Study on the Specific GC Chromatograms of Volatile Oil from Xinyi Biyan Pills
Huiduan LONG Yi LUO Wei WEI


Abstract [Objectives] This study was conducted to establish characteristic chromatograms of of the volatile oil of Xinyi Biyan Pills by gas chromatography, discover possible problems in the production processes of different manufacturers, and further improve the quality control methods.
[Methods]The volatile oil in samples was extracted and tested by gas chromatography to collect chromatograms, which were analyze and evaluated by the similarity evaluation software of chromatographic fingerprints of traditional Chinese medicine.
[Results] Nineteen common peaks were calibrated in the characteristic chromatograms; and the characteristic chromatograms of samples produced by different manufacturers were obviously different.
[Conclusions]Controlling the volatile components in Xinyi Biyan Pills by the established characteristic chromatograms of GC is accurate and feasible, and can be used as a quality control method for Xinyi Biyan Pills.
Key words Xinyi Biyan Pills; Volatile oil; Gas chromatography; Characteristic chromatogram
Received: February 27, 2021 Accepted: April 29, 2021
Supported by Guangxi Key R&D Program Project (GK AB19110027).
Huiduan LONG (1982-), male, P. R. China, pharmacist-in-charge, devoted to research about analysis and testing on active ingredients of medicines.
*Corresponding author.
Xinyi Biyan Pills belong to varieties in the national essential drugs list, and have the effects of dispelling pathogenic wind and clearing away heat and toxic materials. Its standard is included in the 2015 edition of Chinese Pharmacopoeia[1], and the prescription consists of thirteen traditional Chinese medicines. Among them, four medicinal materials, Magnoliae Flos, Menthae Haplocalycis Herba, Pogostemonis Herba[2-3], Folium Perillae[4] are extracted for volatile oils which are used as medicines. Volatile oil is an important factor for these medicinal materials to exert their pharmacological effects, but the inspection items for volatile oil in the quality standard are missing or not perfect. In order to effectively control the quality of the volatile oil in the preparation, we used gas chromatography to conduct a large number of systematic experiments. Through the study of the characteristic chromatograms, we compared and analyzed the differences between samples from the same manufacturer and different manufacturers, and preliminarily established a general method for the inspection and detection of volatile oil in preparations containing volatile oil, and the similarity table of the characteristic chromatograms can reflect the differences between multiple batches of samples.
Materials and Methods
Experimental materials
Instruments
American Agilent 6890N gas chromatograph, hydrogen flame ionization detector (FID); electronic analytical balance (one ten-thousandth); Milli-Q pure water meter (Merck Millipore, Germany); ABS-6G dual range one hundred-thousandth electronic analytical balance (Mettler-Toledo, Switzerland).
Medicine and reagents
Xinyi Biyan Pills (company A, specifications: 0.75 g/10 pills, 38 batches, numbered A1-A38; company B, specifications: 3 g/bag, 10 batches, numbered B1-B10; company C, specifications: 0.75 g/10 pills, 4 batches, numbered C1-C4), all purchased from the market; Magnoliae Flos (121079-200704), Menthae Haplocalycis Herba (120916-201310), Pogostemonis Herba (121135-201005), Folium Perillae (120914-201411), menthol (110728-200506), menthone (111705-200501), patchoulenone (111822-201102) and patchouli alcohol (110772-201407) were provided by National Institutes for Food and Drug Control; and Herba Menthae Spicatae was used after identification by associate chief pharmacist Tang Xiuling and pharmacist in charge Huang Qingquan from Traditional Chinese Medicine and Ethnomedicine Department of Guangxi Zhuang Autonomous Region Institute of Food and Drug Inspection. Carvone (934510) was provided by Beijing Bailingwei Technology Co., Ltd.; water was ultrapure water; and other reagents were analytically pure.
Experimental methods
Chromatographic conditions
The capillary column was a 5% phenyl-methyl polysiloxane (DB-5) column (column length: 60 m, inner diameter: 0.25 mm, film thickness: 0.25 μm); the column temperature adopted temperature programming, which started with the initial temperature of 50 ℃, which was increased to 130 ℃ at a rate of 1.5 ℃/min, held for 5 min and then increased at a rate of 1.5 ℃/min to 150 ℃, which was held for 5 min, and finally increased at a rate of 1.5 ℃/min to 240 ℃, which was held for 5 min; the temperature of the injection port was 250 ℃; the detector temperature was 280 ℃; the injection volume was 1 μl; and the split injection was adopted with a split ratio of 10∶1.
Preparation of control solutions
Appropriate amounts of menthol, menthone, patchoulione, patchouli alcohol and carvone reference substances were accurately weighed, and added with methanol, obtaining a 1 mg/ml reference substance solution. Menthae Haplocalycis Herba reference medicinal material (about 4.33 g), Magnoliae Flos reference medicinal material (about 0.42 g), Pogostemonis Herba reference medicinal material (about 4.33 g), Folium Perillae reference medicinal material (about 3.17 g) and Herba Menthae Spicatae reference medicinal material (about 4.33 g) were accurately weighed, respectively. Each of the material was added into a volatile oil extractor, added with 2 ml ethyl acetate, and tested according to the volatile oil determination method (2204 Volatile Oil Determination Method in part 4 of the 2015 edition of Chinese Pharmacopoeia). The extraction was performed for 3 h, and the ethyl acetate layers of the medicinal materials were obtained as the Menthae Haplocalycis Herba reference medicinal solution, the Magnoliae Flos reference medicinal material solution, Pogostemonis Herba reference medicinal material solution, Folium Perillae reference medicinal material solution and Herba Menthae Spicatae reference medicinal material solution, respectively.
Preparation of test solution
An appropriate amount of a product was taken and finely grinded, and about 10 g of the powder was accurately weighed, added in a volatile oil extractor, added with 2 ml of ethyl acetate, and tested according to the volatile oil determination method (2204 Volatile Oil Determination Method in part 4 of the 2015 edition of Chinese Pharmacopoeia). The extraction was performed for 3 h, and the ethyl acetate layer was obtained as the test solution.
Precision test
An appropriate amount of the same test product (A26) was weighed, grinded carefully, and prepared into a product solution, which was tested according to the method under "Preparation of test solution". According to the chromatographic conditions under "Chromatographic conditions", the sample was injected for 6 times consecutively. With menthol as the reference peak, and the relative retention time of the common peaks and the relative peak areas of the main common peaks were calculated. The RSDs were all less than 1.0%. The fingerprints obtained from the first injection were used as a reference, and the similarity of the fingerprints obtained from the last five injections was calculated, and the similarity was not less than 0.99. The results all met the requirements of fingerprint determination, indicating that the precision of the instrument was good.
Repeatability test
Appropriate amounts of the same test product (A26) were weighed, grinded carefully, and prepared into six test solutions, which were injected once and tested according to the chromatographic conditions under "Chromatographic conditions", and the fingerprints were recorded. With menthol as the reference peak, and the relative retention time of the common peaks and the relative peak areas of the main common peaks were calculated. The RSDs were all less than 1.0%, indicating that the repeatability of the method was good.
Stability test
An appropriate amount of the same test product (A26) was weighed, grinded carefully, and prepared into a test solution, which was tested according to the chromatographic conditions under "Chromatographic conditions" at 0, 4, 8, 12, 18 and 24, respectively, and the fingerprints were recorded. With menthol as the reference peak, and the relative retention time of the common peaks and the relative peak areas of the main common peaks were calculated. The RSDs were all less than 1.5%, indicating that the test solution of Xinyi Biyan Pills was stable within 24 h.
Results and Analysis
Establishment and analysis of characteristic chromatograms
Selection of reference
Xinyi Biyan Pills showed multiple chromatographic peaks by gas chromatography. Menthol could be confirmed by the reference substance. It was relatively stable and the peak time was appropriate. Therefore, the menthol peak was selected as the S peak of the reference substance peaks.
Generation of control chromatogram
Xinyi Biyan Pills showed multiple chromatographic peaks after GC analysis. Using the Chinese Pharmacopoeia Commission "Chinese Medicine Chromatographic Fingerprint Similarity Evaluation System" software, a characteristic chromatogram was established using the 38 batches of samples from manufacturer A with a large number of tested batches and a large market share. The generated characteristic chromatogram is shown in Fig. 1.
Peak attribution
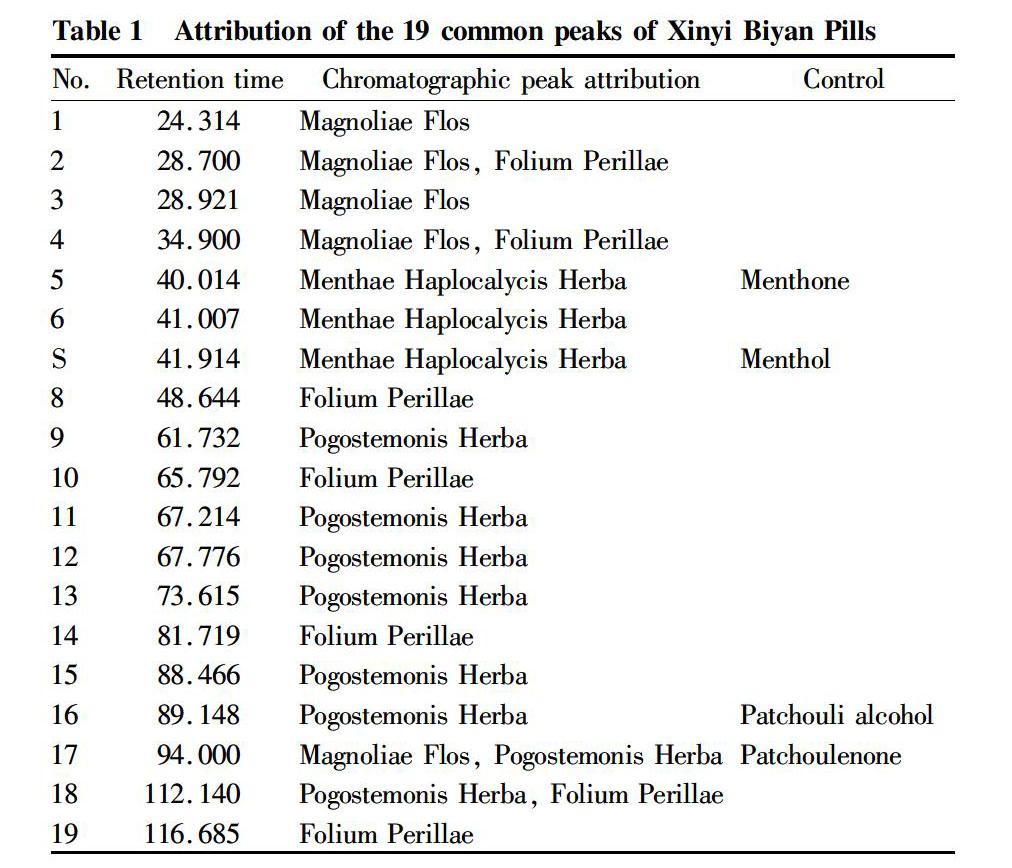
According to the prescription, the reference medicinal materials that needed to extract the volatile oil (Magnoliae Flos, Menthae Haplocalycis Herba, Pogostemonis Herba, Folium Perillae, Herba Menthae Spicatae) were taken with the amounts of various medicinal materials in the preparation. The volatile oils were extracted according to the extraction method of the sample solution, and an appropriate amount was injected into the gas chromatograph. The chromatograms were collected, and the chromatographic peaks were assigned according to the differences of peak retention time[1-3]. Menthol could be confirmed according to the reference substance and was relatively stable, so the menthol peak was selected as the S peak of the reference peaks. The results showed that the similarity of the chromatograms of the varieties produced by the factory was greater than 0.99. The generated characteristic chromatograms had 19 common peaks. The attribution of the 19 common peaks is shown in Tables 1-2.
All the chromatographic peaks of all components of Xinyi Biyan Pills appeared within 130 min. Through the chromatographic analysis of the various medicinal materials in the prescription and reference substances, 19 common peaks were assigned. The characteristic chromatogram included peaks of four medicinal materials in the prescription, Magnoliae Flos, Pogostemonis Herb, Folium Perillae and Menthae Haplocalycis Herba, such as the peaks of menthol, patchoulione, patchouli alcohol and menthone references, and for the Herba Menthae Spicatae, a fake product of Menthae Haplocalycis Herba[5-6], carvone is the exclusive characteristic peak.
Generation of characteristic chromatograms of different manufacturers
By testing the characteristic chromatograms of 38 batches of samples produced by company A with a large number of batches and a large market share, 10 batches of samples produced by company B and 4 batches of samples produced by company C, the differences between different sampling locations and different batches of the same company and the differences between different enterprises were investigated. The characteristic chromatograms are shown in Fig. 2-5.
Similarity of chromatograms of different manufacturers
There should be 19 characteristic peaks in the characteristic chromatograms of the test product. With menthol as the S peak, the relative retention time and relative peak area of each characteristic peak to the S peak were calculated, and the relative retention time should be within ±5% of the specified value. The specified values were 0.580 (peak 1), 0.685 (peak 2), 0.690 (peak 3), 0.832 (peak 4), 0.955 (peak 5), 0.978 (peak 6), 1 (peak 7), 1.161 (peak 8) ), 1.473 (peak 9), 1.570 (peak 10), 1.604 (peak 11), 1.617 (peak 12), 1.756 (peak 13), 1.950 (peak 14), 2.111 (peak 15), 2.127 (peak 16), 2.243 (peak 17), 2.675 (peak 18), and 2.784 (peak 19).
Results and analysis
From the determined chromatograms and results of Xinyi Biyan Pills, all the 38 batches of Xinyi Biyan Pills produced by manufacturer A were detected with 19 exclusive peaks of Magnoliae Flos, Menthae Haplocalycis Herba, Pogostemonis Herba and Folium Perillae, none of which was missing. And the peak areas of the S peaks of various batches were equivalent and relatively stable, and Herba Menthae Spicatae was not detected. Therefore, the overall quality of Xinyi Biyan Pills produced by this factory was relatively good. Manufacturer B produced 12 batches of Xinyi Rhinitis Pills samples, all of which had the exclusive peaks of Menthae Haplocalycis Herba, 4 batches of samples lacked all the attributable peaks of Magnoliae Flos, 8 batches of samples lacked the main peak of Magnoliae Flos, 9 batches of samples lacked the main peak of Pogostemonis Herba, 3 batches of samples lacked part of the characteristic peaks of Pogostemonis Herba, 8 batches of samples lacked the main peak of Folium Perillae, and 4 batches of samples lacked part of the characteristic peaks of Folium Perillae. The peaks of menthol in all 12 batches of samples were small, only about fiftieth to one-tenth of the menthol in the control Menthae Haplocalycis Herba material. It is speculated that the Menthae Haplocalycis Herba, Magnoliae Flos and Folium Perillae invested by the enterprise were of poor quality, and the quality was uneven. Manufacturer C produced two batches of Xinyi Biyan Pill samples, both of which showed the characteristic peaks of Menthae Haplocalycis Herba, Pogostemonis Herba and Folium Perillae, and only part of the characteristic peaks of Magnoliae Flos were missing. However, the S peak (menthol peak) was smaller, only about seventieth of the menthol in the control Menthae Haplocalycis Herba material, and the characteristic peaks No.5 and No.6 of Menthae Haplocalycis Herba were missing. In addition, the characteristic peak of Menthae Haplocalycis Herba (counterfeit of Menthae Haplocalycis Herba), i.e., carvone peak, appeared, indicating that the quality of the Menthae Haplocalycis Herba materials used by the company was poor and it was mixed with Menthae Haplocalycis Herba.
Huiduan LONG et al. Study on the Specific GC Chromatograms of Volatile Oil from Xinyi Biyan Pills
Conclusions and Discussion
Determination of the chromatographic column
Gas chromatography columns of different polarities were compared, including ①polyethylene glycol (DB-WAX) capillary column (30 m×0.53 mm, 1.0 μm), ② polyethylene glycol (DB-WAX) capillary column (60 m×0.53 mm, 1.0 μm), ③dimethyl polysiloxane (DB-1) capillary column (60 m×0.25 mm, 0.25 μm), ④(5% phenyl)-methyl polysiloxane (DB-5) capillary column (60 m×0.25 mm, 0.25 μm) and ⑤ (50% phenyl)-methyl polysiloxane (DB-17) capillary column (60 m×0.25 mm, 0.25 μm). It was found that the separation effect and column efficiency of the chromatographic column ④ for separating each component were good, so the (5% phenyl)-methyl polysiloxane (DB-5) capillary column (60 m×0.25 mm, 0.25 μm) was finally determined as the chromatographic column for the gas chromatography.
Selection of column temperature
Because of the relatively large amounts of materials in the prescription of the test product, the volatile oils extracted by the volatile oil extractor device had many and complicated volatile oil components, and they must be separated for a long time to obtain a satisfactory chromatogram. By comparing the initial temperatures of 40, 50, 60 and 100 ℃, the heating rates of 2, 5 and 10 ℃/min, the holding time of 10 and 15 min, the second heating rates of 2 and 10 ℃/min, and the total running time of 50, 68, 89, 135 and 141.67 min, the final temperature programming was determined. It started with the initial temperature of 50 ℃, which was increased at a rate of 1.5 ℃/min to 130℃, which was held for 5 min, then increased at a rate of 1.5 ℃/min to 150℃, which was held for 5 min, and increased at a rate of 1.5 ℃/min to 150 ℃, which was held for 5 min, and finally increased at a rate of 1.5 ℃/min to 240 ℃, which was held for 5 min, and the total running time was 141.67 min. And through experiments, this temperature programming conditions could get more chromatographic peaks with better resolution.
Determination of extraction solvent
Volatile oil is easily soluble in ethyl acetate, petroleum ether, etc. Ethyl acetate and petroleum ether (60-120 ℃) were added into volatile oil extractors to extract the volatile oil, respectively. It was found that the number of characteristic chromatographic peaks and peak shapes obtained by extracting the volatile oil in the test product with the above two organic solvents were equivalent, but in the test solution extracted with ethyl acetate, the peak areas of chromatographic peaks No. 5, 6, 7, 16, and 18 were significantly larger than those extracted with petroleum ether (60-120 ℃). Therefore, ethyl acetate was determined as the final extraction solvent.
Determination of extraction time
2204 Volatile Oil Determination Method in part 4 of the 2015 edition of Chinese Pharmacopoeia stipulates that the volatile oil extraction time is 5 h. However, considering the saving of time and other resources, 5 and 3 h of extraction were examined in the experiment. It was found that there were no significant changes in the number of chromatographic peaks and peak areas of the test product after extraction for 5 and 3 h, so it was basically determined that the volatile oil was basically completely extracted within 3 h. Therefore, it was finally determined that the extraction time was 3 h.
Characteristic chromatogram result analysis
From the results of the characteristic chromatograms of samples
from different manufacturers, it can be seen that the relative retention time of samples from all manufacturers had little differences, while the relative peak areas of Xinyi Biyan Pill samples from different manufacturers were quite different, indicating that the contents of menthol, menthone, patchouli alcohol, patchoulenone and other ingredients in Xinyi Rhinitis Pills from different manufacturers were quite different, which might be because that the quality of the raw medicinal materials used by the production enterprises for production was uneven, and the production processes were different. There were also certain differences in the relative peak areas between different batches of the same manufacturer, which might be due to the uneven quality of various batches of raw medicinal materials used for production.
In this study, gas chromatography was used to establish a fingerprint analysis method for Xinyi Biyan Pills, which was proved to be capable of easily and effectively identifying the four medicinal materials, Magnoliae Flos, Menthae Haplocalycis Herba, Pogostemonis Herba and Folium Perillae in Xinyi Biyan Pills by methodological investigation. This study provides a basis for the effective control of Xinyi Biyan Pills and the study on the effective material basis of Xinyi Biyan Pills.
References
[1] Chinese Pharmacopoeia Commission. Chinese pharmacopoeia[M]. Beijing: China Medical Science Press, 2015. (in Chinese)
[2] WEI G, FU H. Study on characteristic fingerprint of volatile oil of Pogostemon cablin (Blanco) Benth by GC-MS[J] Chinese Traditional Patent Medicine, 2002, 24(6): 407-410. (in Chinese)
[3] ZENG Z, TAN LX. Study on the chemical constituents and fingerprint of pogostemon cablin from three culture varieties[J]. Chinese Journal of Analytical Chemistry, 2006, 9(34): 1249-1254. (in Chinese)
[4] LI WS. Qualitative analysis of volatile oil from Folium Perillae by GC-MS[J]. Henan Science & Technology, 2010(7): 168. (in Chinese)
[5] CUI YH, GAO TA. Distinguishing Herba Menthae from Herba Menthae Spicatae[J]. Guangming Journal of Chinese Medicine, 2011, 8(26), 8: 1712. (in Chinese)
[6] CAO JH, ZHU CM, LI YL. Distinguishing Herba Menthae from Herba Menthae Spicatae[J]. Journal of Henan University of Chinese Medicine, 2004, 2(1), 19: 110. (in Chinese)
- 农业生物技术(英文版)的其它文章
- Anti-inflammatory Activity and Mechanism of Total Flavonoids from the Phloem of Paulownia elongate S.Y. Hu in LPS-stimulated RAW264.7 Macrophages
- Comparative Genomic Analysis of Boron Transport Gene Family in Arabidopsis and Five Crops
- Effects of Different Water-saving Irrigation Methods on Fruit Quality and Yield of Snow Melon
- Field Control Effects and Crop Safety Assessment of Triazole Fungicides on Apple Rust
- Effects of Acetylacetone Solution Soaking on Agrobacterium-transformed Maize Seed Buds
- Effects of Meteorological Factors on Overwintering Ability, Yield and Quality of Forage Rape

