochemical studies did not support a head or neck origin,our case was not considered as arising from the head or neck. As a pancreatic or head and neck origin could be excluded, and the presence of associated glandular components was revealed, we speculated that our ACC case originated from retroperitoneal multipotential progenitor cells that acquired acinar pancreatic features. A diagnosis of pancreatic-type ACC of the right perinephric space was made.
In contrast to extra-pancreatic pancreatic-type ACC, there are numerous reports on CT and MRI features of pancreatic ACC (PACC). Although presenting a wide range of features, the images of PACC can be summarized as a relatively large oval or round solid mass (average 7.1 cm), exophytic growth, a clear margin with an enhanced capsule, hypovascularity compared with the pancreatic parenchyma, lack of or relatively mild pancreatic ductal dilation or vascular encasement compared with pancreas ductal adenocarcinoma, internal necrosis, cystic changes, always accompanied by invasion of adjacent organs, and extensive metastasis[23-30]. Tumor encapsulation is a specific finding for the diagnosis of PACC, however it may be misleading because of similar characteristics of other tumors[31,32]. Several case reports of extra-pancreatic ACCs have been published by surgeons and pathologists.Therefore, the main focus of those reports was not the imaging features. Consequently,only few cases with radiological features were reported, briefly describing the CT examination (Tables 1 and 2), and no reports of MRI. Those cases merely revealed marked homogeneous or heterogeneous enhancements (Tables 1 and 2) without any detailed information. Here we present detailed images of a case with extra-pancreatic ACC in the perirenal space and summarize the CT and MRI characteristics as an encapsulated solid mass, a well-defined contour, relatively homogenous density or signal, hyperenhancement in the arterial phase and withdrawal enhancement in the portal vein and delayed phases, and an enhanced capsule. However, those imaging manifestations can also be found in other hypervascular peritoneal neoplasms, such as paraganglioma, SFT, or renal oncocytoma. First, considering paraganglioma, the patient's history of hypertension made the differential diagnosis difficult. Retroperitoneal extra-adrenal paragangliomas usually occur in the Zuckerkandl body and the para-aortic sympathetic nervous chain at the renal hilum level[30]. They can synthesize and secrete large amounts of catecholamines, which can cause a clinical syndrome that includes blood pressure elevation. Benign small-volume tumors with a uniform density often appear. Larger oval or lobulated soft tissue masses with clear boundaries are usually accompanied by necrosis and hemorrhage. Tortuous vessels can be seen around or within these hypervascular tumor components[31]. Malignant paragangliomas are characterized by invasiveness and dissemination. However, the presence of homogeneous density without any necrosis or hemorrhage, and relatively well-proportioned enhancement in our case made that diagnosis unlikely. Second,retroperitoneal SFTs typically have a well-circumscribed margin, intense but hetero-geneous enhancement on the arterial phase, and persistent enhancement on the delayed phase[32]. Retroperitoneal SFTs can present with hemorrhage, necrosis, or cystic degeneration. However, the enhanced pattern of our case (fast-in and fast-out)was not consistent with a typical retroperitoneal SFT (persistent enhancement).Finally, renal oncocytomas can show hypointensity in T2WI, have an abundant blood supply, central scar, delayed enhancement, and a capsule, which should be considered. However, renal oncocytoma primarily originates from the renal collecting duct, protruding in the renal contour. The boundary between the tumor and the kidney in our case was smooth without any evidence of a “break sign”, which made the diagnosis of renal oncocytoma unlikely. Hypothetically, the stellate central scar in our case might have been a reaction of fibrosis, blood vessel, or infiltrated inflammatory regions resembling renal oncocytoma or focal nodular hyperplasia[33,34].Consequently, the findings in our case might help to discriminate between extrapancreatic pancreatic-type ACC and other hypervascular perinephric neoplasms.
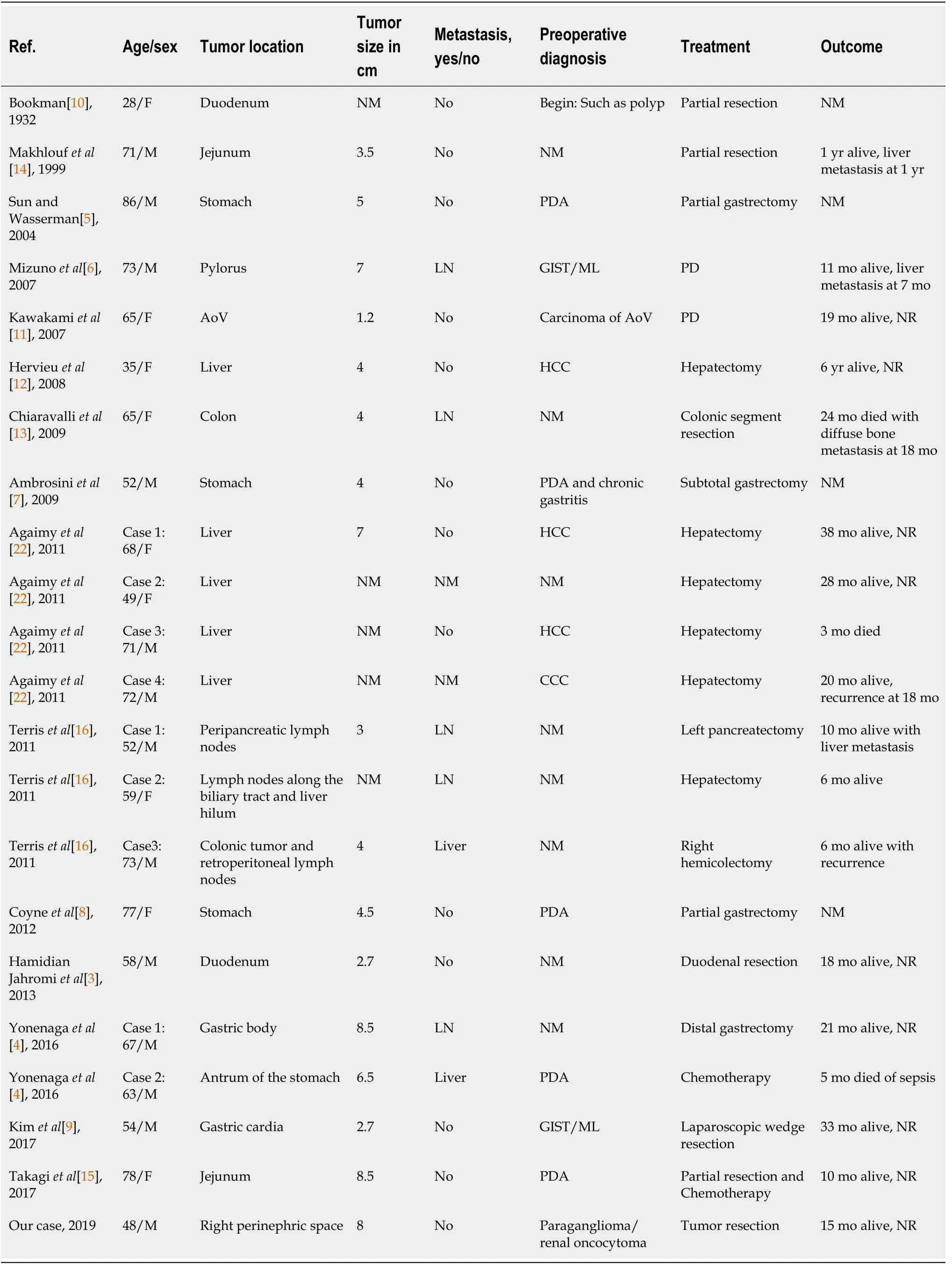
Table 1 Clinical features in reported cases of acinar cell carcinoma arising from extra-pancreatic tissues
CONCLUSION
The findings of a retroperitoneal mass with a relatively homogenous density or signal,fast-in and fast-out enhanced patterns, and an enhanced capsule made extrapancreatic ACC as the most likely diagnosis.
ACKNOWLEDGEMENTS
We thank Dr. Dong K, Key Laboratory of Carcinogenesis and Translational Research(Ministry of Education), Department of Pathology, Peking University Cancer Hospital& Institute, for providing pathological figures of our case.
 World Journal of Clinical Cases2021年20期
World Journal of Clinical Cases2021年20期
- World Journal of Clinical Cases的其它文章
- Obesity in people with diabetes in COVID-19 times: Important considerations and precautions to be taken
- Revisiting delayed appendectomy in patients with acute appendicitis
- Detection of short stature homeobox 2 and RAS-associated domain family 1 subtype A DNA methylation in interventional pulmonology
- Borderline resectable pancreatic cancer and vascular resections in the era of neoadjuvant therapy
- Esophageal manifestation in patients with scleroderma
- Exploration of transmission chain and prevention of the recurrence of coronavirus disease 2019 in Heilongjiang Province due to inhospital transmission
