Application of Nanomaterials in the Rapid Detection of Pathogenic Microorganisms
Shenyan SHI Xiaojuan NIE Dong HUANG Danni ZHAO Jiazhen MEI Tao LI Liyuan ZHANG Rui QIAN Peng LYU
Abstract Pathogenic microorganisms can cause infectious diseases in the human body and cause harm to humans. Under the continuous changes of human life style, sudden infectious diseases and sudden pathogenic microbial contamination in food have brought great challenges to human health. Traditional pathogenic microorganism detection is mainly carried out in laboratories with low speed, and requires experienced operators, which greatly affects the detection efficiency, and real-time monitoring cannot be achieved in the face of epidemics or food contamination. In response to these new situations, new on-site rapid detection methods have become a must, but to achieve the purpose of on-site rapid and immediate detection, higher requirements have been put forward to sensitivity and specificity. Due to their special photoelectric properties and adsorption properties, nanomaterials have gradually been applied in on-site rapid detection methods such as biosensors, and some substantial progress has been made. In this study, we introduced the actual application of nanomaterials in testing and their applications in on-site rapid testing, and made some prospects for the development of on-site rapid testing methods.
Key words Nanomaterials; Rapid on-site testing; Test strip technology
Received: February 23, 2021 Accepted: April 27, 2021
Shenyan SHI (1995-), male, P. R. China, master, devoted to research about nanomaterials.
*Corresponding author. E-mail: penglu@ujs.edu.cn.
Research Background
Pathogenic microorganisms refer to microorganisms that are pathogenic to animals, plants, and humans, including prions, viruses, parasites, fungi, bacteria, mycoplasmas, chlamydiae, etc. After invading the human body, they will cause harm to the human body, some will cause adverse reactions, and some will cause irreversible damage or death to the human body. Therefore, infectious diseases caused by pathogenic microorganisms will seriously threaten human health. With the current rapid economic development of China, peoples lifestyles and habits have changed, and the outbreak of some new infectious diseases has put forward new requirements and challenges for on-site rapid real-time detection.
Due to the rapid development of biotechnology and engineering technology, traditional methods for detecting pathogens are increasingly unable to adapt to the current detection requirements, and many drawbacks have appeared. The traditional detection method of pathogenic microorganisms[1] is usually plate culture, which can separate, culture and identify pathogenic microorganisms according to the morphological characteristics, biochemical reaction and serological reaction of the pathogenic microorganisms. This detection method not only has low specificity, but also consumes a lot of time, and the detection method also has the problem of low sensitivity. For large-scale infectious disease emergencies caused by SARS and the new coronavirus, it is only applicable to laboratory tests and test results, and the long waiting time will become an obstacle to controlling the outbreak. Therefore, the development of detection technology is more biased towards rapid and highly sensitive on-site detection.
Nanomaterials, that are extremely small in size, can exhibit many excellent properties and functions in electrical, mechanics, chemistry, optics, biology and other professional fields, and can enhance the sensitivity and specificity of detection. Meanwhile, nanomaterials are simple and easy to operate in loading and labeling of fluorescent groups, and they are easy to obtain, making it possible to apply them in rapid on-site detection[2]. Compared with molecular dyes, they will not produce negative reactions with non-specifically bound analytes through cellular biological macromolecules. In addition, in recent years, research on composite nanomaterials has gradually increased. Nanomaterials can be modified or combined with other materials or detection methods to achieve better detection results, and such methods make rapid detection on the spot easier.
Nanomaterials
Nanomaterials are extremely small in size and belong to a very typical mesoscopic field. By transforming nanomaterials or combining them with aptamers or antibodies, new on-site rapid detection methods can be constructed. Nanomaterial biosensors have bright prospects in the fields of disease diagnosis and environmental monitoring[3-5].
Metal nanomaterials
Compared with molecular compounds or bulk metals, metal nanomaterials exhibit different optical, electromagnetic and chemical properties due to their shape, spacing, and special surface effects and quantum size effects. The liquid phase synthesis method has become the most widely used synthesis method for metal nanoparticles due to its advantages such as low cost, simple operation, good monodispersity of the prepared particles and high yield.
The unique interaction between gold atoms and sulfhydryl groups helps to modify nanomaterials with oligonucleotides and other compounds[6]. Noble metal nanoparticles coupled with oligonucleotides or antibodies can be used for molecular recognition. The optical properties of metal nanoparticles are excellent, and the colors of the systems change correspondingly when the metal nanoparticles are agglomerated. According to this feature, they have a wide range of application value in the semi-quantitative detection of pathogenic pathogens, and can be used for rapid on-site detection.
Compared with metal nanoparticles, metal nanoclusters are new fluorescent nanomaterials composed of several to several hundred metal atoms, and their core size is generally less than 3 nm. Metal nanoclusters have the advantages of small size, no toxicity and high stability[7]. Common metal nanoclusters include gold clusters, silver clusters, copper clusters, etc.[8-9]. Gold nanoclusters (Au nanoclusters, AuNCs) can be used for disease-related diagnosis (involving biological analysis and biological imaging) and treatment due to their extraordinary physical and chemical properties and excellent biocompatibility[10]. The silver nanoclusters (AgNCs) stabilized with DNA scaffolds have excellent physical and chemical properties and have become a universal tool in biomedical systems. They have adjustable emission fluorescence and are suitable for multifunctional designs. Silver nanoclusters combined with fluorescence and surface-enhanced Raman scattering (SERS) methods have been successfully applied to the detection of microRNA.
Graphene and graphene oxide
Graphene is currently the thinnest material found in nature. It has a two-dimensional structure with a single layer thickness of only 0.335 4 nm[11-12]. It can have a strong interaction with DNA bases, and can adsorb DNA, and its strength is closely related to the DNA molecular structure. In addition, graphene is a super quencher with nano-scale energy transfer characteristics[13-14]. Graphene oxide (GO) is one of the most potential materials in the development of the next generation of bioanalytical equipment. GO is a water-dispersed graphene derivative, usually obtained by oxidation of graphite in a mixture of an oxidant and a strong acid. Graphene and graphene oxide have been successfully used to develop high-efficiency nanosensors for sensitive detection of various proteins. In recent years, fluorescence resonance energy transfer (FRET) sensors based on graphene as an energy acceptor have been well developed due to their efficient energy transfer and electron transfer[15-18].
Quantum dots and composite quantum dots
Quantum dots (QDs), also known as artificial atoms, are a kind of quasi-zero-dimensional nanomaterials with limited three-dimensionality. The synthetic method of QDs is simple, and the surface can be used as a scaffold structure to bind biological macromolecules such as proteins and nucleic acids, so QDs are an ideal fluorescent probe. For example, quantum dots can detect food-borne pathogens by connecting nucleotides and various biological macromolecules (including proteins and antibodies). For example, fluorescent signal changes are used for the detection of Escherichia coli, Salmonella or Shigella and their toxins (such as Staphylococcal enterotoxin B)[19]. This binding method is beneficial to improving the specificity of detection and anti-interference ability.
Quantum dots are generally composed of semiconductor materials of II-VI elements (such as CdS, CdSe, CdTe, ZnSe, ZnS, etc.), III-V elements (such as InP, InAs, etc.) or IV-VI elements (such as PbS, PbSe). Shell/core type quantum dots can enhance the physical and chemical stability of individual quantum dots, protect the core from oxidation in the external environment, and prevent the dissolution and leakage of toxic ions. Meanwhile, the coating shell can also be used as a scaffold structure to conjugate other biobigands. When a semiconductor material or polymer with a higher energy band gap is used as the shell, the fluorescence quantum yield of quantum dots will increase[20-21]. Xiao et al.[22] used microwave-assisted heating to synthesize CdTe/CdS/ZnS water-soluble core/shell quantum dots in an aqueous phase containing a sulfhydryl stabilizer (3-mercaptopropionic acid, MPA). The quantum dots synthesized by this method have high fluorescence quantum yield, good light resistance and good biocompatibility, and no more complicated biological modification is required.
In recent years, studies have found that doping a small amount of metal elements in quantum dots can change the number of emission centers. Based on this characteristic, the research on metal-doped quantum dots has begun to deepen. Among them, the research on manganese-doped quantum dots is the most extensive. Manganese has unique magnetic and optical properties and can provide a good doping system. On the basis of retaining most of the advantages of quantum dots, metal-doped quantum dots can also avoid the problem of self-quenching of fluorescence caused by Stokes shift, so that the fluorescence lifetime is longer and the application range is wider. Quantum dots can also be used in combination with magnetic nanomaterials, which is widely used in the detection of food-borne pathogens.
Up-conversion nanomaterials
Up-conversion phosphors (UCPs) are a kind of nano-scale particles prepared by mixing rare earth metal elements with certain crystal lattices, which have a unique structure and can be excited by infrared rays to emit visible light. Therefore, they can be used as biomarkers for multiplex and quantitative analysis. Different from the traditional luminescence process involving only one ground state and one excited state, up-conversion luminescence requires many intermediate states to accumulate the energy of the low-frequency excitation photons. These processes are all realized by the continuous absorption of one or more photons by the energy levels of the activated particles doped in the crystal particles. UCPs usually have a series of sharp emission peaks, which avoids the impact of overlapping emission peaks. And the emission peak wavelengths are fundamentally not affected by the chemical composition and physical size of the matrix, making UCPs ideal detection materials. UCPs, especially lanthanide metal upconversion materials, have been maturely used in commercial testing products[23].
Application of Nanomaterials in Field Inspection
Nano biosensor
The detection method that uses enzymes, receptors, antibodies and other biological macromolecules with specific recognition capabilities as the original identification is called biosensors. Its characteristics of specific recognition or rapid catalysis can meet the requirements of fast, specific and instant online detection. If antigen, antibody, or enzyme reaction signals are converted into electrochemical signals, and then amplified and processed, rapid and instant detection can be realized using the electrochemical signals. Nanomaterials can play an important role in biosensors due to their special optical, catalytic and photoelectrochemical properties. Nanomaterials are usually used to enhance the specific adsorption of antibodies or enzymes and increase the adsorption capacity, thereby greatly improving the sensitivity of biosensors. Commonly used gold nanoparticles, carbon nanotubes and quantum dots can all have this effect[24-26]. Castaneda et al.[27] designed a sensor that converts the signal generated by the antigen-antibody binding into an electrochemical signal, and then amplified the signal by the p-nitrophenol catalytically adsorbed on gold nanoparticles. In addition, nanomaterials and enzymes can be used to amplify electrochemical signals when the antigens and antibodies are bound. Mackey et al.[28] made an electrochemical immunosensor by labeling human IgG with horseradish peroxidase and then adsorbing it on gold nanoparticles. As a result, the measurement sensitivity of the electrode is higher than that of ELISA, reaching 260 pg/ml. As a result, its measurement sensitivity was higher than that of ELISA, reaching 260 pg/ml. Nano-biosensors combined with the development of some handheld detection equipment can complete the rapid on-site detection of pathogenic microorganisms.
Lateral flow biosensors
The test strip technology is an important on-site rapid detection technology, but its disadvantage is that the detection sensitivity is low, and commercial applications are still mainly qualitative and semi-quantitative. However, due to its less dependence on operation technology and equipment for on-site rapid detection, it is still a mainstream on-site rapid detection technology. In order to improve its detection sensitivity, nanomaterials are applied to test strips, which further increases the application range of the test strip technology.
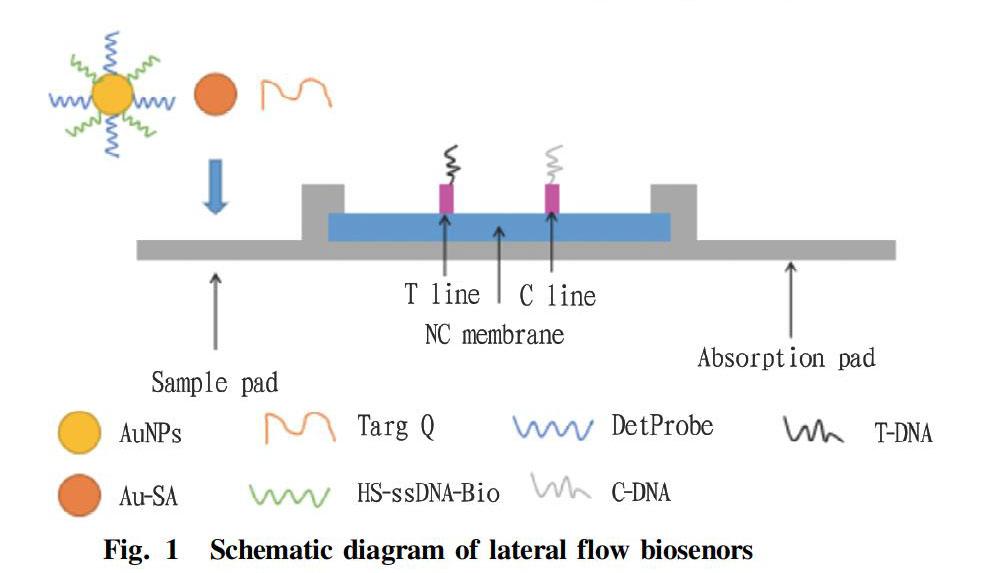
The lateral flow biosenors (LFBs)[29] are detection devices that convert biochemical signals into visual signals and fluorescent signals. AuNPs are usually used as the labeling particles of LFBs due to their special photoelectric properties, and can be used for colorimetric sensing of analytes. The main method of signal amplification is enzyme-catalyzed reaction. The combination of the three can form a DNA-AuNPs three-dimensional network-amplified lateral flow biosensor, which is beneficial to enhance the sensitivity of biosensors, and the method is simple and time-saving.
The test strips are mainly composed of a sample pad, nitrocellulose membrane and an absorbent pad. When a sample to be tested and the detProbe-Au-ssDNA complex (a functionalized probe prepared by two sulfhydryl oligonucleotides DetProbe and HS-ssDNA-Bio with different functions for modifying AuNPs and AuNPs) and Au- After SA (streptavidin-nano-gold complex) are mixed and cultured, and dropped onto a sample membrane, the liquid sample flows to the binding pad, and if a target object exists in the sample, the target object will be ligated to labeled particles, then bind with corresponding specific receptors, pass through the NC membrane with capillary action, and then will be captured by T-DNA on the detection line when it passes through the detection line. The T-DNA and a part of the sample to be tested will form double strands by base complementary pairing, and detProbe and another part of the sample to be tested will also be base complementary paired, thus forming a sandwich structure composed of the test sample, detProbe-Au-ssDNA complex and T-DNA. As Au-SA participates in the reaction, AuNPs and DNA will form a three-dimensional network, so that a red band can be seen at the T line. As the liquid continues to migrate, the detProbe-Au-ssDNA complex and Au-SA will be captured by C-DNA and form a red band at the C line, which means that the test is positive. However, if there is no target in the sample, the complex will not interact when the sample flows through the detection line, and only a red band will appear at the C line, which means that the test is negative.
After adding Au-SA to such LFBs, as functional particles that amplify the signals, they can help reduce the detection limit of target nucleic acids, and improve detection sensitivity, thereby realizing good reproducibility and stability.
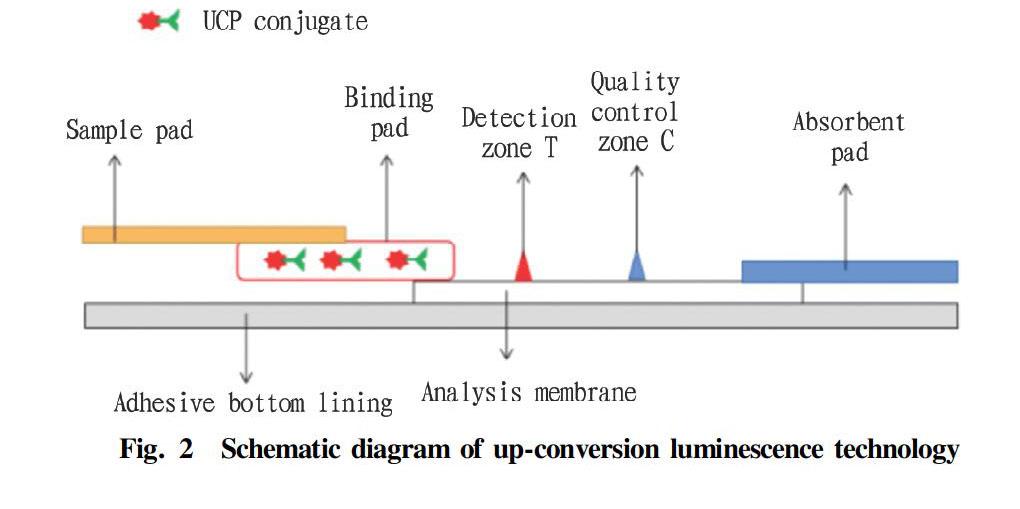
The principle of the up-conversion luminescence technology test strips is to combine up-conversion luminescence technology and immunochromatography. By scanning UCPs luminescent signals, rapid on-site detection of target antibodies or antigens is realized. The test strips are composed of five parts: a sample pad, a binding pad, an analytical membrane, an absorbent pad and an adhesive bottom lining[30], as shown in Fig. 2.
After dropping a sample to be tested on the sample pad, it enters the binding pad through permeation and siphon action, and dissociate the UCP conjugate (marker) in the binding pad, and under the siphon effect of the absorbent pad, the sample flows from the binding pad into the analysis membrane, and then to the absorbent pad. During the whole process, a series of specific immune reactions will occur among the marker, the sample to be tested, the detection zone, and the quality control zone, so that UCP reaction signals can be scanned at the detection zone.
Compared with colloidal gold immunochromatography, UCPs as a marker can meet both qualitative and quantitative requirements, and are suitable for multiplex analysis. And it can still detect targets under the lowest detection limit, with excellent sensitivity; and it can detect nucleic acids of a variety of pathogens, without obvious cross-reaction, and has good specificity.
Prospects
Whether in the field of detecting pathogenic microorganisms such as medical pathogens or food pathogens, rapid on-site instant detection has become a necessity, but new requirements are also put forward for the sensitivity and specificity of detection methods. Nanomaterials have good adsorption capacity, surface effect, small size effect, quantum effect and macro quantum tunneling effect. Combined with biosensors, they greatly improve the sensitivity, stability and antibody molecule adsorption capacity of biosensors, making them a rapid detection method in the field. With a hand-held signal collection device, nano-biosensors are expected to become a fast on-site detection tool. LFBs are easy to operate and does not require professional operators and equipment. It is a very promising on-site rapid detection technology. With the application of nanomaterials to the test strip technology, the detection sensitivity can be greatly improved, and more importantly, quantitative detection can be achieved with the test strip technology, which greatly expands the application scenarios of the test strip technology. Of course, the application of nanomaterials to rapid on-site testing requires continued research and development in terms of cost and stability to speed up the process of application.
References
[1] CHEN Y, QIAN C, LIU C, et al. Nucleic acid amplification free biosensors for pathogen detection[J].Biosensors and Bioelectronics, 2020: 112049.
[2] STASYUK N, SMUTOK O, DEMKIV O, et al. Synthesis, Catalytic properties and application in biosensorics of nanozymes and electronanocatalysts: A review[J]. Sensors (Basel, Switzerland), 2020, 20(16): 4509-4550.
[3] DADASHPOUR M, PILEHVAR-SOLTANAHMADI Y, MOHAMMADI SA, et al. Watercress-based electrospun nanofibrous scaffolds enhance proliferation and stemness preservation of human adipose-derived stem cells[J]. Artificial Cells Nanomedicine & Biotechnology, 2018, 46(4): 819-830.
[4] LOTFI-ATTARI J, PILEHVAR-SOLTANAHMADI Y, DADASHPOUR M, et al. Co-delivery of curcumin and chrysin by polymeric nanoparticles inhibit synergistically growth and hTERT gene expression in human colorectal cancer cells[J]. Nutrition & Cancer, 2017, 69(8): 1290-1299
[5] MEHRJOU B, MO S, DEHGHAN-BANIANI D, et al. Antibacterial and cytocompatible nanoengineered silk-based materials for orthopedic implants and tissue engineering[J]. ACS Applied Materials & Interfaces, 2019, 11(35): 31605-31614.
[6] AURA AM, DAGATA R, SPOTO G. Ultrasensitive detection of Staphylococcus aureus and Listeria monocytogenes genomic DNA by nanoparticle-enhanced surface plasmon resonance imaging[J]. ChemistrySelect, 2017, 2(24): 7024-7030.
[7] XIE J, ZHENG Y, YING JY. Protein-directed synthesis of highly fluorescent gold nanoclusters[J]. Journal of the American Chemical Society, 2009, 131(3): 888-889.
[8] JIN R, ZENG C, ZHOU M, et al. Atomically precise colloidal metal nanoclusters and nanoparticles: Fundamentals and opportunities[J]. Chemical Reviews, 2016, 116(18): 10346-10413.
[9] INDRANATH, CHAKRABORTY, THALAPPIL, et al. Atomically precise clusters of noble metals: Emerging link between atoms and nanoparticles[J]. Chem. Rev, 2017, 117(12): 8208-8271.
[10] NONAPPA. Luminescent gold nanoclusters for bioimaging applications[J]. Beilstein Journal of Nanotechnology, 2020, 11(1): 533-546.
[11] AGER D, ARJUNAN VASANTHA V, CROMBEZ R, et al. Aqueous graphene dispersions–optical properties and stimuli-responsive phase transfer[J]. Acs Nano, 2014, 8(11): 11191-205.
[12] LEE C, WEI X, KYSAR JW, et al. Measurement of the elastic properties and intrinsic strength of monolayer graphene[J]. Science, 2008, 321(5887): 385-388.
[13] LI S, APHALE AN, MACWAN IG, et al. Graphene oxide as a quencher for fluorescent assay of amino acids, peptides, and proteins[J]. ACS Applied Materials & Interfaces, 2012, 4(12): 7069-75.
[14] WANG YH, DENG HH, LIU YH, et al. Partially reduced graphene oxide as highly efficient DNA nanoprobe[J]. Biosensors & Bioelectronics, 2016, 80(3):140-145.
[15] MORALES-NARVAEZ E, MERKOGI A. Graphene oxide as an optical biosensing plattorm[J]. Advanced Materials, 2012 , 24(25): 3298-308.
[16] MA H, WU D, CUI Z, et al. Graphene-based optical and electrochemical biosensors: A review[J]. Analytical Letters, 2013, 46(1): 1-17.
[17] KOCHMANN S, HIRSCH T, WOLFBEIS OS. Graphenes in chemical sensors and biosensors[J]. TrAC Trends in Analytical Chemistry, 2012, 39(none): 87-113.
[18] WEISS NO, ZHOU H, LIAO L, et al. Graphene: An emerging electronic material (Adv. Mater. 43/2012)[J]. Advanced Materials, 2012, 24(43): 5782-825.
[19] WELSHER K, LIU Z, DARANCIANG D, et al. Selective probing and imaging of cells with single walled carbon nanotubes as near-infrared fluorescent molecules[J]. Nano Letters, 2007, 8(2): 586-590.
[20] KIM S, FISHER B, EISLER, HANS-J RGEN, et al. Type-II quantum dots: CdTe/CdSe(core/shell) and CdSe/ZnTe(core/shell) heterostructures[J]. Journal of the American Chemical Society, 2003, 125(38): 11466-11467.
[21] BIJU V, KANEMOTO R, MATSUMOTO Y, et al. Photoinduced photoluminescence variations of CdSe quantum dots in polymer solutions[J]. Journal of Physical Chemistry C, 2007, 111(22): 7924-7932.
[22] XIAO Q, HUANG S, SU W, et al. Facile synthesis and characterization of highly fluorescent and biocompatible N-acetyl-L-cysteine capped CdTe/CdS/ZnS core/shell/shell quantum dots in aqueous phase[J]. Nanotechnology, 2012, 23(49): 495717-27.
[23] WANG F, BANERJEE D, LIU Y, et al. Upconversion nanoparticles in biological labeling, imaging, and therapy[J]. Analyst, 2010, 135(8): 1839-1854.
[24] HOLFORD T, DAVIS F, HIGSON S. Recent trends in antibody based sensors[J]. Biosensors and Bioelectronics, 2012, 34(1): 12-24.
[25] OMIDFAR K, KHORSAND F, AZIZI M. New analytical applications of gold nanoparticles as label in antibody based sensors[J]. Biosensors and Bioelectronics, 2013(43): 336-347.
[26] VISWANATHAN S, RANI C, VIJAY A, et al. Disposable electrochemical immunosensor for carcinombryonic antigen using ferrocene liposome and MWCNT screen-printed electrode[J]. Biosensors and Bioelectronics, 2009, 24(7): 1984-1989.
[27] CASTANEDA M, ALEGRET S, MERKOCI A. Electrochemical sensing of DNA using gold nanoparticles[J]. Electro Analysis, 2007, 19(7/8): 743-753.
[28] MACKEY D, AMBROSI A, KILLARD A, et al. Double-codified gold nanolabels for enhanced immunoanalysis[J]. Analytical Chemistry, 2007, 79(14): 5232-5240.
[29] GAO Y, DENG X, WEN W, et al. Ultrasensitive paper based nucleic acid detection realized by three dimensional DNA-AuNPs network amplification[J]. Biosensors and Bioelectronics, 2017(92): 529-535.
- 农业生物技术(英文版)的其它文章
- Anti-inflammatory Activity and Mechanism of Total Flavonoids from the Phloem of Paulownia elongate S.Y. Hu in LPS-stimulated RAW264.7 Macrophages
- Comparative Genomic Analysis of Boron Transport Gene Family in Arabidopsis and Five Crops
- Effects of Different Water-saving Irrigation Methods on Fruit Quality and Yield of Snow Melon
- Field Control Effects and Crop Safety Assessment of Triazole Fungicides on Apple Rust
- Effects of Acetylacetone Solution Soaking on Agrobacterium-transformed Maize Seed Buds
- Effects of Meteorological Factors on Overwintering Ability, Yield and Quality of Forage Rape

